Evolution at the Origins of Life?
Abstract
1. Introduction
2. Evolutionary Theory beyond Biology
2.1. Some Central Questions
2.2. Generalizing Accounts
2.3. Reducing Accounts
2.3.1. Strong Reducing Accounts
2.3.2. Weak Reducing Accounts
3. The Origins of Life and Its Early Development
3.1. General Considerations
3.2. The Role of Catalysis
3.3. Protometabolic Networks, Genetics, and Protocells
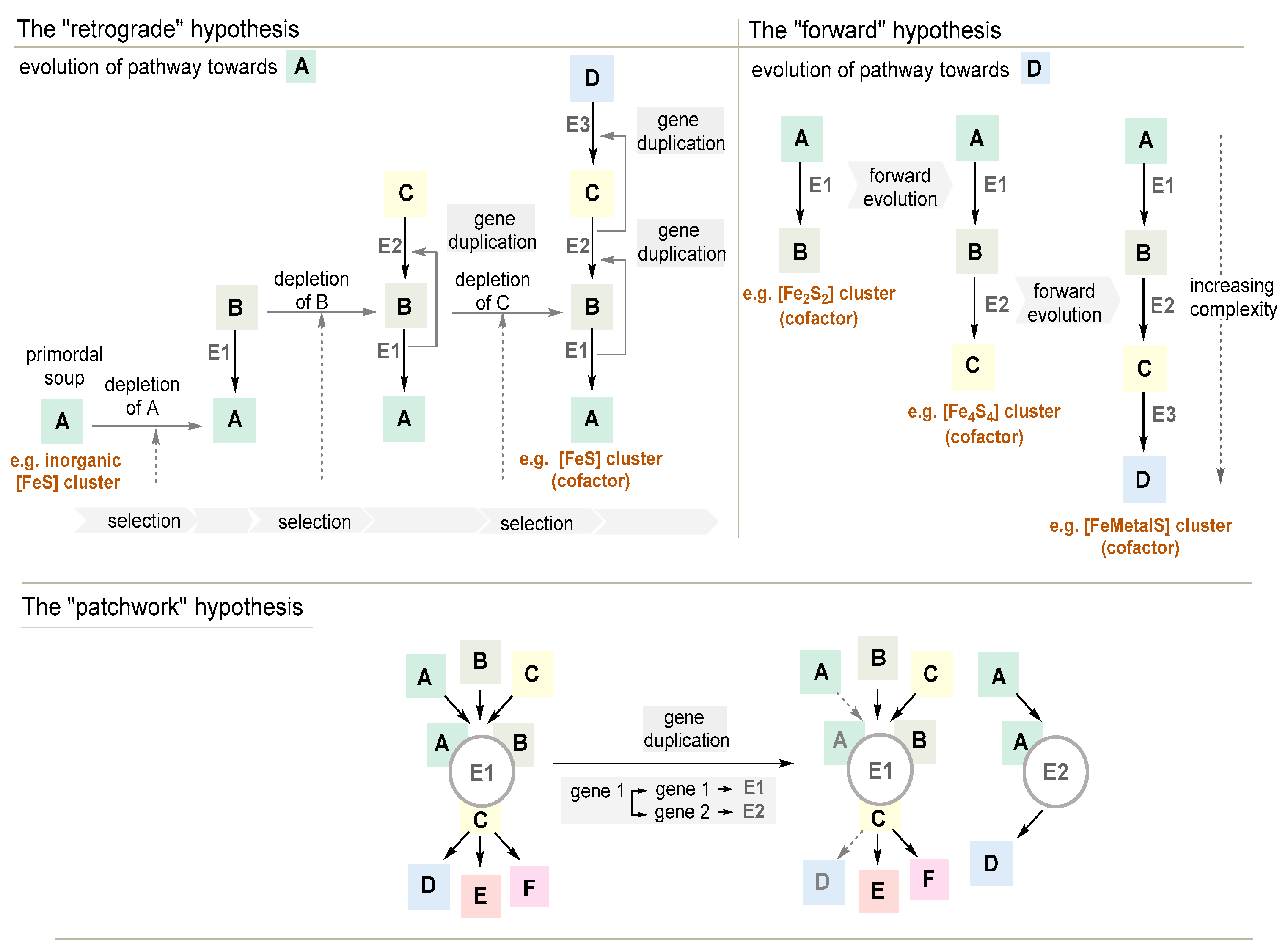
3.3.1. The Retrograde Hypothesis
3.3.2. The Forward Hypothesis
3.3.3. The Patchwork Hypothesis
3.3.4. The Mixed Origin of Metabolic Pathways
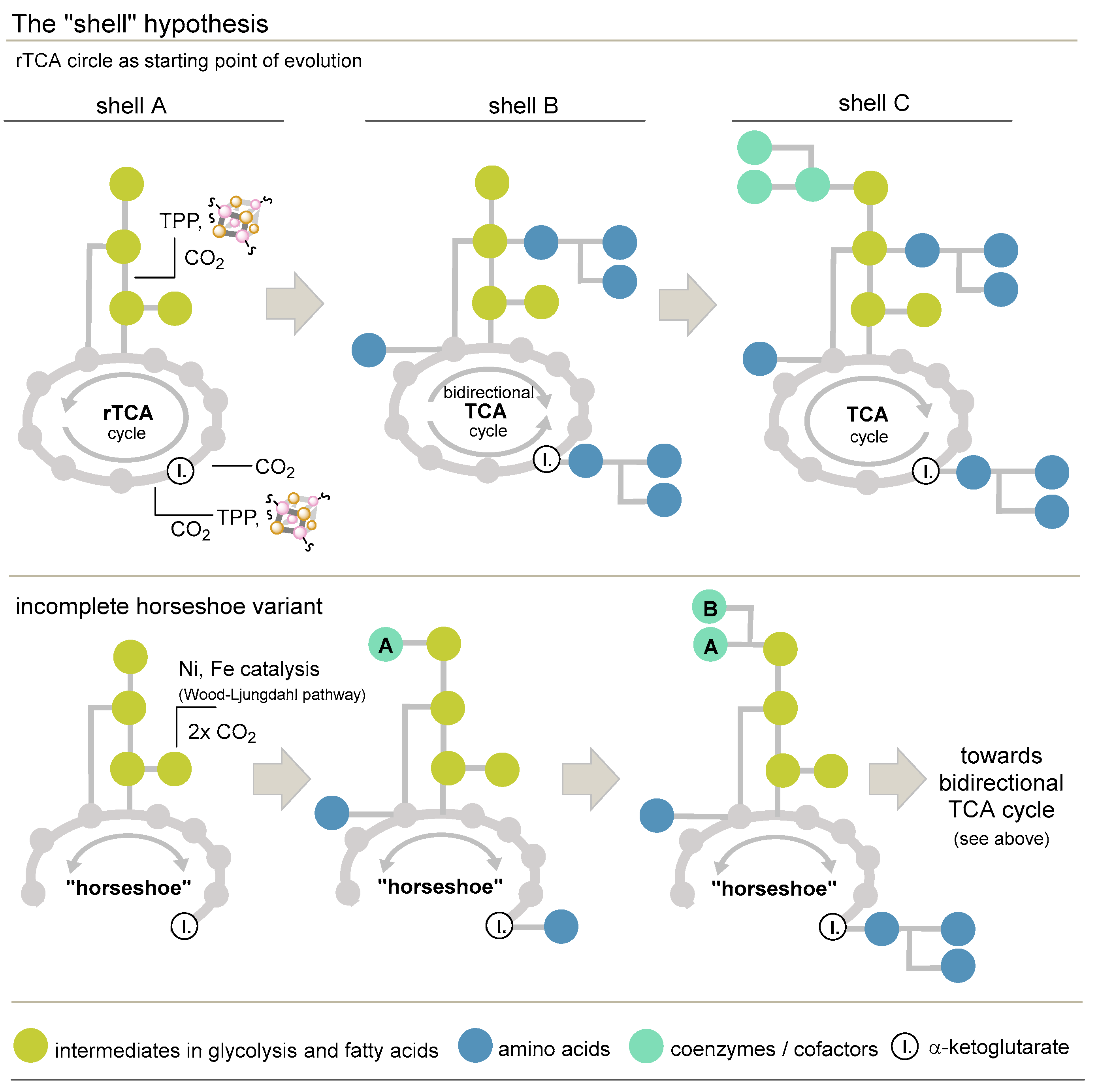
3.3.5. The Shell Hypothesis and a Proposal for a Modification
4. The Use of Evolutionary Concepts in Origins of Life Research
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darwin, C. On the Origin of Species by Means of Natural Selection or the Preservation of Favoured Races in the Struggle for Life, 1st ed.; John Murray, Albemarle Street: London, UK, 1859. [Google Scholar]
- Huxley, J. Evolution: The Modern Synthesis, 1st ed.; Allen & Unwin: London, UK, 1942. [Google Scholar]
- Laland, K.N.; Uller, T.; Feldman, M.W.; Sterelny, K.; Müller, G.B.; Moczek, A.; Jablonka, E.; Odling-Smee, J. The Extended Evolutionary Synthesis: Its Structure, Assumptions and Predictions. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151019. [Google Scholar] [CrossRef] [PubMed]
- Laland, K.; Uller, T.; Feldman, M.; Sterelny, K.; Müller, G.B.; Moczek, A.; Jablonka, E.; Odling-Smee, J.; Wray, G.A.; Hoekstra, H.E.; et al. Does Evolutionary Theory Need a Rethink? Nature 2014, 514, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Pigliucci, M.; Newman, S.A. Evolution—The Extended Synthesis; Pigliucci, M., Müller, G.B., Eds.; The MIT Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Desmond, H.; Ariew, A.; Huneman, P.; Reydon, T.A.C. The Varieties of Darwinism: Explanation, Logic, and Worldview. Q. Rev. Biol. 2024, in press.
- Reydon, T.A.C. Generalized Darwinism as Modest Unification. Am. Philos. Q. 2021, 58, 79–94. [Google Scholar] [CrossRef]
- du Crest, A.; Valković, M.; Ariew, A.; Desmond, H.; Huneman, P.; Reydon, T.A.C. (Eds.) Evolutionary Thinking across Disciplines: Problems and Perspectives in Generalized Darwinism; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Liu, K.; Blokhuis, A.; van Ewijk, C.; Kiani, A.; Wu, J.; Roos, W.H.; Otto, S. Light-Driven Eco-Evolutionary Dynamics in a Synthetic Replicator System. Nat. Chem. 2023, 16, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-C.; Liu, Z.; Chen, I.A. Encapsulation of Ribozymes inside Model Protocells Leads to Faster Evolutionary Adaptation. Proc. Natl. Acad. Sci. USA 2021, 118, e2025054118. [Google Scholar] [CrossRef]
- Abil, Z.; Danelon, C. Roadmap to Building a Cell: An Evolutionary Approach. Front. Bioeng. Biotechnol. 2020, 8, 927. [Google Scholar] [CrossRef]
- Koonin, E.V. The Logic of Chance: The Nature and Origin of Biological Evolution; FT Press: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Koonin, E.V.; Novozhilov, A.S. Origin and Evolution of the Universal Genetic Code. Annu. Rev. Genet. 2017, 51, 45–62. [Google Scholar] [CrossRef]
- Koonin, E.V.; Novozhilov, A.S. Origin and Evolution of the Genetic Code: The Universal Enigma. IUBMB Life 2009, 61, 99–111. [Google Scholar] [CrossRef]
- Koonin, E.V.; Wolf, Y.I. The Fundamental Units, Processes and Patterns of Evolution, and the Tree of Life Conundrum. Biol. Direct 2009, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Findley, A.M.; Findley, G.L. The Generalized Genetic Code. A Note on Order-Isomorphism/Order-Equivalence Relations. Int. J. Quantum Chem. 1982, 22, 59–63. [Google Scholar] [CrossRef]
- Rosandić, M.; Paar, V. Standard Genetic Code vs. Supersymmetry Genetic Code—Alphabetical Table vs. Physicochemical Table. Biosystems 2022, 218, 104695. [Google Scholar] [CrossRef]
- Rosandić, M.; Paar, V. The Supersymmetry Genetic Code Table and Quadruplet Symmetries of DNA Molecules Are Unchangeable and Synchronized with Codon-Free Energy Mapping during Evolution. Genes 2023, 14, 2200. [Google Scholar] [CrossRef]
- Négadi, T. Revealing the Genetic Code Symmetries through Computations Involving Fibonacci-like Sequences and Their Properties. Computation 2023, 11, 154. [Google Scholar] [CrossRef]
- Lewontin, R.C. The Units of Selection. Annu. Rev. Ecol. Syst. 1970, 1, 1–18. [Google Scholar] [CrossRef]
- Godfrey-Smith, P. Conditions for Evolution by Natural Selection. J. Philos. 2007, 104, 489–516. [Google Scholar] [CrossRef]
- Godfrey-Smith, P.; Godfrey-Smith, P. Darwinian Populations and Natural Selection; Oxford University Press: Oxford, NY, USA, 2009. [Google Scholar]
- Lloyd, E. Adaptation; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Godfrey-Smith, P. Three Kinds of Adaptationism. In Adaptationism and Optimality; Orzack, S.H., Sober, E., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 335–357. [Google Scholar]
- Aldrich, H.E.; Hodgson, G.M.; Hull, D.L.; Knudsen, T.; Mokyr, J.; Vanberg, V.J. In Defence of Generalized Darwinism. J. Evol. Econ. 2008, 18, 577–596. [Google Scholar] [CrossRef]
- Hannan, M.T.; Freeman, J. The Population Ecology of Organizations. Am. J. Sociol. 1977, 82, 929–964. [Google Scholar] [CrossRef]
- Hannan, M.T.; Polos, L.; Carroll, G.R. Logics of Organization Theory; Princeton University Press: Princeton, NJ, USA, 2007. [Google Scholar]
- Hodgson, G.M. Darwinism in Economics: From Analogy to Ontology. J. Evol. Econ. 2002, 12, 259–281. [Google Scholar] [CrossRef]
- Hodgson, G.M.; Knudsen, T. Darwin’s Conjecture: The Search for General Principles of Social and Economic Evolution; University of Chicago Press: Chicago, IL, USA, 2013. [Google Scholar]
- Reydon, T.A.C.; Scholz, M. Why Organizational Ecology Is not a Darwinian Research Program. Philos. Soc. Sci. 2009, 39, 408–439. [Google Scholar] [CrossRef]
- Hannan, M. Rethinking Organizational Ecology in Light of Developments in Cognitive Science and Natural Language Processing. SocArXiv 2022. [Google Scholar] [CrossRef]
- Reydon, T.A.C.; Scholz, M. Searching for Darwinism in Generalized Darwinism. Br. J. Philos. Sci. 2015, 66, 561–589. [Google Scholar] [CrossRef]
- Dennett, D. Darwin’s Dangerous Idea; Simon & Schuster: New York, NY, USA, 1996. [Google Scholar]
- Dawkins, R. The Selfish Gene; Oxford University Press: Oxford, UK, 1976. [Google Scholar]
- Hull, D.L. Individuality and Selection. Annu. Rev. Ecol. Syst. 1980, 11, 311–332. [Google Scholar] [CrossRef]
- Smith, J.M.; Szathmary, E. The Major Transitions in Evolution; OUP Oxford: Oxford, UK, 1997. [Google Scholar]
- Szathmáry, E. Toward Major Evolutionary Transitions Theory 2.0. Proc. Natl. Acad. Sci. USA 2015, 112, 10104–10111. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J. The Structure of Evolutionary Theory; Belknap Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Gould, S.J. The New York Review of Books; The New York Review Books: New York, NY, USA, 1997. [Google Scholar]
- Bourrat, P. In What Sense Can There Be Evolution by Natural Selection Without Perfect Inheritance? Int. Stud. Philos. Sci. 2019, 32, 13–31. [Google Scholar] [CrossRef]
- Pross, A. What Is Life? How Chemistry Becomes Biology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Katsnelson, M.I.; Wolf, Y.I.; Koonin, E.V. Towards Physical Principles of Biological Evolution. Phys. Scr. 2018, 93, 043001. [Google Scholar] [CrossRef]
- Vanchurin, V.; Wolf, Y.I.; Koonin, E.V.; Katsnelson, M.I. Thermodynamics of Evolution and the Origin of Life. Proc. Natl. Acad. Sci. USA 2022, 119, e2120042119. [Google Scholar] [CrossRef]
- Vanchurin, V.; Wolf, Y.I.; Katsnelson, M.I.; Koonin, E.V. Toward a Theory of Evolution as Multilevel Learning. Proc. Natl. Acad. Sci. USA 2022, 119, e2120037119. [Google Scholar] [CrossRef]
- Eigen, M. Self-Replication and Molecular Evolution. In Evolution from Molecules to Men; Bendall, D.S., Ed.; Cambridge University Press: Cambridge, UK, 1983; pp. 105–130. [Google Scholar]
- Eigen, M.; Schuster, P. The Hypercycle: A Principle of Natural Self-Organization; Springer: Berlin/Heidelberg, Germany, 1979. [Google Scholar]
- Kauffman, S.A. The Origins of Order: Self-Organization and Selection in Evolution; Oxford University Press: Oxford, NY, USA, 1993. [Google Scholar]
- Pross, A.; Khodorkovsky, V. Extending the Concept of Kinetic Stability: Toward a Paradigm for Life. J. Phys. Org. Chem. 2004, 17, 312–316. [Google Scholar] [CrossRef]
- Pross, A. Toward a General Theory of Evolution: Extending Darwinian Theory to Inanimate Matter. J. Syst. Chem. 2011, 2, 1. [Google Scholar] [CrossRef]
- Pross, A. How Does Biology Emerge From Chemistry? Orig. Life Evol. Biosph. 2012, 42, 433–444. [Google Scholar] [CrossRef]
- Eigen, M. Selforganization of Matter and the Evolution of Biological Macromolecules. Naturwissenschaften 1971, 58, 465–523. [Google Scholar] [CrossRef]
- Camprubí, E.; de Leeuw, J.W.; House, C.H.; Raulin, F.; Russell, M.J.; Spang, A.; Tirumalai, M.R.; Westall, F. The Emergence of Life. Space Sci. Rev. 2019, 215, 56. [Google Scholar] [CrossRef]
- Gilbert, W. Origin of Life: The RNA World. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Robertson, M.P.; Joyce, G.F. The Origins of the RNA World. Cold Spring Harb. Perspect. Biol. 2012, 4, a003608. [Google Scholar] [CrossRef]
- Anet, F.A. The Place of Metabolism in the Origin of Life. Curr. Opin. Chem. Biol. 2004, 8, 654–659. [Google Scholar] [CrossRef]
- Weiss, M.C.; Sousa, F.L.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W.F. The Physiology and Habitat of the Last Universal Common Ancestor. Nat. Microbiol. 2016, 1, 16116. [Google Scholar] [CrossRef]
- Koonin, E.V. Frozen Accident Pushing 50: Stereochemistry, Expansion, and Chance in the Evolution of the Genetic Code. Life 2017, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Kirschning, A. On the Evolutionary History of the Twenty Encoded Amino Acids. Chem. A Eur. J. 2022, 28, e202201419. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.A.; Piedrafita, G.; Ralser, M. The Widespread Role of Non-Enzymatic Reactions in Cellular Metabolism. Curr. Opin. Biotechnol. 2015, 34, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Jürjens, G.; Kirschning, A.; Candito, D.A. Lessons from the Synthetic Chemist Nature. Nat. Prod. Rep. 2015, 32, 723–737. [Google Scholar] [CrossRef][Green Version]
- Miller, S.L. A Production of Amino Acids Under Possible Primitive Earth Conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef]
- Wächtershäuser, G. Before Enzymes and Templates: Theory of Surface Metabolism. Microbiol. Rev. 1988, 52, 452–484. [Google Scholar] [CrossRef] [PubMed]
- Wächtershäuser, G. Evolution of the First Metabolic Cycles. Proc. Natl. Acad. Sci. USA 1990, 87, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Kirschning, A. Coenzymes and Their Role in the Evolution of Life. Angew. Chem. Int. Ed. 2021, 60, 6242–6269. [Google Scholar] [CrossRef] [PubMed]
- McCown, P.J.; Corbino, K.A.; Stav, S.; Sherlock, M.E.; Breaker, R.R. Riboswitch Diversity and Distribution. RNA 2017, 23, 995–1011. [Google Scholar] [CrossRef] [PubMed]
- Serganov, A.; Patel, D.J. Metabolite Recognition Principles and Molecular Mechanisms Underlying Riboswitch Function. Annu. Rev. Biophys. 2012, 41, 343–370. [Google Scholar] [CrossRef]
- Camprubi, E.; Jordan, S.F.; Vasiliadou, R.; Lane, N. Iron Catalysis at the Origin of Life. IUBMB Life 2017, 69, 373–381. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gatreddi, S.; Gupta, S.; Nevarez, J.L.; Rankin, J.A.; Turmo, A.; Hu, J.; Hausinger, R.P. Unveiling the Mechanisms and Biosynthesis of a Novel Nickel-Pincer Enzyme. Biochem. Soc. Trans. 2022, 50, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Hanson, R.E.; Cronan, J.E. Biotin Synthesis Begins by Hijacking the Fatty Acid Synthetic Pathway. Nat. Chem. Biol. 2010, 6, 682–688. [Google Scholar] [CrossRef]
- Cronan, J.E. Assembly of Lipoic Acid on Its Cognate Enzymes: An Extraordinary and Essential Biosynthetic Pathway. Microbiol. Mol. Biol. Rev. 2016, 80, 429–450. [Google Scholar] [CrossRef]
- Vignais, P.M.; Billoud, B. Occurrence, Classification, and Biological Function of Hydrogenases: An Overview. Chem. Rev. 2007, 107, 4206–4272. [Google Scholar] [CrossRef] [PubMed]
- Gruber, N.; Galloway, J.N. An Earth-System Perspective of the Global Nitrogen Cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef]
- Raymond, J.; Siefert, J.L.; Staples, C.R.; Blankenship, R.E. The Natural History of Nitrogen Fixation. Mol. Biol. Evol. 2004, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W. Enzymology of the Wood–Ljungdahl Pathway of Acetogenesis. Ann. N. Y. Acad. Sci. 2008, 1125, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood–Ljungdahl Pathway of CO2 Fixation. Biochim. Biophys. Acta Proteins Proteom. 2008, 1784, 1873–1898. [Google Scholar] [CrossRef]
- Deamer, D.W.; Deamer, D.W. Assembling Life: How Can Life Begin on Earth and Other Habitable Planets? Oxford University Press: Oxford, NY, USA, 2019. [Google Scholar]
- Sutherland, J.D. Opinion: Studies on the Origin of Life—The End of the Beginning. Nat. Rev. Chem. 2017, 1, 0012. [Google Scholar] [CrossRef]
- Benner, S.A.; Kim, H.-J.; Biondi, E. Prebiotic Chemistry That Could not not Have Happened. Life 2019, 9, 84. [Google Scholar] [CrossRef]
- Wołos, A.; Roszak, R.; Żądło-Dobrowolska, A.; Beker, W.; Mikulak-Klucznik, B.; Spólnik, G.; Dygas, M.; Szymkuć, S.; Grzybowski, B.A. Synthetic Connectivity, Emergence, and Self-Regeneration in the Network of Prebiotic Chemistry. Science 2020, 369, eaaw1955. [Google Scholar] [CrossRef]
- Harrison, S.A.; Lane, N. Life as a Guide to Prebiotic Nucleotide Synthesis. Nat. Commun. 2018, 9, 5176. [Google Scholar] [CrossRef]
- Copley, S.D.; Smith, E.; Morowitz, H.J. The Origin of the RNA World: Co-Evolution of Genes and Metabolism. Bioorg. Chem. 2007, 35, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.T.; Yadav, M.; Krishnamurthy, R.; Springsteen, G. A Plausible Metal-Free Ancestral Analogue of the Krebs Cycle Composed Entirely of α-Ketoacids. Nat. Chem. 2020, 12, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Muchowska, K.B.; Varma, S.J.; Chevallot-Beroux, E.; Lethuillier-Karl, L.; Li, G.; Moran, J. Metals Promote Sequences of the Reverse Krebs Cycle. Nat. Ecol. Evol. 2017, 1, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, G. Alternative Pathways of Carbon Dioxide Fixation: Insights into the Early Evolution of Life? Annu. Rev. Microbiol. 2011, 65, 631–658. [Google Scholar] [CrossRef]
- Karp, P.D.; Keseler, I.M.; Shearer, A.; Latendresse, M.; Krummenacker, M.; Paley, S.M.; Paulsen, I.; Collado-Vides, J.; Gama-Castro, S.; Peralta-Gil, M.; et al. Multidimensional Annotation of the Escherichia Coli K-12 Genome. Nucleic Acids Res. 2007, 35, 7577–7590. [Google Scholar] [CrossRef] [PubMed]
- Ralser, M. The RNA World and the Origin of Metabolic Enzymes. Biochem. Soc. Trans. 2014, 42, 985–988. [Google Scholar] [CrossRef]
- Muchowska, K.B.; Chevallot-Beroux, E.; Moran, J. Recreating Ancient Metabolic Pathways before Enzymes. Bioorg. Med. Chem. 2019, 27, 2292–2297. [Google Scholar] [CrossRef]
- Wu, L.-F.; Sutherland, J.D. Provisioning the Origin and Early Evolution of Life. Emerg. Top. Life Sci. 2019, 3, 459–468. [Google Scholar] [CrossRef]
- Orgel, L.E. The Implausibility of Metabolic Cycles on the Prebiotic Earth. PLoS Biol. 2008, 6, e18. [Google Scholar] [CrossRef]
- Horowitz, N.H. On the Evolution of Biochemical Syntheses. Proc. Natl. Acad. Sci. USA 1945, 31, 153–157. [Google Scholar] [CrossRef]
- King, G.A.M. Symbiosis and the Origin of Life. Orig. Life Evol. Biosph. 1977, 8, 39–53. [Google Scholar] [CrossRef]
- Granick, S. Speculations on the Origins and Evolution of Photosynthesis. Ann. N. Y. Acad. Sci. 1957, 69, 292–308. [Google Scholar] [CrossRef]
- Fani, R.; Fondi, M. Origin and Evolution of Metabolic Pathways. Phys. Life Rev. 2009, 6, 23–52. [Google Scholar] [CrossRef]
- Kirschning, A. On the Evolution of Coenzyme Biosynthesis. Nat. Prod. Rep. 2022, 39, 2175–2199. [Google Scholar] [CrossRef]
- Jensen, R.A. Enzyme Recruitment in Evolution of New Function. Annu. Rev. Microbiol. 1976, 30, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Yčas, M. On Earlier States of the Biochemical System. J. Theor. Biol. 1974, 44, 145–160. [Google Scholar] [CrossRef]
- Bromke, M.A. Amino Acid Biosynthesis Pathways in Diatoms. Metabolites 2013, 3, 294–311. [Google Scholar] [CrossRef] [PubMed]
- Lazcano, A.; Miller, S.L. On the Origin of Metabolic Pathways. J. Mol. Evol. 1999, 49, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Delaye, L.; Lazcano, A. Prebiological Evolution and the Physics of the Origin of Life. Phys. Life Rev. 2005, 2, 47–64. [Google Scholar] [CrossRef]
- Muchowska, K.B.; Varma, S.J.; Moran, J. Nonenzymatic Metabolic Reactions and Life’s Origins. Chem. Rev. 2020, 120, 7708–7744. [Google Scholar] [CrossRef] [PubMed]
- Kitadai, N.; Maruyama, S. Origins of Building Blocks of Life: A Review. Geosci. Front. 2018, 9, 1117–1153. [Google Scholar] [CrossRef]
- Esquilin-Lebron, K.; Dubrac, S.; Barras, F.; Boyd, J.M. Bacterial Approaches for Assembling Iron-Sulfur Proteins. mBio 2021, 12, e02425-21. [Google Scholar] [CrossRef]
- Morowitz, H.J. A Theory of Biochemical Organization, Metabolic Pathways, and Evolution. Complexity 1999, 4, 39–53. [Google Scholar] [CrossRef]
- He, X.; Sha, R.; Zhuo, R.; Mi, Y.; Chaikin, P.M.; Seeman, N.C. Exponential Growth and Selection in Self-Replicating Materials from DNA Origami Rafts. Nat. Mater. 2017, 16, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, M.Y.; Cournoyer, J.E.; Bram, S.; Mehta, A.P. Evolution and Synthetic Biology. Curr. Opin. Microbiol. 2023, 76, 102394. [Google Scholar] [CrossRef]
- Yewdall, N.A.; Mason, A.F.; van Hest, J.C.M. The Hallmarks of Living Systems: Towards Creating Artificial Cells. Interface Focus. 2018, 8, 20180023. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J.W.; Bartel, D.P.; Luisi, P.L. Synthesizing Life. Nature 2001, 409, 387–390. [Google Scholar] [CrossRef]
- Spitzer, J.; Pielak, G.J.; Poolman, B. Emergence of Life: Physical Chemistry Changes the Paradigm. Biol. Direct 2015, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, N.; Usui, K.; Kazuta, Y.; Sunami, T.; Matsuura, T.; Yomo, T. Darwinian Evolution in a Translation-Coupled RNA Replication System within a Cell-like Compartment. Nat. Commun. 2013, 4, 2494. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, S.; Chaput, J.C. Darwinian Evolution of an Alternative Genetic System Provides Support for TNA as an RNA Progenitor. Nat. Chem. 2012, 4, 183–187. [Google Scholar] [CrossRef]
- Paegel, B.M.; Joyce, G.F. Darwinian Evolution on a Chip. PLoS Biol. 2008, 6, e85. [Google Scholar] [CrossRef] [PubMed]
- Mizuuchi, R.; Furubayashi, T.; Ichihashi, N. Evolutionary Transition from a Single RNA Replicator to a Multiple Replicator Network. Nat. Commun. 2022, 13, 1460. [Google Scholar] [CrossRef]
- Mayer, C.; Schreiber, U.; Dávila, M.J.; Schmitz, O.J.; Bronja, A.; Meyer, M.; Klein, J.; Meckelmann, S.W. Molecular Evolution in a Peptide-Vesicle System. Life 2018, 8, 16. [Google Scholar] [CrossRef]
- Pinheiro, V.B.; Taylor, A.I.; Cozens, C.; Abramov, M.; Renders, M.; Zhang, S.; Chaput, J.C.; Wengel, J.; Peak-Chew, S.-Y.; McLaughlin, S.H.; et al. Synthetic Genetic Polymers Capable of Heredity and Evolution. Science 2012, 336, 341–344. [Google Scholar] [CrossRef]
- Attwater, J.; Wochner, A.; Holliger, P. In-Ice Evolution of RNA Polymerase Ribozyme Activity. Nat. Chem. 2013, 5, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.P.; Joyce, G.F. Highly Efficient Self-Replicating RNA Enzymes. Chem. Biol. 2014, 21, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Adamala, K.; Szostak, J.W. Competition between Model Protocells Driven by an Encapsulated Catalyst. Nat. Chem. 2013, 5, 495–501. [Google Scholar] [CrossRef]
- Matsumura, S.; Kun, Á.; Ryckelynck, M.; Coldren, F.; Szilágyi, A.; Jossinet, F.; Rick, C.; Nghe, P.; Szathmáry, E.; Griffiths, A.D. Transient Compartmentalization of RNA Replicators Prevents Extinction Due to Parasites. Science 2016, 354, 1293–1296. [Google Scholar] [CrossRef]
- Sadownik, J.W.; Mattia, E.; Nowak, P.; Otto, S. Diversification of Self-Replicating Molecules. Nat. Chem. 2016, 8, 264–269. [Google Scholar] [CrossRef]
- Lu, H.; Blokhuis, A.; Turk-MacLeod, R.; Karuppusamy, J.; Franconi, A.; Woronoff, G.; Jeancolas, C.; Abrishamkar, A.; Loire, E.; Ferrage, F.; et al. Small-Molecule Autocatalysis Drives Compartment Growth, Competition and Reproduction. Nat. Chem. 2023, 16, 70–78. [Google Scholar] [CrossRef]
- Ameta, S.; Matsubara, Y.J.; Chakraborty, N.; Krishna, S.; Thutupalli, S. Self-Reproduction and Darwinian Evolution in Autocatalytic Chemical Reaction Systems. Life 2021, 11, 308. [Google Scholar] [CrossRef]
- Adamski, P.; Eleveld, M.; Sood, A.; Kun, Á.; Szilágyi, A.; Czárán, T.; Szathmáry, E.; Otto, S. From Self-Replication to Replicator Systems En Route to de Novo Life. Nat. Rev. Chem. 2020, 4, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Danger, G.; d’Hendecourt, L.L.S.; Pascal, R. On the Conditions for Mimicking Natural Selection in Chemical Systems. Nat. Rev. Chem. 2020, 4, 102–109. [Google Scholar] [CrossRef]
- Meléndez-Hevia, E.; Montero-Gómez, N.; Montero, F. From Prebiotic Chemistry to Cellular Metabolism—The Chemical Evolution of Metabolism before Darwinian Natural Selection. J. Theor. Biol. 2008, 252, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Lazcano, A. Prebiotic Evolution and Self-Assembly of Nucleic Acids. ACS Nano 2018, 12, 9643–9647. [Google Scholar] [CrossRef]
- Loakes, D.; Holliger, P. Darwinian Chemistry: Towards the Synthesis of a Simple Cell. Mol. Biosyst. 2009, 5, 686–694. [Google Scholar] [CrossRef]
- Carnall, J.M.A.; Waudby, C.A.; Belenguer, A.M.; Stuart, M.C.A.; Peyralans, J.J.-P.; Otto, S. Mechanosensitive Self-Replication Driven by Self-Organization. Science 2010, 327, 1502–1506. [Google Scholar] [CrossRef]
- Malakoutikhah, M.; Peyralans, J.J.-P.; Colomb-Delsuc, M.; Fanlo-Virgós, H.; Stuart, M.C.A.; Otto, S. Uncovering the Selection Criteria for the Emergence of Multi-Building-Block Replicators from Dynamic Combinatorial Libraries. J. Am. Chem. Soc. 2013, 135, 18406–18417. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, G.; Otto, S. Solvent Composition Dictates Emergence in Dynamic Molecular Networks Containing Competing Replicators. J. Am. Chem. Soc. 2015, 137, 2067–2072. [Google Scholar] [CrossRef]
- Duim, H.; Otto, S. Towards Open-Ended Evolution in Self-Replicating Molecular Systems. Beilstein J. Org. Chem. 2017, 13, 1189–1203. [Google Scholar] [CrossRef]
- Altay, M.; Altay, Y.; Otto, S. Parasitic Behavior of Self-Replicating Molecules. Angew. Chem. Int. Ed. 2018, 57, 10564–10568. [Google Scholar] [CrossRef]
- Altay, Y.; Altay, M.; Otto, S. Existing Self-Replicators Can Direct the Emergence of New Ones. Chem. A Eur. J. 2018, 24, 11911–11915. [Google Scholar] [CrossRef]
- Ottelé, J.; Hussain, A.S.; Mayer, C.; Otto, S. Chance Emergence of Catalytic Activity and Promiscuity in a Self-Replicator. Nat. Catal. 2020, 3, 547–553. [Google Scholar] [CrossRef]
- Monreal Santiago, G.; Liu, K.; Browne, W.R.; Otto, S. Emergence of Light-Driven Protometabolism on Recruitment of a Photocatalytic Cofactor by a Self-Replicator. Nat. Chem. 2020, 12, 603–607. [Google Scholar] [CrossRef]
- Bartolec, B.; Kiani, A.; Beatty, M.A.; Altay, M.; Santiago, G.M.; Otto, S. Selection of Diverse Polymorphic Structures from a Small Dynamic Molecular Network Controlled by the Environment. Chem. Sci. 2022, 13, 14300–14304. [Google Scholar] [CrossRef]
- Otto, S. An Approach to the De Novo Synthesis of Life. Acc. Chem. Res. 2022, 55, 145–155. [Google Scholar] [CrossRef]
- Segré, D.; Ben-Eli, D.; Lancet, D. Compositional Genomes: Prebiotic Information Transfer in Mutually Catalytic Noncovalent Assemblies. Proc. Natl. Acad. Sci. USA 2000, 97, 4112–4117. [Google Scholar] [CrossRef] [PubMed]
- Segré, D.; Shenhav, B.; Kafri, R.; Lancet, D. The Molecular Roots of Compositional Inheritance. J. Theor. Biol. 2001, 213, 481–491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Segré, D.; Ben-Eli, D.; Deamer, D.W.; Lancet, D. The Lipid World. Orig. Life Evol. Biosph. 2001, 31, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Vasas, V.; Szathmáry, E.; Santos, M. Lack of Evolvability in Self-Sustaining Autocatalytic Networks Constraints Metabolism-First Scenarios for the Origin of Life. Proc. Natl. Acad. Sci. USA 2010, 107, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Vasas, V.; Fernando, C.; Szilágyi, A.; Zachár, I.; Santos, M.; Szathmáry, E. Primordial Evolvability: Impasses and Challenges. J. Theor. Biol. 2015, 381, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Vasas, V.; Fernando, C.; Santos, M.; Kauffman, S.; Szathmáry, E. Evolution before Genes. Biol. Direct 2012, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Fernando, C.; Rowe, J. Natural Selection in Chemical Evolution. J. Theor. Biol. 2007, 247, 152–167. [Google Scholar] [CrossRef][Green Version]
- Nunes Palmeira, R.; Colnaghi, M.; Harrison, S.A.; Pomiankowski, A.; Lane, N. The Limits of Metabolic Heredity in Protocells. Proc. R Soc. B Biol. Sci. 2022, 289, 20221469. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoenmakers, L.L.J.; Reydon, T.A.C.; Kirschning, A. Evolution at the Origins of Life? Life 2024, 14, 175. https://doi.org/10.3390/life14020175
Schoenmakers LLJ, Reydon TAC, Kirschning A. Evolution at the Origins of Life? Life. 2024; 14(2):175. https://doi.org/10.3390/life14020175
Chicago/Turabian StyleSchoenmakers, Ludo L. J., Thomas A. C. Reydon, and Andreas Kirschning. 2024. "Evolution at the Origins of Life?" Life 14, no. 2: 175. https://doi.org/10.3390/life14020175
APA StyleSchoenmakers, L. L. J., Reydon, T. A. C., & Kirschning, A. (2024). Evolution at the Origins of Life? Life, 14(2), 175. https://doi.org/10.3390/life14020175







