Development of Imaging Complexity Biomarkers for Prediction of Symptomatic Radiation Pneumonitis in Patients with Non-Small Cell Lung Cancer, Focusing on Underlying Lung Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Scheme and Surveillance
2.3. Measurement of Morphometric Complexity
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Clinical Risk Factor Analysis
3.3. Morphometric Complexity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Claude, L.; Pérol, D.; Ginestet, C.; Falchero, L.; Arpin, D.; Vincent, M.; Martel, I.; Hominal, S.; Cordier, J.F.; Carrie, C. A prospective study on radiation pneumonitis following conformal radiation therapy in non-small-cell lung cancer: Clinical and dosimetric factors analysis. Radiother. Oncol. 2004, 71, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Zhu, G.; Wu, H.; Yu, R.; Li, F.; Xu, B. Analysis of clinical and dosimetric factors associated with severe acute radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with concurrent chemotherapy and intensity-modulated radiotherapy. Radiat. Oncol. 2010, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, J.S. Predictors of radiation pneumonitis and pulmonary function changes after concurrent chemoradiotherapy of non-small cell lung cancer. Radiat. Oncol. J. 2013, 31, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Leprieur, E.G.; Fernandez, D.; Chatellier, G.; Klotz, S.; Giraud, P.; Durdux, C. Acute radiation pneumonitis after conformational radiotherapy for nonsmall cell lung cancer: Clinical, dosimetric, and associated-treatment risk factors. J. Cancer Res. Ther. 2013, 9, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Han, G.; Sarangkasiri, S.; DeMarco, M.; Turke, C.; Stevens, C.W.; Dilling, T.J. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Kobayashi-Shibata, S.; Terahara, A.; Okuma, K.; Haga, A.; Wakui, R.; Ohtomo, K.; Nakagawa, K. Prescreening based on the presence of CT-scan abnormalities and biomarkers (KL-6 and SP-D) may reduce severe radiation pneumonitis after stereotactic radiotherapy. Radiat. Oncol. 2010, 5, 32. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Ohguri, T.; Ide, S.; Aoki, T.; Imada, H.; Yahara, K.; Narisada, H.; Korogi, Y. Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: The potential risk of extensive radiation pneumonitis. Lung Cancer. 2013, 82, 260–265. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, Y.S.; Lee, S.N.; Lee, H.C.; Oh, S.J.; Kim, S.J.; Kim, Y.K.; Han, D.H.; Yoo Ie, R.; Kang, J.H.; et al. Interstitial Lung Change in Pre-radiation Therapy Computed Tomography Is a Risk Factor for Severe Radiation Pneumonitis. Cancer Res. Treat. 2015, 47, 676–686. [Google Scholar] [CrossRef]
- Rancati, T.; Ceresoli, G.L.; Gagliardi, G.; Schipani, S.; Cattaneo, G.M. Factors predicting radiation pneumonitis in lung cancer patients: A retrospective study. Radiother. Oncol. 2003, 67, 275–283. [Google Scholar] [CrossRef]
- Takeda, A.; Kunieda, E.; Ohashi, T.; Aoki, Y.; Oku, Y.; Enomoto, T.; Nomura, K.; Sugiura, M. Severe COPD is correlated with mild radiation pneumonitis following stereotactic body radiotherapy. Chest. 2012, 141, 858–866. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, X.; Wu, A.; Liu, H.; Wu, H.; Wu, Q.; Liu, Y.; Li, Y.; Cai, Y.; Liang, S. Pulmonary emphysema is a risk factor for radiation pneumonitis in NSCLC patients with squamous cell carcinoma after thoracic radiation therapy. Sci. Rep. 2017, 7, 2748. [Google Scholar] [CrossRef] [PubMed]
- Kanaji, N.; Tadokoro, A.; Kita, N.; Murota, M.; Ishii, T.; Takagi, T.; Watanabe, N.; Tojo, Y.; Harada, S.; Hasui, Y.; et al. Impact of idiopathic pulmonary fibrosis on advanced non-small cell lung cancer survival. J. Cancer Res. Clin. Oncol. 2016, 142, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Park, J.Y.; Lee, H.Y.; Cho, Y.J.; Yoon, H.I.; Lee, J.H.; Jheon, S.; Lee, C.T.; Park, J.S. Lung cancer in patients with idiopathic pulmonary fibrosis: Clinical characteristics and impact on survival. Respir. Med. 2014, 108, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.W.; Park, M.S.; Kim, Y.S.; Jang, J.; Lee, J.H.; Lee, C.T.; Chung, J.H.; Shim, H.S.; Lee, K.W.; Kim, S.S.; et al. Combined pulmonary fibrosis and emphysema and idiopathic pulmonary fibrosis in non-small cell lung cancer: Impact on survival and acute exacerbation. BMC Pulm. Med. 2019, 19, 177. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, S.; Gurioli, C.; Ryu, J.H.; Decker, P.A.; Ravaglia, C.; Tantalocco, P.; Buccioli, M.; Piciucchi, S.; Sverzellati, N.; Dubini, A.; et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest. 2015, 147, 157–164. [Google Scholar] [CrossRef]
- Kim, H.; Pyo, H.; Noh, J.M.; Lee, W.; Park, B.; Park, H.Y.; Yoo, H. Preliminary result of definitive radiotherapy in patients with non-small cell lung cancer who have underlying idiopathic pulmonary fibrosis: Comparison between X-ray and proton therapy. Radiat. Oncol. 2019, 14, 19. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, H.; Pyo, H.; Ahn, Y.C.; Noh, J.M.; Ju, S.G.; Lee, W.; Park, B.; Kim, J.M.; Kang, N.; et al. Impact Of Underlying Pulmonary Diseases On Treatment Outcomes In Early-Stage Non-Small Cell Lung Cancer Treated with Definitive Radiotherapy. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 2273–2281. [Google Scholar] [CrossRef]
- Hwang, J.; Oh, Y.M.; Lee, M.; Choi, S.; Seo, J.B.; Lee, S.M.; Kim, N. Low morphometric complexity of emphysematous lesions predicts survival in chronic obstructive pulmonary disease patients. Eur. Radiol. 2019, 29, 176–185. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, H.; Kim, S.M.; Yang, D.S. Preliminary Results of Developing Imaging Complexity Biomarkers for the Incidence of Severe Radiation Pneumonitis Following Radiotherapy in Non-Small Cell Lung Cancer Patients with Underlying Idiopathic Pulmonary Fibrosis. Life 2024, 14, 897. [Google Scholar] [CrossRef]
- Grassberger, P. On the fractal dimension of the Henon attractor. Phys. Lett. A. 1983, 97, 224–226. [Google Scholar] [CrossRef]
- Ott, E. Strange attractors and fractal dimensions. In Chaos in Dynamical Systems; Ott, E., Ed.; Cambridge University Press: Cambridge, UK, 1993; pp. 69–107. [Google Scholar]
- Kozma, G.; Lotker, Z.; Stupp, G. The minimal spanning tree and the upper box dimension. Proc. Am. Math. Soc. 2006, 134, 1183–1187. [Google Scholar] [CrossRef]
- Martínez, V.J.; Domínguez-Tenreiro, R.; Roy, L.J. Hausdorff dimension from the minimal spanning tree. Phys. Rev. E 1993, 47, 735–738. [Google Scholar] [CrossRef] [PubMed]
- van de Weygaert, R.; Jones, B.J.T.; Martinez, V.J. The minimal spanning tree as an estimator for generalized dimensions. Phys. Lett. A 1992, 169, 145–150. [Google Scholar] [CrossRef]
- Hatabu, H.; Hunninghake, G.M.; Richeldi, L.; Brown, K.K.; Wells, A.U.; Remy-Jardin, M.; Verschakelen, J.; Nicholson, A.G.; Beasley, M.B.; Christiani, D.C.; et al. Interstitial lung abnormalities detected incidentally on CT: A Position Paper from the Fleischner Society. Lancet Respir. Med. 2020, 8, 726–737. [Google Scholar] [CrossRef]
| Characteristics | Number | % |
|---|---|---|
| Age [years; median (range)] | 76 (38–93) | |
| Gender | ||
| Female | 42 | 24.0% |
| Male | 133 | 76.0% |
| Smoking status | ||
| Never smoker | 81 | 46.3% |
| Current or ex-smoker | 94 | 53.7% |
| ECOG performance status | ||
| 0–1 | 152 | 86.9% |
| 2 | 23 | 13.1% |
| Underlying lung diseases | ||
| None | 109 | 62.3% |
| COPD | 51 | 29.1% |
| IPF | 15 | 8.6% |
| Clinical stage | ||
| I–II | 93 | 53.1% |
| III | 82 | 46.9% |
| Histology | ||
| Squamous cell carcinoma | 81 | 46.3% |
| Adenocarcinoma | 88 | 50.3% |
| Others | 6 | 3.4% |
| Radiotherapy technique | ||
| Stereotactic ablative radiotherapy | 36 | 20.6% |
| Intensity-modulated radiotherapy | 139 | 79.4% |
| Characteristics | ≤Grade 2 RP | ≥Grade 3 RP | p-Value |
|---|---|---|---|
| Age [years; median (range)] | 76 (38–93) | 75 (65–87) | 0.099 |
| Gender | 0.350 | ||
| Female | 38 (90.5%) | 4 (9.5%) | |
| Male | 115 (86.5%) | 18 (13.5%) | |
| Smoking status | 0.318 | ||
| Never smoker | 73 (90.1%) | 8 (9.9%) | |
| Current or ex-smoker | 80 (85.1%) | 14 (14.9%) | |
| ECOG performance status | 0.202 | ||
| 0–1 | 131 (86.2%) | 21 (13.8%) | |
| 2 | 22 (95.7%) | 1 (4.3%) | |
| Underlying lung diseases | <0.001 | ||
| None | 100 (91.7%) | 9 (8.3%) | |
| COPD | 47 (92.2%) | 4 (7.8%) | |
| IPF | 6 (40.0%) | 9 (60.0%) | |
| Clinical stage | 0.009 | ||
| I-II | 87 (93.5%) | 6 (6.5%) | |
| III | 66 (80.5%) | 16 (19.5%) | |
| Histology | 0.637 | ||
| Squamous cell carcinoma | 69 (85.2%) | 12 (14.8%) | |
| Adenocarcinoma | 79 (89.8%) | 9 (10.2%) | |
| Others | 5 (83.3%) | 1 (16.7%) | |
| Pulmonary function test (mean) | |||
| FEV1 | 2.1L | 2.1L | 0.469 |
| FEV1 % predicted | 73.9% | 80.9% | 0.082 |
| FVC | 3.1L | 2.9L | 0.213 |
| FVC % predicted | 78.9% | 77.4% | 0.093 |
| FEV1/FVC | 64.7% | 71.8% | 0.533 |
| DLco | 70.4% | 67.4% | 0.274 |
| Planning parameter | 198.8 | ||
| Total lung_MLD | 745.5 cGy | 977.9 cGy | 0.027 |
| Total lung_V5 | 36.2% | 47.7% | 0.030 |
| Total lung_V20 | 14.3% | 16.7% | 0.431 |
| Characteristics | Severe RP | p-Value | Retained |
|---|---|---|---|
| Age | 4.16 × 10−2 (−2.72 × 10−2–0.120) | 0.265 | |
| Male gender | 0.931 (0.167–5.20) | 0.934 | |
| Current or ex-smoker | 0.718 (0.181–2.97) | 0.636 | |
| Histology | |||
| SqCC | 0.753 (0.199–2.72) | 0.668 | |
| ADC | 0.960 (0.042–8.68) | 0.974 | |
| Others | |||
| Clinical stage III | 3.69 (0.721–21.8) | 0.129 | Yes |
| Underlying lung diseases | |||
| None | |||
| COPD | 1.32 (0.292–5.59) | 0.706 | |
| IPF | 48.4 (9.09–347) | <0.001 * | Yes |
| DLco < 80% | 0.264 (0.060–1.00) | 0.056 | Yes |
| Plan parameter | |||
| Total lung_MLD | 1.21 × 10−3 | 0.539 | |
| (−2.58 × 10−3–7.01× 10−3) | |||
| Total lung_V5 | −9.39 × 10−3 | 0.793 | |
| (−8.29 × 10−2–5.94 × 10−2) | |||
| Total lung_V20 | −1.27 × 10−2 | 0.674 | |
| (−3.49 × 10−2–9.45 × 10−3) |
| HR b (95% CI c) | C-Index d | p-Value | ||||
|---|---|---|---|---|---|---|
| Grade ≥ 2 (# of events = 63) | Unadjusted model | Box-counting fractal dimension | 2.173–2.363 (N = 88) | Reference | ||
| 1.956–2.173 (N = 87) | 1.321 (0.786–2.220) | 0.522 | 0.294 | |||
| Lacunarity | 0.461–0.743 (N = 88) | Reference | ||||
| 0.317–0.456 (N = 87) | 0.735 (0.438–1.234) | 0.522 | 0.245 | |||
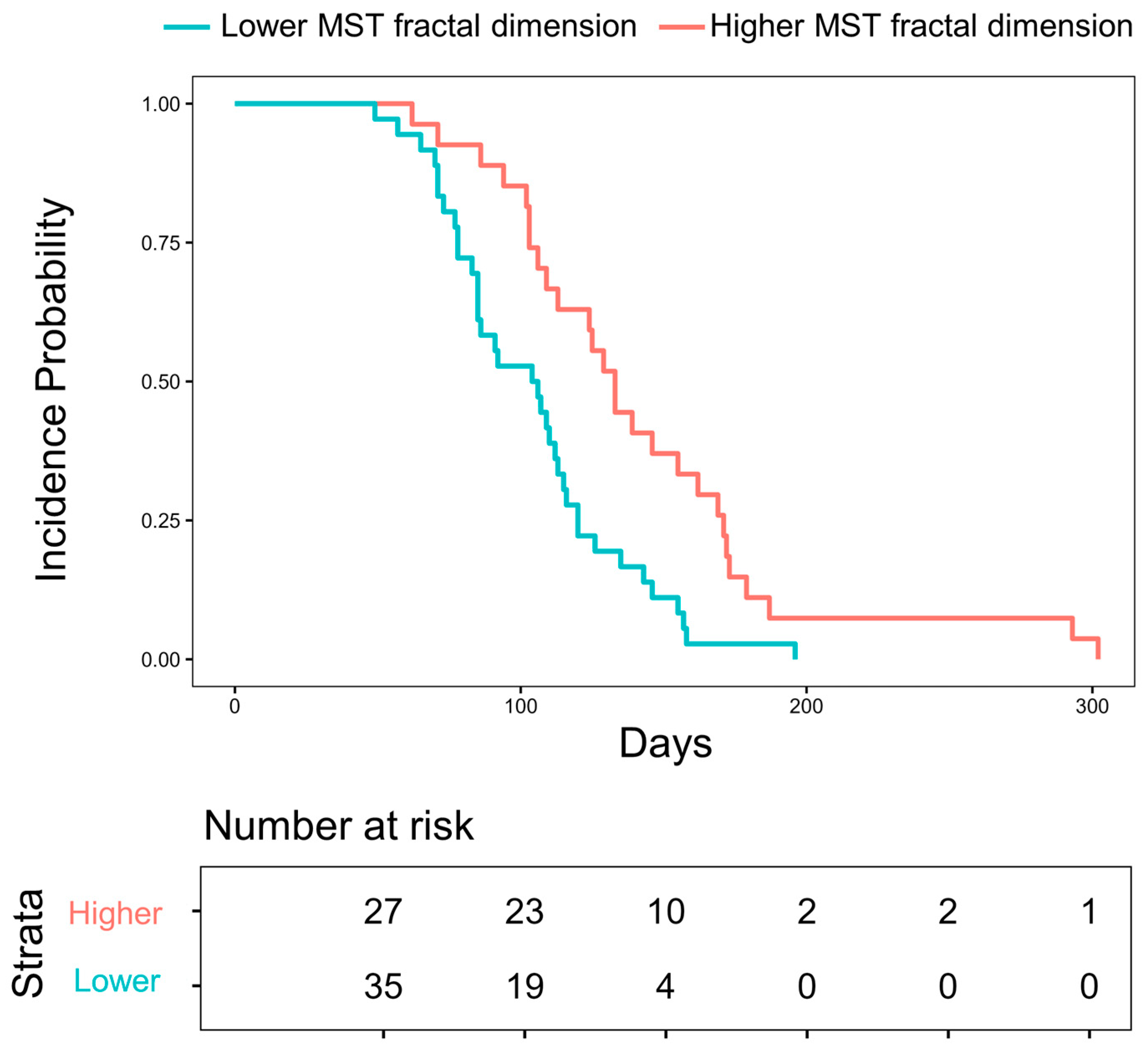
| MST fractal dimension | 2.716–2.833 (N = 88) | Reference | ||||
| 2.534–2.716 (N = 87) | 2.296 (1.348–3.910) | 0.614 | 0.002 | |||
| Adjusted model a | Box-counting fractal dimension | 2.173–2.363 (N = 88) | Reference | |||
| 1.956–2.173 (N = 87) | 1.565 (0.841–2.911) | 0.610 | 0.157 | |||
| Lacunarity | 0.461–0.743 (N = 88) | Reference | ||||
| 0.317–0.456 (N = 87) | 0.711 (0.387–1.307) | 0.606 | 0.272 | |||
| MST fractal dimension | 2.716–2.833 (N = 88) | Reference | ||||
| 2.534–2.716 (N = 87) | 3.292 (1.722–6.294) | 0.666 | < 0.001 | |||
| Grade ≥ 3 (# of events = 22) | Unadjusted model | Box-counting fractal dimension | 2.173–2.363 (N = 88) | Reference | ||
| 1.956–2.173 (N = 87) | 0.892 (0.375–2.122) | 0.480 | 0.796 | |||
| Lacunarity | 0.461–0.743 (N = 88) | Reference | ||||
| 0.317–0.456 (N = 87) | 1.126 (0.476–2.663) | 0.491 | 0.788 | |||
| MST fractal dimension | 2.716–2.833 (N = 88) | Reference | ||||
| 2.534–2.716 (N = 87) | 1.614 (0.671–3.882) | 0.609 | 0.285 | |||
| Adjusted model | Box-counting fractal dimension | 2.173–2.363 (N = 88) | Reference | |||
| 1.956–2.173 (N = 87) | 1.024 (0.222–4.722) | 0.678 | 0.976 | |||
| Lacunarity | 0.461–0.743 (N = 88) | Reference | ||||
| 0.317–0.456 (N = 87) | 0.091 (0.015–0.573) | 0.776 | 0.002 | |||
| MST fractal dimension | 2.716–2.833 (N = 88) | Reference | ||||
| 2.534–2.716 (N = 87) | 7.952 (1.722–36.733) | 0.803 | 0.008 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.; Kim, H.; Moon, J.-Y.; Kim, S.M.; Yang, D.S. Development of Imaging Complexity Biomarkers for Prediction of Symptomatic Radiation Pneumonitis in Patients with Non-Small Cell Lung Cancer, Focusing on Underlying Lung Disease. Life 2024, 14, 1497. https://doi.org/10.3390/life14111497
Hwang J, Kim H, Moon J-Y, Kim SM, Yang DS. Development of Imaging Complexity Biomarkers for Prediction of Symptomatic Radiation Pneumonitis in Patients with Non-Small Cell Lung Cancer, Focusing on Underlying Lung Disease. Life. 2024; 14(11):1497. https://doi.org/10.3390/life14111497
Chicago/Turabian StyleHwang, Jeongeun, Hakyoung Kim, Joon-Young Moon, Sun Myung Kim, and Dae Sik Yang. 2024. "Development of Imaging Complexity Biomarkers for Prediction of Symptomatic Radiation Pneumonitis in Patients with Non-Small Cell Lung Cancer, Focusing on Underlying Lung Disease" Life 14, no. 11: 1497. https://doi.org/10.3390/life14111497
APA StyleHwang, J., Kim, H., Moon, J.-Y., Kim, S. M., & Yang, D. S. (2024). Development of Imaging Complexity Biomarkers for Prediction of Symptomatic Radiation Pneumonitis in Patients with Non-Small Cell Lung Cancer, Focusing on Underlying Lung Disease. Life, 14(11), 1497. https://doi.org/10.3390/life14111497






