Non-Thermal Atmospheric Pressure Plasma as an Adjunct to Intestinal Anastomosis: A Pilot Study on Preventing Anastomotic Leaks
Abstract
1. Introduction
2. Materials and Methods
2.1. NTAPP-Generation System
2.2. Application Protocol
2.3. Safety Considerations
2.4. Patients
2.5. Patient Characteristics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Mu, T.; Deng, X.; Guo, R.; Xia, B.; Jiang, L.; Wu, Z.; Liu, M. New insights of biological functions of natural polyphenols in inflammatory intestinal diseases. Int. J. Mol. Sci. 2023, 24, 9581. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, S.; Damian, G.; Glancy, D.G. Intestinal obstruction. Surgery 2023, 41, 47–54. [Google Scholar]
- Aniruthan, D.; Pranavi, A.R.; Sreenath, G.S.; Kate, V. Efficacy of single layered intestinal anastomosis over double layered intestinal anastomosis-an open labelled, randomized controlled trial. Int. J. Surg. 2020, 78, 173–178. [Google Scholar] [CrossRef]
- Molfetta, R.; Paolini, R. The controversial role of intestinal mast cells in colon cancer. Cells 2023, 12, 459. [Google Scholar] [CrossRef]
- Tasdighi, E.; Barzin, M.; Mahawar, K.K.; Hosseinpanah, F.; Ebadinejad, A.; Taraghikhah, N.; Mansoori, A.; Khalaj, A.; Niroomand, M.; Valizadeh, M.; et al. Effect of biliopancreatic limb length on weight loss, postoperative complications, and remission of comorbidities in one anastomosis gastric bypass: Systematic review and meta-analysis. Obes. Surg. 2022, 32, 892–903. [Google Scholar] [CrossRef]
- Romary, D.J.; Landsberger, S.A.; Bradner, K.N.; Ramirez, M.; Brian, R.; Leon, B.R. Liquid ozone therapies for the treatment of epithelial wounds: A systematic review and meta-analysis. Int. Wound J. 2023, 20, 1235–1252. [Google Scholar] [CrossRef]
- Steyer, G.E.; Puchinger, M.; Pfeifer, J. Successful clinical avoidance of colorectal anastomotic leakage through local decontamination. Antibiotics 2024, 13, 79. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Zhu, J.; Zhang, C.; Liu, G.; Wang, X.; Sun, Y.; Guo, Z. Clinical features and management of post-necrotizing enterocolitis strictures in infants: A multicentre retrospective study. Medicine 2020, 99, 20209. [Google Scholar] [CrossRef]
- Reischl, S.; Wilhelm, D.; Friess, H.; Neumann, P.-A. Innovative approaches for induction of gastrointestinal anastomotic healing: An update on experimental and clinical aspects. Langenbeck’s Arch. Surg. 2021, 406, 971–980. [Google Scholar] [CrossRef]
- Gómez-Garnica, D.G.; Rey-Chaves, C.E.; Barco-Castillo, C.; Gutierrez, J.A.; Falla, A. Negative pressure wound therapy after intestinal anastomosis: A risk factor analysis for dehiscence. J. Surg. Res. 2024, 296, 223–229. [Google Scholar] [CrossRef]
- Trébol, J.; Georgiev-Hristov, T.; Pascual-Miguelañez, I.; Guadalajara, H.; García-Arranz, M.; García-Olmo, D. Stem cell therapy applied for digestive anastomosis: Current state and future perspectives. World J. Stem Cells 2022, 14, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Uzunlu, O.; Aydii, E.; Çomut, E.; Avci, E.; Şenol, H. The comparison of the suture materials on intestinal anastomotic healing: An experimental study. Ulus. Travma Acil Cerrahi Derg. 2023, 29, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Steger, J.; Jell, A.; Ficht, S.; Ostler, D.; Eblenkamp, M.; Mela, P.; Wilhelm, D. Systematic review and meta-analysis on colorectal anastomotic techniques. Ther. Clin. Risk Manag. 2022, 18, 523–539. [Google Scholar] [CrossRef]
- Foppa, C.; Carvello, M.; Maroli, A.; Sacchi, M.; Gramellini, M.; Montorsi, M.; Spinelli, A. Single-stapled anastomosis is associated with a lower anastomotic leak rate than double-stapled technique after minimally invasive total mesorectal excision for MRI-defined low rectal cancer. Surgery 2023, 173, 1367–1373. [Google Scholar] [CrossRef]
- Adams, E.D.; Zaghiyan, K.N.; Fleshner, P.R. End-to-end stapled technique for Kono-S anastomosis. Tech. Coloproctol. 2023, 27, 1383–1386. [Google Scholar] [CrossRef]
- Valsamidis, T.N.; Rasmussen, T.; Eriksen, J.D.; Iversen, L.H. The role of tissue adhesives and sealants in colorectal anastomotic healing—A scoping review. Int. J. Colorectal Dis. 2023, 38, 265. [Google Scholar] [CrossRef]
- Kawahara, M.; Kuramoto, S.; Ryan, P.; Stillwell, R. First experimental sutureless laser anastomosis of the large bowel. Dis. Colon Rectum 1996, 39, 556–561. [Google Scholar] [CrossRef]
- Ashbell, I.; Agam, N.; Katzir, A.; Basov, S.; Platkov, M.; Avital, I.; Nisky, I.; Netz, U. Laser tissue soldering of the gastrointestinal tract: A systematic review. Heliyon 2023, 9, e16018. [Google Scholar] [CrossRef]
- Mbaidjol, Z.; Stoffel, M.H.; Frenz, M.; Constantinescu, M.A. A novel technique for laser-assisted revascularization: An in vitro pilot study. Lasers Med. Sci. 2021, 36, 855–862. [Google Scholar] [CrossRef]
- Cipolato, O.; Dosnon, L.; Rosendorf, J.; Sarcevic, S.; Zäch, M.; Bondi, A.; Liska, V.; Schlegel, A.A.; Herrmann, I.K. Nanothermometry-enabled intelligent laser tissue soldering. Small Methods 2023, 7, 2300693. [Google Scholar] [CrossRef]
- Mallela, D.P.; Bose, S.; Shallal, C.C.; Goldsborough, E.; Xun, H.; Chen, J.; Stonko, D.P.; Brandacher, G.; Sacks, J.; Kang, S.H.; et al. A systematic review of sutureless vascular anastomosis technologies. Semin. Vasc. Surg. 2021, 34, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Shams, F.; Moravvej, H.; Hosseinzadeh, S.; Mostafavi, E.; Bayat, H.; Kazemi, B.; Bandehpour, M.; Rostami, E.; Rahimpour, A.; Moosavian, H. Overexpression of VEGF in dermal fibroblast cells accelerates the angiogenesis and wound healing function: In vitro and in vivo studies. Sci. Rep. 2022, 12, 18529. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, U.A.; Chen, L.; Ma, D.; Haywood, V.A.; Barakat, M.; Urao, N.; DiPietro, L.A. Compromised angiogenesis and vascular integrity in impaired diabetic wound healing. PLoS ONE 2020, 15, e0231962. [Google Scholar] [CrossRef]
- Moszczyńska, J.; Roszek, K.; Wiśniewski, M. Non-thermal plasma application in medicine-focus on reactive species involvement. Int. J. Mol. Sci. 2023, 24, 12667. [Google Scholar] [CrossRef]
- García-Alcantara, E.; López-Callejas, R.; Morales-Ramírez, P.R.; Peña-Eguiluz, R.; Fajardo-Muñoz, R.; Mercado-Cabrera, A.; Barocio, S.R.; Valencia-Alvarado, R.; Rodríguez-Méndez, B.G.; Muñoz-Castro, A.E.; et al. Accelerated mice skin acute wound healing in vivo by combined treatment of argon and helium plasma needle. Arch. Med. Res. 2013, 44, 169–177. [Google Scholar] [CrossRef]
- López-Callejas, R.; Peña-Eguiluz, R.; Valencia-Alvarado, R.; Mercado-Cabrera, A.; Rodríguez-Méndez, B.G.; Serment-Guerrero, J.H.; Cabral-Prieto, A.; González-Garduño, A.C.; Domínguez-Cadena, N.A.; Muñoz-Infante, J.; et al. Alternative method for healing the diabetic foot by means of a plasma needle. Clin. Plasma Med. 2018, 9, 19–23. [Google Scholar] [CrossRef]
- Peña Eguiluz, R.; López-Callejas, R.; González-Arciniega, E.; Rodríguez-Méndez, B.G.; Mercado-Cabrera, A.; Guakil-Haber, A.; Kuri García, A.; Espinosa Mancilla, A.E.; Valencia-Alvarado, R. Non-thermal plasma wound healing after removal of a neck tumor in a patient with HIV: A case report. Otolaryngol. Case Rep. 2022, 22, 100391. [Google Scholar] [CrossRef]
- Ibáñez-Mancera, N.G.; López-Callejas, R.; Pena-Eguiluz, R.; Rodríguez-Méndez, B.G.; Mercado-Cabrera, A.; Toral-Rizo, V.; Lara-Carrillo, E.; Valencia-Alvarado, R. Wound healing after biopsy in the mobile oral mucosa using a non-thermal atmospheric pressure plasma. IEEE Trans. Rad. Plasma Med. Sci. 2022, 6, 928–935. [Google Scholar] [CrossRef]
- López-Callejas, R.; Velasco-García, P.S.; Betancourt-Ángeles, M.; Rodríguez-Méndez, B.G.; Berrones-Stringel, G.; Jaramillo-Martínez, C.; Farías-López, F.E.; Mercado-Cabrera, A.; Valencia-Alvarado, R. Use of -thermal plasma as postoperative therapy in anal fistula: Clinical experience and results. Biomedicines 2024, 12, 1866. [Google Scholar] [CrossRef]
- Lee, H.G.; Choi, J.H.; Jang, Y.S.; Kim, U.K.; Kim, G.C.; Hwang, D.S. Non-thermal plasma accelerates the healing process of peripheral nerve crush injury in rats. Int. J. Med. Sci. 2020, 17, 1112–1120. [Google Scholar] [CrossRef]
- Kusakci-Seker, B.; Demirayak-Akdemir, M. The effect of non-thermal atmospheric pressure plasma application on wound healing after gingivectomy. Int. Wound J. 2020, 17, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Boekema, B.; Stoop, M.; Vlig, M.; van Liempt, J.; Sobota, A.; Middelkoop, E. Antibacterial and safety tests of a flexible cold atmospheric plasma device for the stimulation of wound healing. Appl. Microbiol. Biotechnol. 2021, 105, 2057–2070. [Google Scholar] [CrossRef] [PubMed]
- Sliney, D.; Aron-Rosa, D.; DeLori, F.; Fankhauser, F.; Landry, R.; Mainster, M.; Marshall, J.; Rassow, B.; Stuck, B.; Trokel, S.; et al. Adjustment of guidelines for exposure of the eye to optical radiation from ocular instruments: Statement from a task group of the International Commission on Non-Ionizing Radiation Protection (ICNIRP). Appl. Opt. 2005, 44, 2162–2176. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, P.; Acharya, T.R.; Kaushik, N.; Nguyen, L.N.; Lim, J.S.; Hessel, V.; Kaushik, N.K.; Choi, E.H. Non-thermal argon plasma jets of various lengths for selective reactive oxygen and nitrogen species production. J. Environ. Chem. Eng. 2022, 10, 107782. [Google Scholar] [CrossRef]
- Maho, T.; Binois, R.; Brulé-Morabito, F.; Demasure, M.; Douat, C.; Dozias, S.; Bocanegra, P.E.; Goard, I.; Hocqueloux, L.; Helloco, C.L.; et al. Anti-bacterial action of plasma multi-jets in the context of chronic wound healing. Appl. Sci. 2021, 11, 9598. [Google Scholar] [CrossRef]
- Eggers, B.; Stope, M.B.; Marciniak, J.; Mustea, A.; Eick, S.; Deschner, J.; Nokhbehsaim, M.; Kramer, F.J. Non-invasive physical plasma reduces the inflammatory response in microbially prestimulated human gingival fibroblasts. Int. J. Mol. Sci. 2023, 24, 16156. [Google Scholar] [CrossRef]
- Noguchi, T.; Emoto, S.; Kawai, K.; Nishikawa, T.; Shuno, Y.; Sasaki, K.; Kaneko, M.; Murono, K.; Ishii, H.; Sonoda, H.; et al. Anastomotic bleeding following ileocolic end-to-side anastomosis using a circular stapler: Incidence and risk factors. Surg. Today 2020, 50, 1368–1374. [Google Scholar] [CrossRef]
- Alverdy, J.C.; Schardey, H.M. Anastomotic leak: Toward an understanding of its root causes. J. Gastrointest. Surg. 2021, 25, 2966–2975. [Google Scholar] [CrossRef]
- Skovsen, A.P.; Jensen, T.K.; Gögenur, I.; Tolstrup, M.-B. Small bowel anastomosis in emergency surgery. World J. Surg. 2024, 48, 341–349. [Google Scholar] [CrossRef]
- Mittelstädt, A.; von Loeffelholz, T.; Weber, K.; Denz, A.; Krautz, C.; Grützmann, R.; Weber, G.F.; Brunner, M. Influence of interrupted versus continuous suture technique on intestinal anastomotic leakage rate in patients with Crohn’s disease—A propensity score matched analysis. Int. J. Colorectal Dis. 2022, 37, 2245–2253. [Google Scholar] [CrossRef]
- Morgan, R.B.; Shogan, B.D. The science of anastomotic healing. Semin. Colon Rectal Surg. 2022, 33, 100879. [Google Scholar] [CrossRef] [PubMed]
- Facile, I.; Galli, R.; Dinter, P.; Rosenberg, R.; Von Flüe, M.; Steinemann, D.C.; Posabella, A.; Droeser, R.A. Short- and long-term outcomes for primary anastomosis versus Hartmann’s procedure in Hinchey III and IV diverticulitis: A multivariate logistic regression analysis of risk factors. Langenbeck’s Arch. Surg. 2021, 406, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Axt, S.; Haller, K.; Wilhelm, P.; Falch, C.; Martus, P.; Johannink, J.; Rolinger, J.; Beltzer, C.; Axt, L.; Königsrainer, A.; et al. Early postoperative endoscopic evaluation of rectal anastomoses: A prospective cross-sectional study. Surg. Endosc. 2022, 36, 8881–8892. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liang, L.; Liu, L.; Zhu, Z. Does intraoperative indocyanine green fluorescence angiography decrease the incidence of anastomotic leakage in colorectal surgery? A systematic review and meta-analysis. Int. J. Colorectal Dis. 2021, 36, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yin, L.; Xu, J.; Liu, H.; Xiang, X.; Zhao, H.; Qiu, J.; Liu, K. An ex vivo preliminary investigation into the impact of parameters on tissue welding strength in small intestine mucosa-mucosa end-to-end anastomosis. Front. Bioeng. Biotechnol. 2023, 11, 1200239. [Google Scholar] [CrossRef]
- Heeman, W.; Wildeboer, A.C.L.; Al-Taher, M.; Joost, E.M.; Calon, J.E.M.; Stassen, L.P.S.; Michele Diana, M.; Derikx, J.P.M.; van Dam, G.M.; Boerma, E.C.; et al. Experimental evaluation of laparoscopic laser speckle contrast imaging to visualize perfusion deficits during intestinal surgery. Surg. Endosc. 2023, 37, 950–957. [Google Scholar] [CrossRef]
- Sun, R.; Wang, Y.; Lv, Z.; Li, H.; Zhang, S.; Dang, Q.; Zhao, X.; Yue, T.; Yuan, Y. Construction of Fu brick tea polysaccharide-cold plasma modified alginate microgels for probiotic delivery: Enhancing viability and colonization. Int. J. Biol. Macromol. 2024, 268, 131899. [Google Scholar] [CrossRef]
- Naoi, D.; Horie, H.; Sadatomo, A.; Koinuma, K.; Ota, G.; Oshiro, K.; Tahara, M.; Mori, K.; Ito, H.; Inoue, Y.; et al. The effect of staple height and rectal-wall thickness on anastomotic leakage after laparoscopic low anterior resection. Asian J. Surg. 2023, 46, 1577–1582. [Google Scholar] [CrossRef]
- Tsalikidis, C.; Mitsala, A.; Mentonis, V.I.; Romanidis, K.; Pappas-Gogos, G.; Tsaroucha, A.K.; Pitiakoudis, M. Predictive factors for anastomotic leakage following colorectal cancer surgery: Where are we and where are we going? Curr. Oncol. 2023, 30, 3111–3137. [Google Scholar] [CrossRef]
- Shineh, G.; Mobaraki, M.; Perves Bappy, M.J.; Mills, D.K. Biofilm formation, and related impacts on healthcare, food processing and packaging, industrial manufacturing, marine industries, and sanitation—A review. Appl. Microbiol. 2023, 3, 629–665. [Google Scholar] [CrossRef]
- Ju, J.-W.; Lee, H.-J.; Kim, M.-J.; Ryoo, S.-B.; Kim, W.H.; Jeong, S.-Y.; Park, K.J.; Park, J.W. Postoperative NSAIDs use and the risk of anastomotic leakage after restorative resection for colorectal cancer. Asian J. Surg. 2023, 46, 4749–4754. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Leth, M.F.; Laursen, C.C.W.; Larsen, M.E.; Tornøe, A.S.; Eriksen, V.R.; Hovmand, A.E.K.; Jakobsen, J.C.; Mathiesen, M.M.O. Risks of serious adverse events withnon-steroidal anti-inflammatory drugs ingastro intestinal surgery: Asystematic review with meta-analysis and trial sequential analysis. Acta Anaesthesiol. Scand. 2024, 68, 871–887. [Google Scholar] [CrossRef]
- Mehboob, A.; Perveen, S.; Iqbal, M.; Bux, M.K.; Waheed, A. Frequency and complications of Ileostomy. Cureus 2020, 12, e11249. [Google Scholar] [CrossRef] [PubMed]
- Bananzade, A.; Dehghankhalili, M.; Bahrami, F.; Tadayon, S.M.K.; Ghaffarpasand, F. Outcome of early versus late ileostomy closure in patients with rectal cancers undergoing low anterior resection: A prospective cohort study. Asian J. Surg. 2023, 46, 4277–4282. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Zhao, X.; Liu, Z.; Yang, G.; Wu, Q.; Wang, X.; Zhang, X.; Cheng, Z.; Wang, K. Complications of preventive loop ileostomy versus colostomy: A meta-analysis, trial sequential analysis, and systematic review. BMC Surg. 2023, 23, 235. [Google Scholar] [CrossRef] [PubMed]
- Zeman, M.; Czarnecki, M.; Grajek, M.; Idasiak, A.; Tukiendorf, A.; Czarniecka, A. Evaluation of risk factors for postoperative complications in rectal cancer patients. Pol. J. Surg. 2020, 92, 24–30. [Google Scholar] [CrossRef]
- Ju, H.E.; Lee, C.S.; Bae, J.H.; Lee, H.J.; Yoon, M.R.; Al-Sawat, A.; Lee, D.S.; Lee, I.K.; Lee, Y.S.; Song, I.H.; et al. High incidence of late anastomosis leakage in patients for rectal cancer after neoadjuvant chemoradiotherapy: A comparative study. Asian J. Surg. 2022, 45, 1832–1842. [Google Scholar] [CrossRef]
- Gerdin, A.; Park, J.; Häggström, J.; Segelman, J.; Matthiessen, P.; Lydrup, M.L.; Rutegård, M. Preoperative beta blockers and other drugs in relation to anastomotic leakage after anterior resection for rectal cancer. Colorectal Dis. 2024, 26, 974–986. [Google Scholar] [CrossRef]
- La Regina, D.; Di Giuseppe, M.; Lucchelli, M.; Saporito, A.; Boni, L.; Efthymiou, C.; Cafarotti, S.; Marengo, M.; Mongelli, F. Financial impact of anastomotic leakage in colorectal surgery. J. Gastrointest. Surg. 2019, 23, 580–586. [Google Scholar] [CrossRef]
- Mukai, T.; Maki, A.; Shimizu, H.; Kim, H. The economic burdens of anastomotic leakage for patients undergoing colorectal surgery in Japan. Asian J. Surg. 2023, 46, 4323–4329. [Google Scholar] [CrossRef]

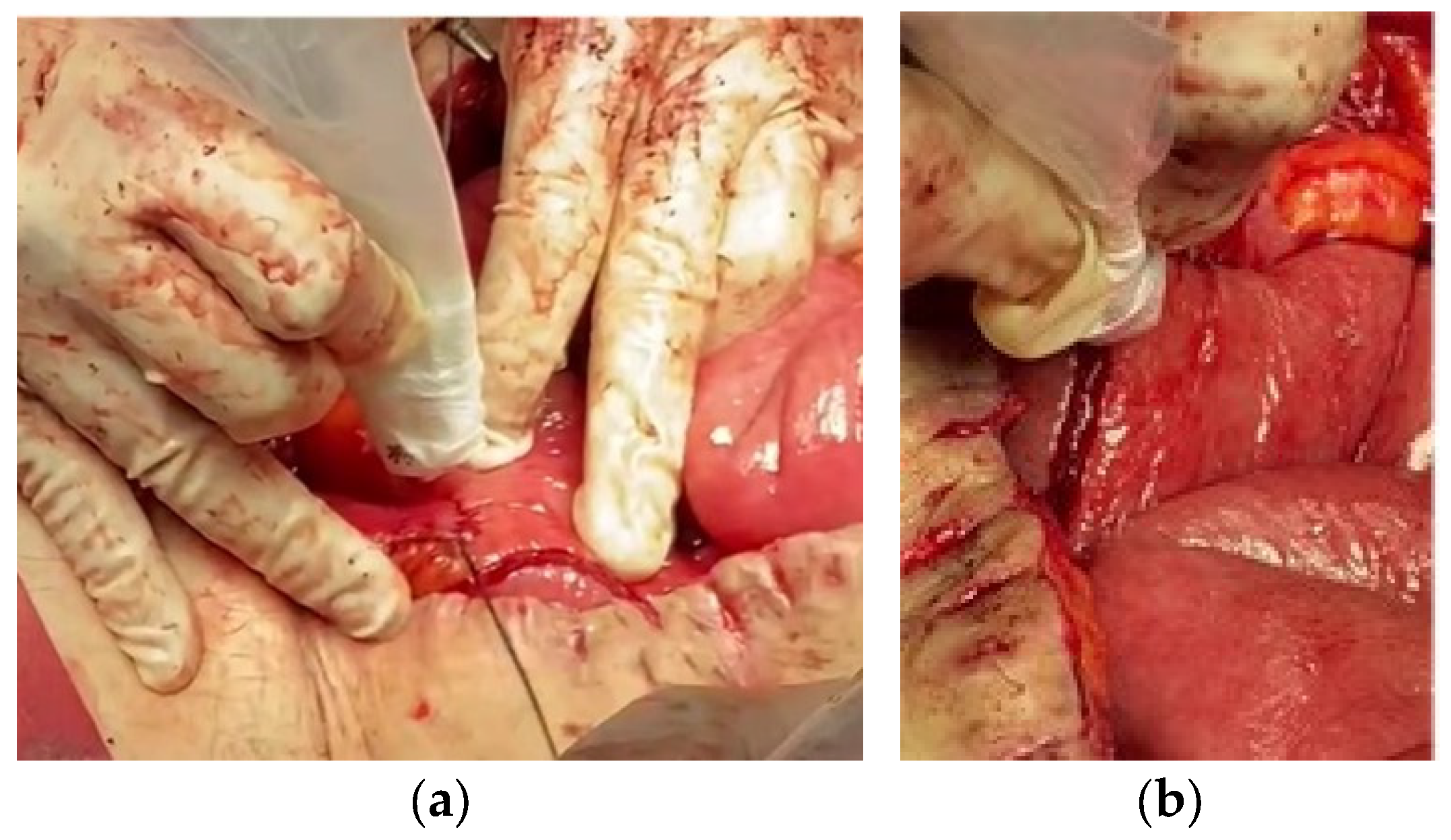
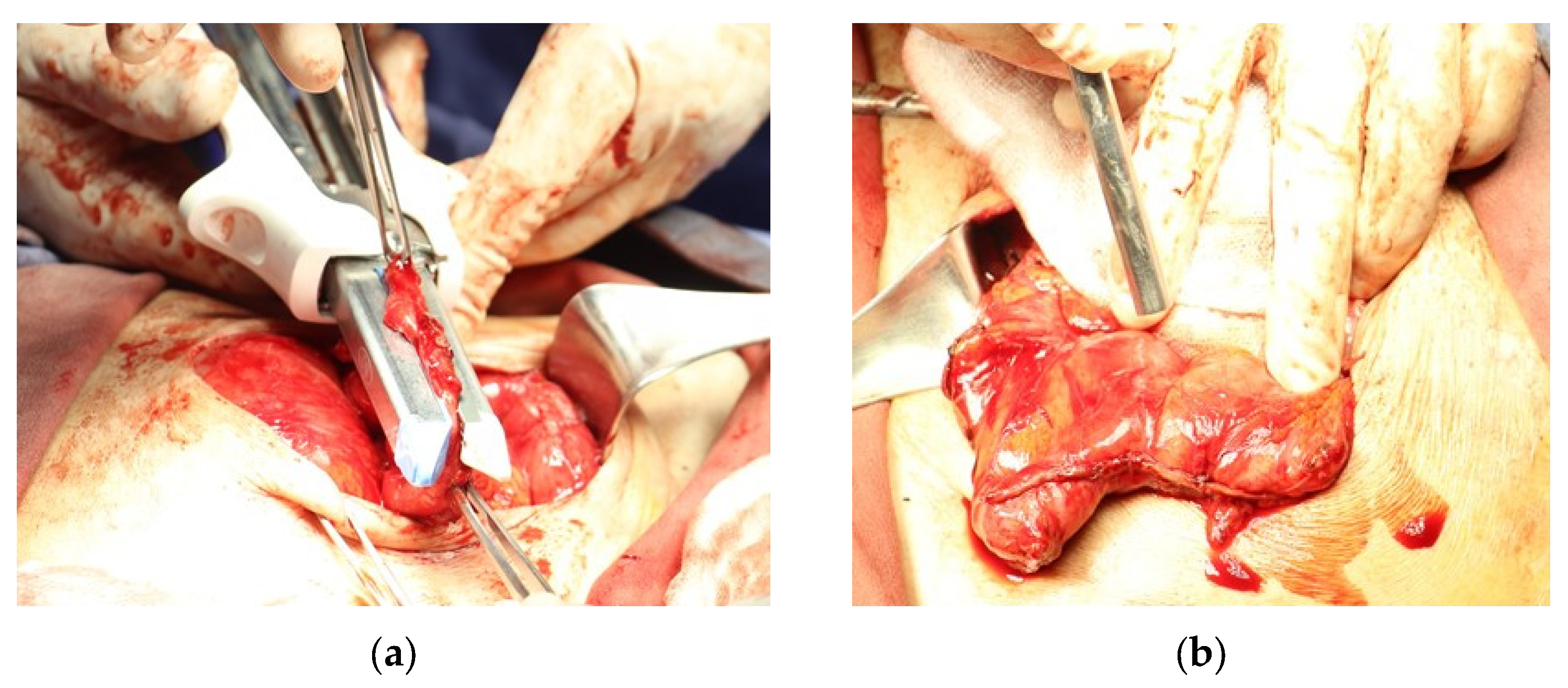

| Parameter | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age [years] | 78 | 49 | 78 |
| Sex | Male | Female | Female |
| Surgery type | Partial colectomy | Ileocecal segmental resection | Laparoscopic resection of Meckel’s diverticulum |
| Anastomosis type | Terminal Term | Laterolateral entero-enteric | Terminoterminal entero-enteric |
| Surgical technique | Techniques combination | Techniques combination | Laparoscopic using four trocars |
| Stapler | linear | linear | linear |
| Suture material | Polyglactin 910 (Vicryl) | Polyglactin 910 (Vicryl) | Polyglactin 910 (Vicryl) |
| Suture gauge | 2-0 | 2-0 | 2-0 |
| Number of planes | 2 (stapler and manual) | 2 (stapler and manual) | 2 (stapler and manual) |
| Irrigation | saline solution | saline solution | saline solution |
| Drain | Jackson-Pratt | Jackson-Pratt | Not placed |
| Surgical time | 120 min | 135 min | 100 min |
| Blood loss | 200 mL | 50 mL | 25 mL |
| Intraoperative complications | Neither | Neither | Adhesions, Meckel’s diverticulum |
| Postoperative complications | None mentioned | None mentioned | None mentioned |
| Hospital stay time | 7 days | 7 days | 3 days |
| Epithelialization | Complete | Complete | Complete |
| Neovascularization | Observed | Observed | Observed |
| Inflammation | slight | slight | very slight |
| Fibrosis | It is observed | It is observed | It is observed |
| Signs of infection | Not found | Not found | Not found |
| Dehiscence | Not | Not | Not |
| Stenosis | Not | Not | Not |
| Necrosis | Not | Not | Not |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betancourt-Ángeles, M.; López-Callejas, R.; Berrones-Stringel, G.; Jaramillo-Martínez, C.; Navarro-Luna, B.; Rodríguez-Méndez, B.G.; Mercado-Cabrera, A.; Valencia-Alvarado, R. Non-Thermal Atmospheric Pressure Plasma as an Adjunct to Intestinal Anastomosis: A Pilot Study on Preventing Anastomotic Leaks. Life 2024, 14, 1450. https://doi.org/10.3390/life14111450
Betancourt-Ángeles M, López-Callejas R, Berrones-Stringel G, Jaramillo-Martínez C, Navarro-Luna B, Rodríguez-Méndez BG, Mercado-Cabrera A, Valencia-Alvarado R. Non-Thermal Atmospheric Pressure Plasma as an Adjunct to Intestinal Anastomosis: A Pilot Study on Preventing Anastomotic Leaks. Life. 2024; 14(11):1450. https://doi.org/10.3390/life14111450
Chicago/Turabian StyleBetancourt-Ángeles, Mario, Régulo López-Callejas, Guillermo Berrones-Stringel, César Jaramillo-Martínez, Bryan Navarro-Luna, Benjamín Gonzalo Rodríguez-Méndez, Antonio Mercado-Cabrera, and Raúl Valencia-Alvarado. 2024. "Non-Thermal Atmospheric Pressure Plasma as an Adjunct to Intestinal Anastomosis: A Pilot Study on Preventing Anastomotic Leaks" Life 14, no. 11: 1450. https://doi.org/10.3390/life14111450
APA StyleBetancourt-Ángeles, M., López-Callejas, R., Berrones-Stringel, G., Jaramillo-Martínez, C., Navarro-Luna, B., Rodríguez-Méndez, B. G., Mercado-Cabrera, A., & Valencia-Alvarado, R. (2024). Non-Thermal Atmospheric Pressure Plasma as an Adjunct to Intestinal Anastomosis: A Pilot Study on Preventing Anastomotic Leaks. Life, 14(11), 1450. https://doi.org/10.3390/life14111450






