The Intensity of BCL2A1 Expression Increases According to the Stage Progression of Acute Histologic Chorioamnionitis in the Extra-Placental Membranes of Spontaneous Preterm Birth
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Clinical Characteristics and Pregnancy Outcomes
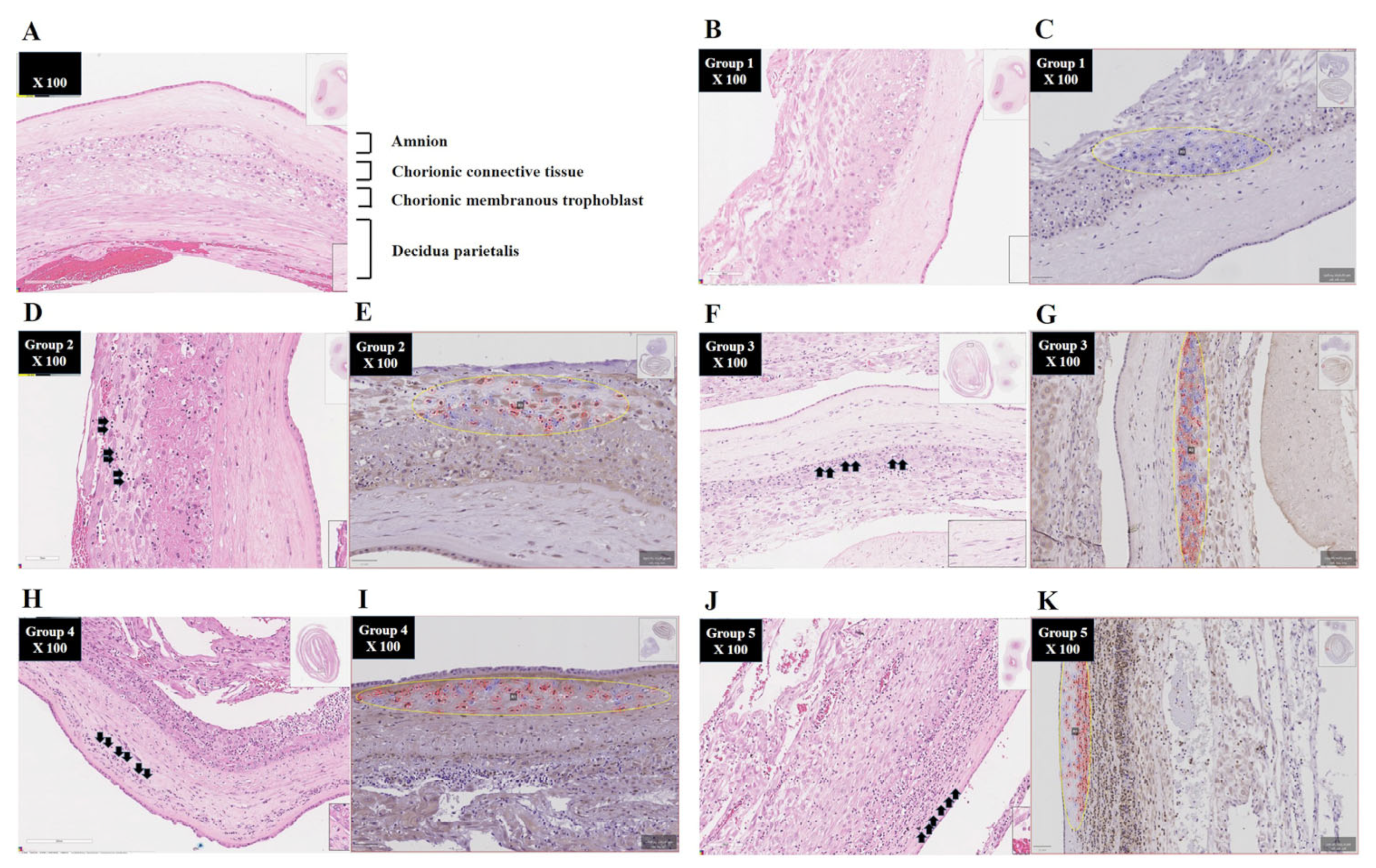
2.3. Diagnosis of Acute Histologic Chorioamnionitis (Acute HCA) in Extra-Placental Membranes (EPMs)
2.4. Immunohistochemistry of Extra-Placental Membranes (EPMs)
2.5. Digital Image Analysis
2.6. Amniotic Fluid (AF)
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics and Pregnancy Outcomes
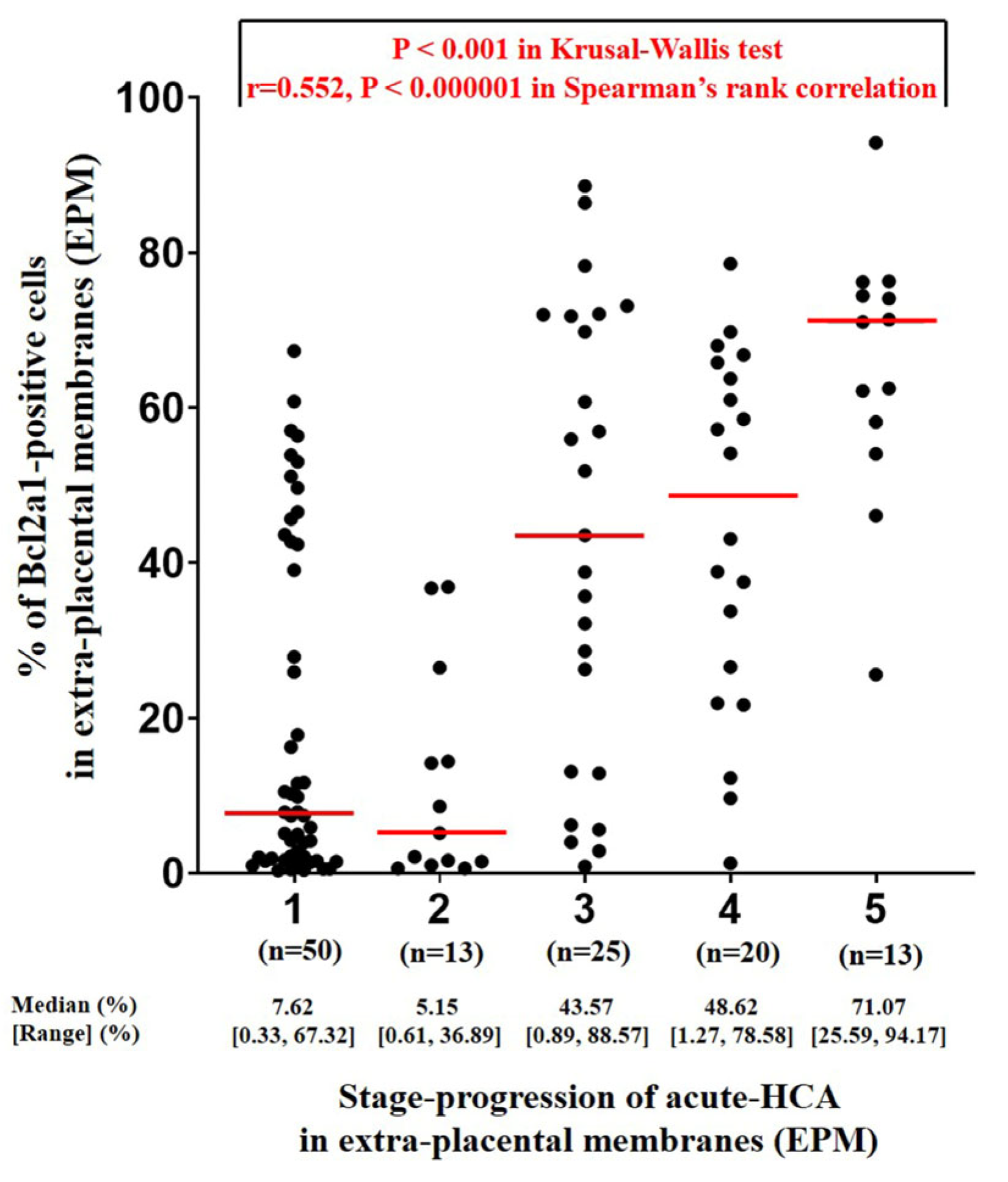
3.2. Determination of the Percentage of BCL2A1-Positive Cells Using QuPath Digital Image Analysis According to the Stage Progression of Acute HCA in EPM
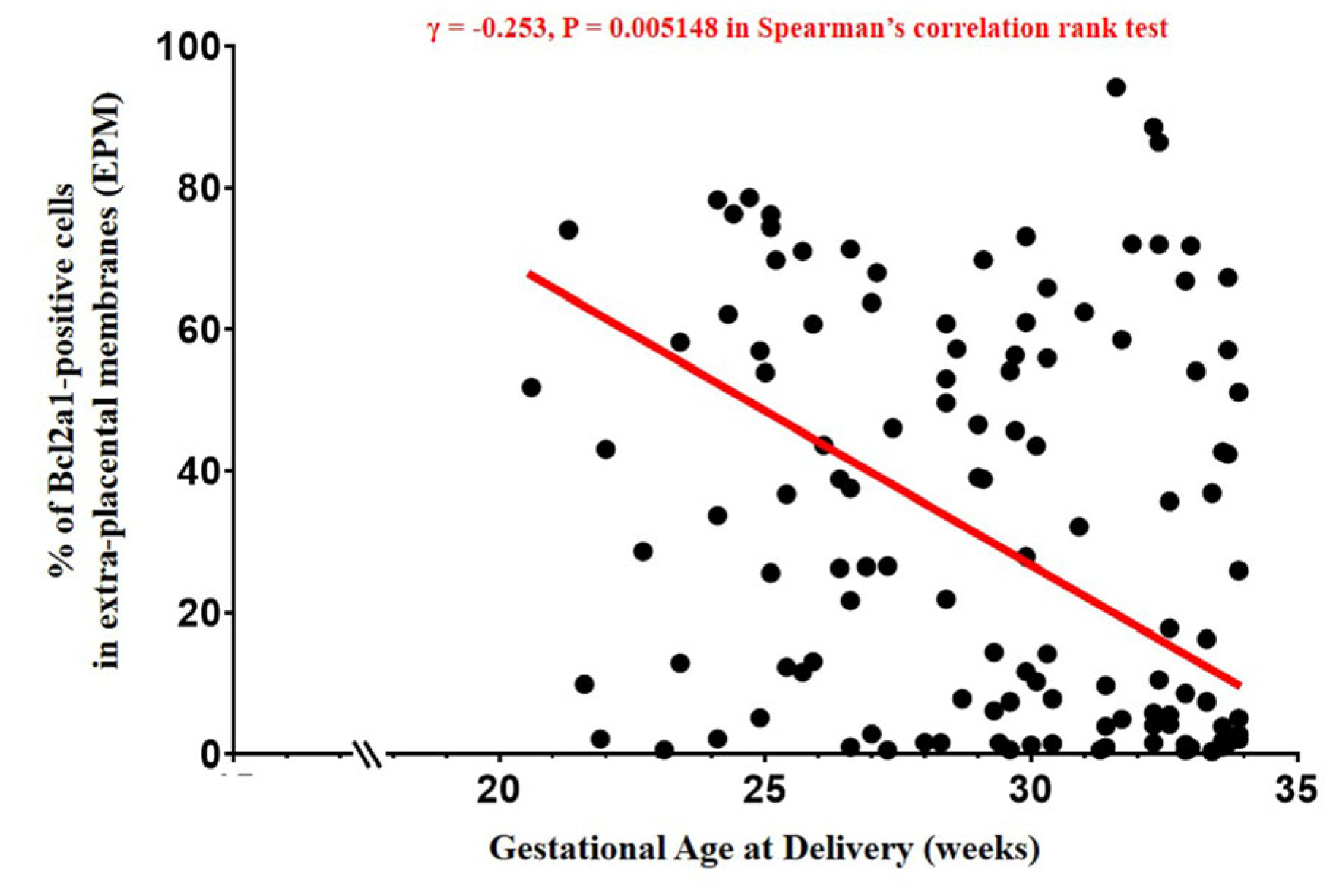
3.3. The Percentage of BCL2A1-Positive Cells in EPM, AFWBC, and Gestational Age at Delivery
4. Discussion
4.1. Principal Findings
4.2. Digital Image Analysis Using QuPath Can Calculate the Percentage of BCL2A1-Positive Cells as a Surrogate Marker for the Intensity of Neutrophils Infiltrating the Most Advanced Region of Acute HCA in EPM
4.3. BCL2A1 Could Enhance Its Value as a Therapeutic Target for Acute HCA
4.4. Reconfirmation of Findings Consistent with the Results of Previous Studies
4.5. Major Strengths and Limitations of This Study
4.6. Significance of This Study
4.7. Unanswered Questions and Further Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Mazor, M. Infection and preterm labor. Clin. Obstet. Gynecol. 1988, 31, 553–584. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, L.F.; Chaiworapongsa, T.; Romero, R. Intrauterine infection and prematurity. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S29–S52. [Google Scholar] [CrossRef]
- Oh, J.W.; Moon, K.C.; Park, C.-W.; Park, J.S.; Jun, J.K. Acute chorioamnionitis and intra-amniotic inflammation are more severe according to outside-in neutrophil migration within the same chorio-decidua. Taiwan. J. Obstet. Gynecol. 2021, 60, 639–652. [Google Scholar] [CrossRef]
- Park, C.-W.; Oh, J.W.; Moon, K.C.; Park, J.S.; Jun, J.K. Amniotic necrosis is associated with severe and advanced acute histologic chorioamnionitis. Placenta 2017, 57, 285. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Moon, J.B.; Shim, S.S.; Kim, M.; Kim, G.; Jun, J.K. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 2001, 185, 1130–1136. [Google Scholar] [CrossRef]
- Shim, S.S.; Romero, R.; Hong, J.S.; Park, C.-W.; Jun, J.K.; Kim, B.I.; Yoon, B.H. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 2004, 191, 1339–1345. [Google Scholar] [CrossRef]
- Sohn, J.W.; Choi, E.S.; Park, C.-W.; Moon, K.C.; Park, J.S.; Jun, J.K. Preterm Labor and Preterm-PROM at a Lower Gestational Age Are Associated with a Longer Latency-to-Delivery Even in Patients with the Same Intensity of Intra-Amniotic Inflammation: “Carroll-Model” Revisited. Life 2022, 12, 1329. [Google Scholar] [CrossRef]
- Park, C.-W.; Kim, S.M.; Park, J.S.; Jun, J.K.; Yoon, B.H. Fetal, amniotic and maternal inflammatory responses in early stage of ascending intrauterine infection, inflammation restricted to chorio-decidua, in preterm gestation. J. Matern. Fetal. Neonatal. Med. 2014, 27, 98–105. [Google Scholar] [CrossRef]
- Park, C.-W.; Moon, K.C.; Park, J.S.; Jun, J.K.; Romero, R.; Yoon, B.H. The involvement of human amnion in histologic chorioamnionitis is an indicator that a fetal and an intra-amniotic inflammatory response is more likely and severe: Clinical implications. Placenta 2009, 30, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-W.; Yoon, B.H.; Kim, S.M.; Park, J.S.; Jun, J.K. Which is more important for the intensity of intra-amniotic in-flammation between total grade or involved anatomical region in preterm gestations with acute histologic chorioam-nionitis? Obstet. Gynecol. Sci. 2013, 56, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, C.-W.; Moon, K.C.; Park, J.S.; Jun, J.K. The Inflammatory Milieu of Amniotic Fluid Increases with Cho-rio-Deciduitis Grade in Inflammation-Restricted to Choriodecidua, but Not Amnionitis, of Extra-Placental Membranes. J. Clin. Med. 2021, 10, 3041. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.R.; Loison, F. Constitutive neutrophil apoptosis: Mechanisms and regulation. Am. J. Hematol. 2008, 83, 288–295. [Google Scholar] [CrossRef]
- Vogler, M. BCL2A1: The underdog in the BCL2 family. Cell. Death. Differ. 2012, 19, 67–74. [Google Scholar] [CrossRef]
- Vier, J.; Groth, M.; Sochalska, M.; Kirschnek, S. The anti-apoptotic Bcl-2 family protein A1/Bfl-1 regulates neutrophil survival and homeostasis and is controlled via PI3K and JAK/STAT signaling. Cell. Death. Dis. 2016, 7, e2103. [Google Scholar] [CrossRef]
- Presicce, P.; Park, C.-W.; Senthamaraikannan, P.; Bhattacharyya, S.; Jackson, C.; Kong, F.; Rueda, C.M.; DeFranco, E.; Miller, L.A.; Hildeman, D.A.; et al. IL-1 signaling mediates intra-uterine inflammation and chorio-decidua neutrophil recruitment and activation. JCI Insight. 2018, 3, e98306. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Kim, C.J.; Jun, J.K.; Gomez, R.; Choi, J.H.; Syn, H.C. Amniotic fluid interleukin-6: A sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am. J. Obstet. Gynecol. 1995, 172, 960–970. [Google Scholar] [CrossRef]
- Hein, A.L.; Mukherjee, M.; Talmon, G.A.; Natarajan, S.K.; Nordgren, T.M.; Lyden, E.; Hanson, C.K.; Cox, J.L.; Santia-go-Pintado, A.; Molani, M.A.; et al. QuPath Digital Immunohistochemical Analysis of Placental Tissue. J. Pathol. Inform. 2021, 12, 40. [Google Scholar] [CrossRef]
- Porter, R.J.; Din, S.; Bankhead, P.; Oniscu, A.; Arends, M.J. QuPath Algorithm Accurately Identifies MLH1-Deficient Inflammatory Bowel Disease-Associated Colorectal Cancers in a Tissue Microarray. Diagnostics 2023, 13, 1890. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Yang, S.H.; Jun, J.K.; Park, K.H.; Kim, C.J.; Romero, R. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: A comparison with amniotic fluid white blood cell count. Obstet. Gynecol. 1996, 87, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Jun, J.K.; Park, K.H.; Syn, H.C.; Gomez, R.; Romero, R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet. Gynecol. 1996, 88, 1034–1140. [Google Scholar] [CrossRef]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.; Boyd, T.K.; Brundler, M.A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef]
- Park, C.-W.; Lee, S.M.; Park, J.S.; Jun, J.K. A more frequent advanced-stage, but not early-stage, ascending intra-uterine infection is associated with preterm birth due to preterm labor and preterm-PROM at lower gestational age. In Reproductive Sciences; Sage Publications: New York, NY, USA, 2016; Volume 23, p. 243A. [Google Scholar]
- Russell, P. Inflammatory lesions of the human placenta: Clinical significance of acute chorioamnionitis. Am. J. Diagn. Gynecol. Obstet. 1979, 2, 127–137. [Google Scholar]
- Park, C.-W.; Park, J.S.; Jun, J.K.; Yoon, B.H. The inflammatory milieu of amniotic fluid in acute-chorioamnionitis de-creases with increasing gestational age. Placenta 2015, 36, 1283–1290. [Google Scholar] [CrossRef]
- The Human Protein Atlas. BCL2A1. Available online: https://www.proteinatlas.org/ENSG00000140379-BCL2A1 (accessed on 18 September 2024).
- Smith, F.G.; Wade, A.W.; Lewis, M.L.; Qi, W. Cyclooxygenase (COX) Inhibitors and the Newborn Kidney. Pharmaceuticals 2012, 5, 1160–1176. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, C.-W.; Moon, K.C.; Park, J.S.; Jun, J.K. Neutrophil to Lymphocyte Ratio in Maternal Blood: A Clue to Suspect Amnionitis. J. Clin. Med. 2021, 10, 2673. [Google Scholar] [CrossRef]
- Bezemer, R.E.; Schoots, M.H.; Timmer, A.; Scherjon, S.A.; Erwich, J.J.H.M.; van Goor, H.; Gordijn, S.J.; Prins, J.R. Altered Levels of Decidual Immune Cell Subsets in Fetal Growth Restriction, Stillbirth, and Placental Pathology. Front. Immunol. 2020, 11, 1898. [Google Scholar] [CrossRef]

| Group-1 † | Group-2 † | Group-3 † | Group-4 † | Group-5 † | p Value †† | |
|---|---|---|---|---|---|---|
| 41.3% (50/121) | 10.7% (13/121) | 20.7% (25/121) | 16.5% (20/121) | 10.7% (13/121) | ||
| Maternal age, year (mean ± SD) | 33.3 ± 4.6 | 34.0 ± 4.0 | 32.3 ± 4.1 | 33.1 ± 4.0 | 31.5 ± 4.8 | NS (0.552) |
| Nulliparity | 46.0% (23/50) | 46.2% (6/13) | 32.0% (8/25) | 30.0% (6/20) | 53.8% (7/13) | NS (0.498) |
| Preterm PROM as a cause of PTB | 46.0% (23/50) | 30.8% (4/13) | 56.0% (14/25) | 45.0% (9/20) | 46.2% (6/13) | NS (0.695) |
| Male newborn | 56.0% (28/50) | 53.8% (7/13) | 72.0% (18/25) | 40.0% (8/20) | 92.3% (12/13) a | 0.026 |
| Cesarean delivery | 46.0% (23/50) | 23.1% (3/13) | 24.0% (6/25) | 30.0% (6/20) | 46.2% (6/13) | NS (0.236) |
| GA at delivery, weeks (median [range]) | 31.6 [21.6–33.9] | 29.1 [23.1–33.9] | 29.9 [20.6–33.0] | 27.2 [22.0–32.9] b | 25.1 [21.3–33.1] c | <0.001 |
| Birth weight, g (mean ± SD) | 1615 ± 543 | 1375 ± 638 | 1409 ± 595 | 1160 ± 409 d | 1028 ± 549 e | 0.003 |
| 1 m Apgar score of <7 | 74.0% (37/50) | 61.5% (8/13) | 72.0% (18/25) | 90.0% (18/20) | 92.3% (12/13) | NS (0.202) |
| 5 m Apgar score of <7 | 30.0% (15/50) | 38.5% (5/13) | 24.0% (6/25) | 50.0% (10/20) | 76.9% (10/13) f, g | <0.05 |
| Antenatal use of corticosteroids | 74.0% (37/50) | 76.9% (10/13) | 80.0% (20/25) | 85.0% (17/20) | 76.9% (10/13) | NS (0.895) |
| Antenatal use of antibiotics | 66.0 (33/50) | 61.5% (8/13) | 80.0% (20/25) | 80% (16/20) | 84.6% (11/13) | NS (0.394) |
| Antenatal use of tocolytics | 52.0% (26/50) | 76.9% (10/13) | 84.0% (21/25) | 90.0% (18/20) h | 92.3% (12/13) | <0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.-W.; Lee, E.-M.; Shin, S.-H.; Lee, C.; Won, J.-K. The Intensity of BCL2A1 Expression Increases According to the Stage Progression of Acute Histologic Chorioamnionitis in the Extra-Placental Membranes of Spontaneous Preterm Birth. Life 2024, 14, 1535. https://doi.org/10.3390/life14121535
Park C-W, Lee E-M, Shin S-H, Lee C, Won J-K. The Intensity of BCL2A1 Expression Increases According to the Stage Progression of Acute Histologic Chorioamnionitis in the Extra-Placental Membranes of Spontaneous Preterm Birth. Life. 2024; 14(12):1535. https://doi.org/10.3390/life14121535
Chicago/Turabian StylePark, Chan-Wook, Eun-Mi Lee, Seung-Han Shin, Chul Lee, and Jae-Kyung Won. 2024. "The Intensity of BCL2A1 Expression Increases According to the Stage Progression of Acute Histologic Chorioamnionitis in the Extra-Placental Membranes of Spontaneous Preterm Birth" Life 14, no. 12: 1535. https://doi.org/10.3390/life14121535
APA StylePark, C.-W., Lee, E.-M., Shin, S.-H., Lee, C., & Won, J.-K. (2024). The Intensity of BCL2A1 Expression Increases According to the Stage Progression of Acute Histologic Chorioamnionitis in the Extra-Placental Membranes of Spontaneous Preterm Birth. Life, 14(12), 1535. https://doi.org/10.3390/life14121535






