A Real-Life Study in Patients Newly Diagnosed with Autoimmune Hashimoto’s Thyroiditis: Analysis of Asthenia as Admission Complaint
Abstract
1. Introduction
2. Materials and Methods
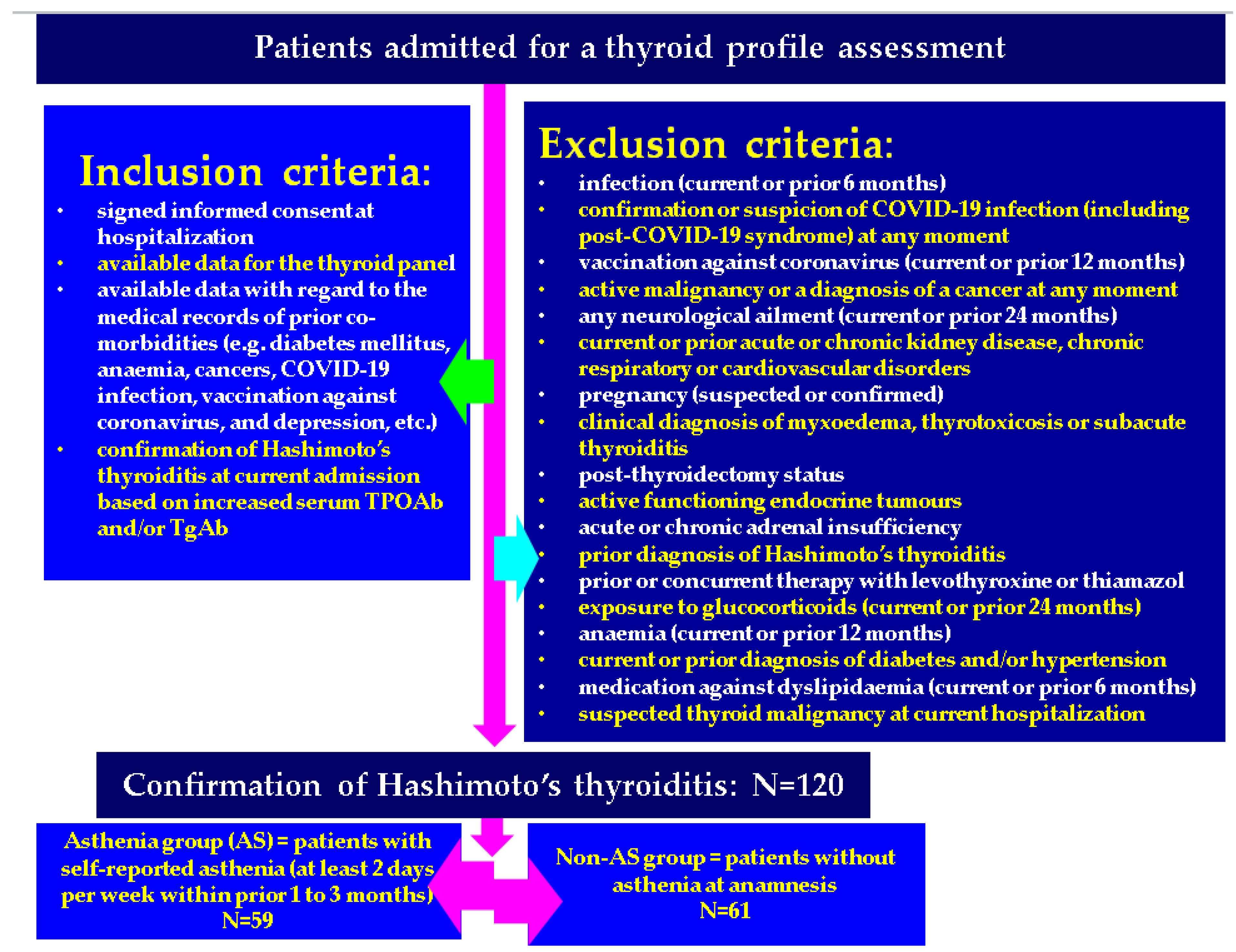
2.1. Study Design and Studied Population
2.2. Statistical Analysis
2.3. Ethical Aspects
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | asthenia (the subjects group with asthenia) |
| FT4 | free levothyroxine |
| HT | Hashimoto’s thyroiditis |
| M | median |
| N | number of patients |
| Q | quartiles |
| TPOAb | anti-thyroperoxidase antibodies |
| TgAb | anti-thyroglobulin antibodies |
| TSH | Thyroid Stimulating Hormone |
Appendix A

References
- Tan, N.C.; Chew, R.Q.; Subramanian, R.C.; Sankari, U.; Koh, Y.L.E.; Cho, L.W. Patients on levothyroxine replacement in the community: Association between hypothyroidism symptoms, co-morbidities and their quality of life. Fam. Pract. 2019, 36, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Perros, P.; Nagy, E.V.; Papini, E.; Van Der Feltz-Cornelis, C.M.; Weetman, A.P.; Hay, H.A.; Abad-Madroñero, J.; Tallett, A.J.; Bilas, M.; Lakwijk, P.; et al. Hypothyroidism and Somatization: Results from E-Mode Patient Self-Assessment of Thyroid Therapy, a Cross-Sectional, International Online Patient Survey. Thyroid 2023, 33, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Samuels, M.H.; Bernstein, L.J. Brain Fog in Hypothyroidism: What Is It, How Is It Measured, and What Can Be Done About It. Thyroid 2022, 32, 752–763. [Google Scholar] [CrossRef]
- Young, P.; Finn, B.C.; Bruetman, J.; Pellegrini, D.; Kremer, A. The chronic asthenia syndrome: A clinical approach. Medicina 2010, 70, 284–292. [Google Scholar] [PubMed]
- Vasenina, E.E.; Gankina, O.A.; Levin, O.S. Stress, asthenia and cognitive disorders. S.S. Korsakov J. Neurol. Psychiatry 2022, 122, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Maoz, D.; Shoenfeld, Y. Chronic fatigue syndrome. Harefuah 2006, 145, 272–275. [Google Scholar]
- Harris, K.; Band, R.J.; Cooper, H.; Macintyre, V.G.; Mejia, A.; Wearden, A.J. Distress in significant others of patients with chronic fatigue syndrome: A systematic review of the literature. Br. J. Health Psychol. 2016, 21, 881–893. [Google Scholar] [CrossRef]
- Bansal, R.A.; Tadros, S.; Bansal, A.S. The presence of overlapping quality of life symptoms in primary antibody deficiency (PAD) and chronic fatigue syndrome (CFS). Allergy Asthma Clin. Immunol. 2020, 16, 21. [Google Scholar] [CrossRef]
- Jansen, H.I.; Boelen, A.; Heijboer, A.C.; Bruinstroop, E.; Fliers, E. Hypothyroidism: The difficulty in attributing symptoms to their underlying cause. Front. Endocrinol. 2023, 14, 1130661. [Google Scholar] [CrossRef]
- Nistor, C.E.; Pantile, D.; Gavan, C.S.; Ciuche, A. Pneumothorax on COVID-19 patients-retrospective clinical observations. Rom. J. Leg. Med. 2022, 30, 112–116. [Google Scholar] [CrossRef]
- Ramonfaur, D.; Ayad, N.; Liu, P.H.Z.; Zhou, J.; Wu, Y.; Li, J.; Chen, G. The global clinical studies of long COVID. Int. J. Infect. Dis. 2024, 146, 107105. [Google Scholar] [CrossRef] [PubMed]
- Golzardi, M.; Hromić-Jahjefendić, A.; Šutković, J.; Aydin, O.; Ünal-Aydın, P.; Bećirević, T.; Redwan, E.M.; Rubio-Casillas, A.; Uversky, V.N. The Aftermath of COVID-19: Exploring the Long-Term Effects on Organ Systems. Biomedicines 2024, 12, 913. [Google Scholar] [CrossRef] [PubMed]
- Sk Abd Razak, R.; Ismail, A.; Abdul Aziz, A.F.; Suddin, L.S.; Azzeri, A.; Sha’ari, N.I. Post-COVID syndrome prevalence: A systematic review and meta-analysis. BMC Public Health 2024, 24, 1785. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A. Exploring the Pathophysiology of Long COVID: The Central Role of Low-Grade Inflammation and Multisystem Involvement. Int. J. Mol. Sci. 2024, 25, 6389. [Google Scholar] [CrossRef]
- Harris, E. Review Calls for Standardized Long COVID Definition. JAMA 2024, 332, 97. [Google Scholar] [CrossRef]
- Stevens, J.M.; Montgomery, K.; Miller, M.; Saeidzadeh, S.; Kwekkeboom, K.L. Common patient-reported sources of cancer-related distress in adults with cancer: A systematic review. Cancer Med. 2024, 13, e7450. [Google Scholar] [CrossRef]
- Nistor, C.E.; Găvan, C.S.; Ciritel, A.A.; Nemes, A.F.; Ciuche, A. The Association of Minimally Invasive Surgical Approaches and Mortality in Patients with Malignant Pleuropericarditis—A 10 Year Retrospective Observational Study. Medicina 2022, 58, 718. [Google Scholar] [CrossRef]
- Ee, C.; Kay, S.; Reynolds, A.; Lovato, N.; Lacey, J.; Koczwara, B. Lifestyle and integrative oncology interventions for cancer-related fatigue and sleep disturbances. Maturitas 2024, 187, 108056. [Google Scholar] [CrossRef]
- Bower, J.E.; Lacchetti, C.; Alici, Y.; Barton, D.L.; Bruner, D.; Canin, B.E.; Escalante, C.P.; Ganz, P.A.; Garland, S.N.; Gupta, S.; et al. Management of Fatigue in Adult Survivors of Cancer: ASCO-Society for Integrative Oncology Guideline Update. J. Clin. Oncol. 2024, 42, 2456–2487. [Google Scholar] [CrossRef]
- Arring, N.M.; Barton, D.L.; Brooks, T.; Zick, S.M. Integrative Therapies for Cancer-Related Fatigue. Cancer J. 2019, 25, 349–356. [Google Scholar] [CrossRef]
- Available online: https://lege5.ro/Gratuit/geztsmzyge/ghidul-de-diagnostic-si-terapie-al-nodulilor-tiroidieni-pentru-romania-din-18102010 (accessed on 8 August 2024).
- Klubo-Gwiezdzinska, J.; Wartofsky, L. Hashimoto thyroiditis: An evidence-based guide to etiology, diagnosis and treatment. Pol. Arch. Intern. Med. 2022, 132, 16222. [Google Scholar] [CrossRef] [PubMed]
- Koehler, V.F.; Bojunga, J. Autoimmune thyroid disease. Dtsch. Med. Wochenschr. 2021, 146, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.E.; Pantile, D.; Stanciu-Gavan, C.; Ciuche, A.; Moldovan, H. Diagnostic and Therapeutic Characteristics in Patients with Pneumotorax Associated with COVID-19 versus Non-COVID-19 Pneumotorax. Med. Kaunas 2022, 58, 1242. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, M.; Szczepanek-Parulska, E.; Ruchala, M. Lipoprotein alterations in endocrine disorders—A review of the recent developments in the field. Front. Endocrinol. 2024, 15, 1354098. [Google Scholar] [CrossRef] [PubMed]
- Szczepanek-Parulska, E.; Sokolowski, J.; Dmowska, D.; Klimek, J.; Stasikowski, T.; Zdebski, P.; Olejarz, M.; Gac, A.; Bartecki, M.; Ruchała, M. Lipid profile abnormalities associated with endocrine disorders. Endokrynol. Pol. 2022, 73, 863–871. [Google Scholar] [CrossRef]
- Newman, C.B. Effects of endocrine disorders on lipids and lipoproteins. Best. Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101667. [Google Scholar] [CrossRef]
- Sumino, H.; Murakami, M. Causes and Abnormal Lipid Laboratory Values of Secondary Hyperlipidemia: Endocrine Disease. Rinsho Byori. Jpn. J. Clin. Pathol. 2016, 64, 513–517. [Google Scholar]
- Biondi, B. Subclinical Hypothyroidism in Patients with Obesity and Metabolic Syndrome: A Narrative Review. Nutrients 2023, 16, 87. [Google Scholar] [CrossRef]
- Siemińska, L.; Wojciechowska, C.; Walczak, K.; Borowski, A.; Marek, B.; Nowak, M.; Kajdaniuk, D.; Foltyn, W.; Kos-Kudła, B. Associations between metabolic syndrome, serum thyrotropin, and thyroid antibodies status in postmenopausal women, and the role of interleukin-6. Endokrynol. Pol. 2015, 66, 394–403. [Google Scholar] [CrossRef][Green Version]
- Biondi, B.; Cappola, A.R.; Cooper, D.S. Subclinical Hypothyroidism: A Review. JAMA 2019, 322, 153–160. [Google Scholar] [CrossRef]
- Gajda, S.N.; Kuryłowicz, A.; Żach, M.; Bednarczuk, T.; Wyleżoł, M. Diagnosis and treatment of thyroid disorders in obese patients—What do we know? Endokrynol. Pol. 2019, 70, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Bambini, F.; Gatta, E.; D’Alessio, R.; Dondi, F.; Pignata, G.; Pirola, I.; Bertagna, F.; Cappelli, C. Thyroid disease and autoimmunity in obese patients: A narrative review. Endokrynol. Pol. 2023, 74. [Google Scholar] [CrossRef]
- Pomahacova, R.; Paterova, P.; Nykodymova, E.; Polak, P.; Sladkova, E.; Skalicka, E.; Sykora, J. Overweight and obesity in children and adolescents with endocrine disorders. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2023, 167, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Feldt-Rasmussen, U.; Effraimidis, G.; Bliddal, S.; Klose, M. Consequences of undertreatment of hypothyroidism. Endocrine 2024, 84, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Ahima, R.S. Endocrine disorders associated with obesity. Best. Pract. Res. Clin. Obstet. Gynaecol. 2023, 90, 102394. [Google Scholar] [CrossRef]
- Zhong, L.; Liu, S.; Yang, Y.; Xie, T.; Liu, J.; Zhao, H.; Tan, G. Metabolic syndrome and risk of subclinical hypothyroidism: A systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1399236. [Google Scholar] [CrossRef]
- Grigoriadis, G.; Koufakis, T.; Kotsa, K. Epidemiological, Pathophysiological, and Clinical Considerations on the Interplay between Thyroid Disorders and Type 2 Diabetes Mellitus. Medicina 2023, 59, 2013. [Google Scholar] [CrossRef]
- Alwan, H.; Ribero, V.A.; Efthimiou, O.; Del Giovane, C.; Rodondi, N.; Duntas, L. A systematic review and meta-analysis investigating the relationship between metabolic syndrome and the incidence of thyroid diseases. Endocrine 2024, 84, 320–327. [Google Scholar] [CrossRef]
- Mohammed Hussein, S.M.; AbdElmageed, R.M. The Relationship Between Type 2 Diabetes Mellitus and Related Thyroid Diseases. Cureus 2021, 13, e20697. [Google Scholar] [CrossRef]
- Zenoaga-Barbăroșie, C.; Berca, L.; Vassu-Dimov, T.; Toma, M.; Nica, M.I.; Alexiu-Toma, O.A.; Ciornei, C.; Albu, A.; Nica, S.; Nistor, C.; et al. The Predisposition for Type 2 Diabetes Mellitus and Metabolic Syndrome. Balk. J. Med. Genet. 2023, 26, 21–26. [Google Scholar] [CrossRef]
- Gramont, B.; Goutte, J.; Féasson, L.; Millet, G.; Hupin, D.; Cathébras, P. Chronic fatigue: What investigations? And what for? Rev. Med. Interne 2023, 44, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Craig, T.; Kakumanu, S. Chronic fatigue syndrome: Evaluation and treatment. Am. Fam. Physician 2002, 65, 1083–1090. [Google Scholar] [PubMed]
- Bansal, A.S. Investigating unexplained fatigue in general practice with a particular focus on CFS/ME. BMC Fam. Pract. 2016, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Reshetova, T.V.; Lapteva, E.S.; Lukashkova, V.V.; Reshetov, A.V. The problems of older people and directions of medical, social and psychological support during the COVID-19 pandemic. Adv. Gerontol. 2021, 34, 679–693. [Google Scholar] [PubMed]
- Chutko, L.S.; Surushkina, S.Y. Asthenic disorders. History and modernity. S.S. Korsakov J. Neurol. Psychiatry 2020, 120, 131–136. [Google Scholar] [CrossRef]
- Sharif, K.; Watad, A.; Bragazzi, N.L.; Lichtbroun, M.; Martini, M.; Perricone, C.; Amital, H.; Shoenfeld, Y. On chronic fatigue syndrome and nosological categories. Clin. Rheumatol. 2018, 37, 1161–1170. [Google Scholar] [CrossRef]
- Nistor, C.; Ciuche, A.; Constantinescu, I. Emergency surgical tracheal decompression in a huge retrosternal goiter. Acta Endocrinol. 2017, 13, 370–374. [Google Scholar] [CrossRef]
- Carsote, M.; Valea, A.; Dumitru, N.; Terzea, D.; Petrova, E.; Albu, S.; Buruiana, A.; Ghemigian, A. Metastases in daily endocrine practice. Arch. Balk. Med. Union. 2016, 51, 476–480. [Google Scholar]
- Unger, N.; Theodoropoulou, M.; Schilbach, K. Clinically active pituitary tumors. Inn. Med. 2024, 65, 672–680. [Google Scholar] [CrossRef]
- Donald, J.; Bilasy, S.E.; Yang, C.; El-Shamy, A. Exploring the Complexities of Long COVID. Viruses 2024, 16, 1060. [Google Scholar] [CrossRef]
- Ashmawy, R.; Hammouda, E.A.; El-Maradny, Y.A.; Aboelsaad, I.; Hussein, M.; Uversky, V.N.; Redwan, E.M. Interplay between Comorbidities and Long COVID: Challenges and Multidisciplinary Approaches. Biomolecules 2024, 14, 835. [Google Scholar] [CrossRef] [PubMed]
- Gutzeit, J.; Weiß, M.; Nürnberger, C.; Lemhöfer, C.; Appel, K.S.; Pracht, E.; Reese, J.P.; Lehmann, C.; Polidori, M.C.; Hein, G.; et al. Definitions and symptoms of the post-COVID syndrome: An updated systematic umbrella review. Eur. Arch. Psychiatry Clin. Neurosci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M.; Rosko, S.; Jaeger, B.; Gerlach, J.; Rausch, H. Unraveling the enigma of long COVID: Novel aspects in pathogenesis, diagnosis, and treatment protocols. Inflammopharmacology 2024, 32, 2075–2090. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, P.; Leszczyńska, A.; Ciepiela, O. Long COVID Definition, Symptoms, Risk Factors, Epidemiology and Autoimmunity: A Narrative Review. Am. J. Med. Open 2024, 11, 100068. [Google Scholar] [CrossRef]
- Tsay, G.J.; Zouali, M. Cellular pathways and molecular events that shape autoantibody production in COVID-19. J. Autoimmun. 2024, 147, 103276. [Google Scholar] [CrossRef]
- Chou, R.; Herman, E.; Ahmed, A.; Anderson, J.; Selph, S.; Dana, T.; Williams, L.; Ivlev, I. Long COVID Definitions and Models of Care: A Scoping Review. Ann. Intern. Med. 2024, 177, 929–940. [Google Scholar] [CrossRef]
- Ruiz-Pablos, M.; Paiva, B.; Zabaleta, A. Hypocortisolemic ASIA: A vaccine- and chronic infection-induced syndrome behind the origin of long COVID and myalgic encephalomyelitis. Front. Immunol. 2024, 15, 1422940. [Google Scholar] [CrossRef]
- Dhiman, N.R.; Joshi, D.; Singh, R.; Gyanpuri, V.; Kumar, A. Post-COVID-19 headache- NDPH phenotype: A systematic review of case reports. Front. Pain. Res. 2024, 5, 1376506. [Google Scholar] [CrossRef]
- Cheema, S.; Stubberud, A.; Rantell, K.; Nachev, P.; Tronvik, E.; Matharu, M. Phenotype of new daily persistent headache: Subtypes and comparison to transformed chronic daily headache. J. Headache Pain. 2023, 24, 109. [Google Scholar] [CrossRef]
- Peng, K.P.; Wang, S.J. Update of New Daily Persistent Headache. Curr. Pain. Headache Rep. 2022, 26, 79–84. [Google Scholar] [CrossRef]
- Goldenberg, D.L. How to understand the overlap of long COVID, chronic fatigue syndrome/myalgic encephalomyelitis, fibromyalgia and irritable bowel syndromes. Semin. Arthritis Rheum. 2024, 67, 152455. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.L. Applying Lessons From Rheumatology to Better Understand Long COVID. Arthritis Care Res. 2024, 76, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, A.L.; Lipkin, W.I. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol. Med. 2021, 27, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.A.; Maksoud, R.; Beeraka, N.M.; Madhunapantula, S.V.; Sinelnikov, M.; Nikolenko, V.N.; Neganova, M.E.; Klochkov, S.G.; Amjad Kamal, M.; Staines, D.R.; et al. Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome. J. Adv. Res. 2022, 40, 179–196. [Google Scholar] [CrossRef]
- Anghel, A.; Stanciu, S.; Ciobica, M.L.; Stoicescu, D.; Muresan, M.M. Contrast-enhanced ultrasound-clinical applications. Rom. J. Mil. Med. 2011, 114, 25–30. [Google Scholar]
- Liu, J.; Guo, Y.; Xiao, J.; Chen, L.; Liang, Z. Comparison of the Efficacy and Safety of the American Thyroid Association Guidelines and American College of Radiology TI-RADS. Endocr. Pract. 2021, 27, 661–667. [Google Scholar] [CrossRef]
- Stanciu, S.; Enciu, C.; Raduta, I.; Stoicescu, D.; Anghel, A.; Anghel, D.; Olan, B.; Ciobica, L. The role of contrast-enhanced ultrasound in risk assessment of carotid atheroma. Rom. J. Mil. Med. 2016, 119, 9–11. [Google Scholar] [CrossRef]
- Zlobina, I.A.; Krivtsunov, A.N.; Bogat, S.V.; Prashchayeu, K.I. Musculoskeletal system as a target organ of a frailty processes. Adv. Gerontol. 2015, 28, 725–728. [Google Scholar] [CrossRef]
- Tan, L.F.; Lim, Z.Y.; Choe, R.; Seetharaman, S.; Merchant, R. Screening for Frailty and Sarcopenia Among Older Persons in Medical Outpatient Clinics and its Associations With Healthcare Burden. J. Am. Med. Dir. Assoc. 2017, 18, 583–587. [Google Scholar] [CrossRef]
- Massari, M.C.; Bimonte, V.M.; Falcioni, L.; Moretti, A.; Baldari, C.; Iolascon, G.; Migliaccio, S. Nutritional and physical activity issues in frailty syndrome during the COVID-19 pandemic. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X231152648. [Google Scholar] [CrossRef]
- Lozupone, M.; La Montagna, M.; Di Gioia, I.; Sardone, R.; Resta, E.; Daniele, A.; Giannelli, G.; Bellomo, A.; Panza, F. Social Frailty in the COVID-19 Pandemic Era. Front. Psychiatry 2020, 11, 577113. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, N.C.; Dikbaş, O.; Kulaklı, F.; Sarı, I.F.; Sengul, D.; Sengul, I. Revisiting femoral cartilage thickness in cases with Hashimoto’s thyroiditis in thyroidology: A single institute experience. Rev. Assoc. Med. Bras. 2023, 69, e20221615. [Google Scholar] [CrossRef] [PubMed]
- Valea, A.; Carsote, M.; Moldovan, C.; Georgescu, C. Chronic autoimmune thyroiditis and obesity. Arch. Balk. Med. Union. 2018, 53, 64–69. [Google Scholar]
- Sengul, D.; Sengul, I.; Soares Junior, J.M. Repercussion of thyroid dysfunctions in thyroidology on the reproductive system: Conditio sine qua non? Rev. Assoc. Med. Bras. 2022, 68, 721–722. [Google Scholar] [CrossRef]
- Gutic, B.; Bozanovic, T.; Mandic, A.; Dugalic, S.; Todorovic, J.; Stanisavljevic, D.; Dugalic, M.G.; Sengul, D.; Detanac, D.A.; Sengul, I.; et al. Programmed cell death-1 and its ligands: Current knowledge and possibilities in immunotherapy. Clinics 2023, 78, 100177. [Google Scholar] [CrossRef]
- Pradhan, R.; Kundu, A.; Kundu, C.N. The cytokines in tumor microenvironment: From cancer initiation-elongation-progression to metastatic outgrowth. Crit. Rev. Oncol. Hematol. 2024, 196, 104311. [Google Scholar] [CrossRef]
- Valea, A.; Ghervan, C.; Morar, A.; Pop, D.D.; Carsote, M.; Albu, S.E.; Georgescu, C.E.; Chiorean, A. Hashimoto’s thyroiditis and breast cancer: Coincidence or correlation? Arch. Balk. Med. Union. 2016, 51, 129–132. [Google Scholar]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Fallahi, P.; Elia, G.; Ragusa, F.; Ruffilli, I.; Camastra, S.; Giusti, C.; Paparo, S.R.; Gonnella, D.; Shoenfeld, Y.; Ferrari, S.M.; et al. The aggregation between AITD with rheumatologic, or dermatologic, autoimmune diseases. Best. Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101372. [Google Scholar] [CrossRef]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best. Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef]
- Sengul, D.; Sengul, I. World Thyroid Day 2023 in thyroidology: No overlook thyroid dis-eases to opt for “thyroid health” purposes. Rev. Assoc. Med. Bras. 2023, 69, e20230864. [Google Scholar] [CrossRef]
| Parameter | Total Cohort (N = 120) | AS Group (N = 59) | AS-Negative Group (N = 61) | p-Value Between AS and AS-Negative Groups |
|---|---|---|---|---|
| Age (mean ± SD) | 48.2 ± 14.8 | 49.3 ± 14.7 | 47.1 ± 14.8 | 0.426 |
| Area of residence (rural, %) | 33 (27.5) | 25 (42.3) | 8 (13.1) | 0.001 |
| Autoimmune diseases (number, %) | 16 (13.3) | 6 (10.1) | 9 (14.8) | 0.448 |
| Obesity (number, %) | 43 (35.8) | 23 (39.0) | 20 (32.8) | 0.479 |
| Osteoporosis (number, %) | 27 (22.5) | 15 (25.4) | 12 (19.7) | 0.451 |
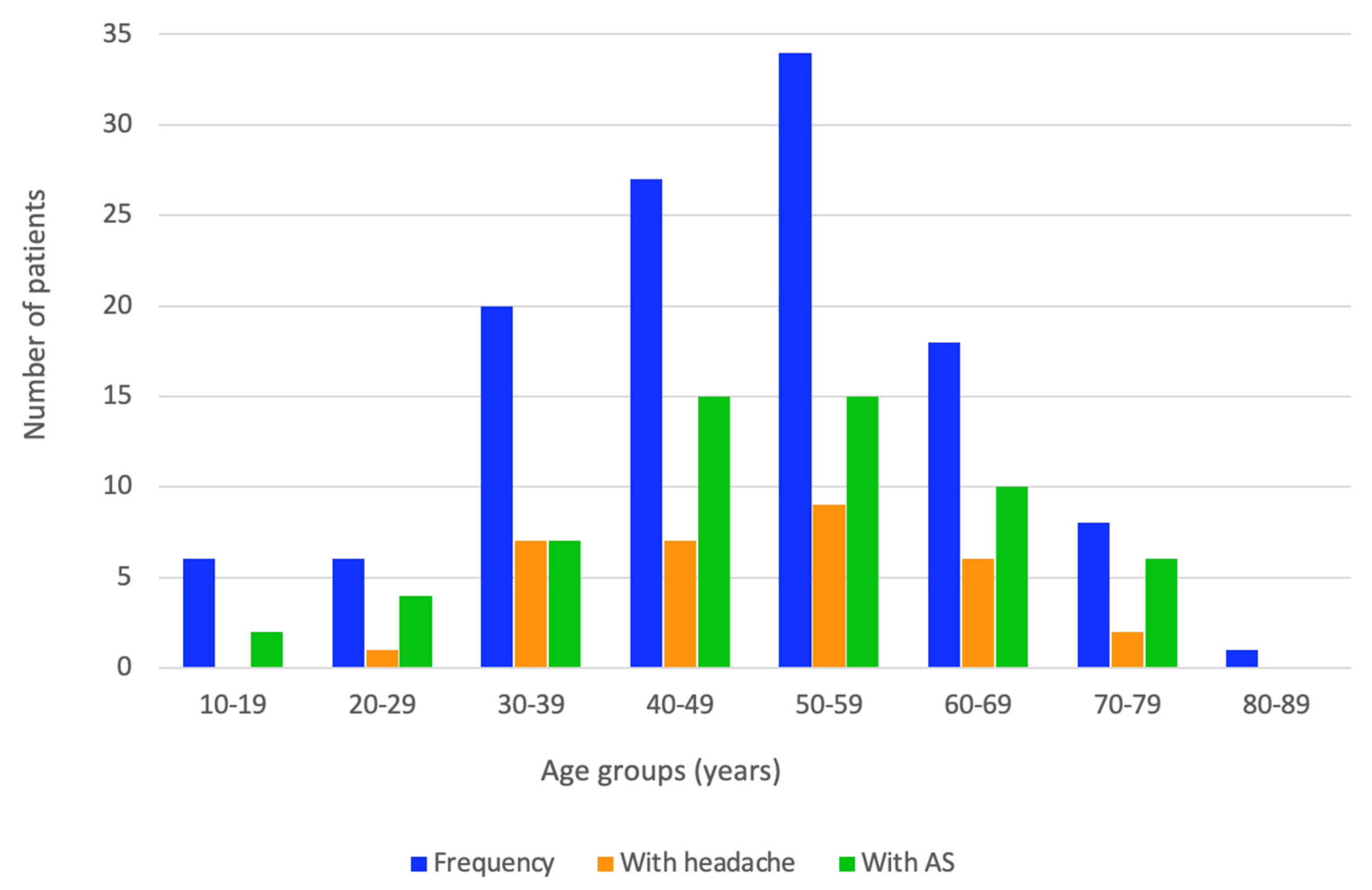
| Headache (number, %) | 32 (26.7) | 21 (35.6) | 11 (18.0) | 0.030 |
| Obesity Grade | Total Cohort Number (% from Obese Patients) | AS Group Number (% from Obese Patients with AS) | AS-Negative Group Number (% from Obese Patients Without AS) | p-Value |
|---|---|---|---|---|
| Grade I | 20 (46.5) | 7 (30.4) | 13 (65.0) | 0.035 |
| Grade II | 15 (34.9) | 9 (39.1) | 6 (30.0) | |
| Grade III | 8 (18.6) | 7 (30.4) | 1 (5.0) |
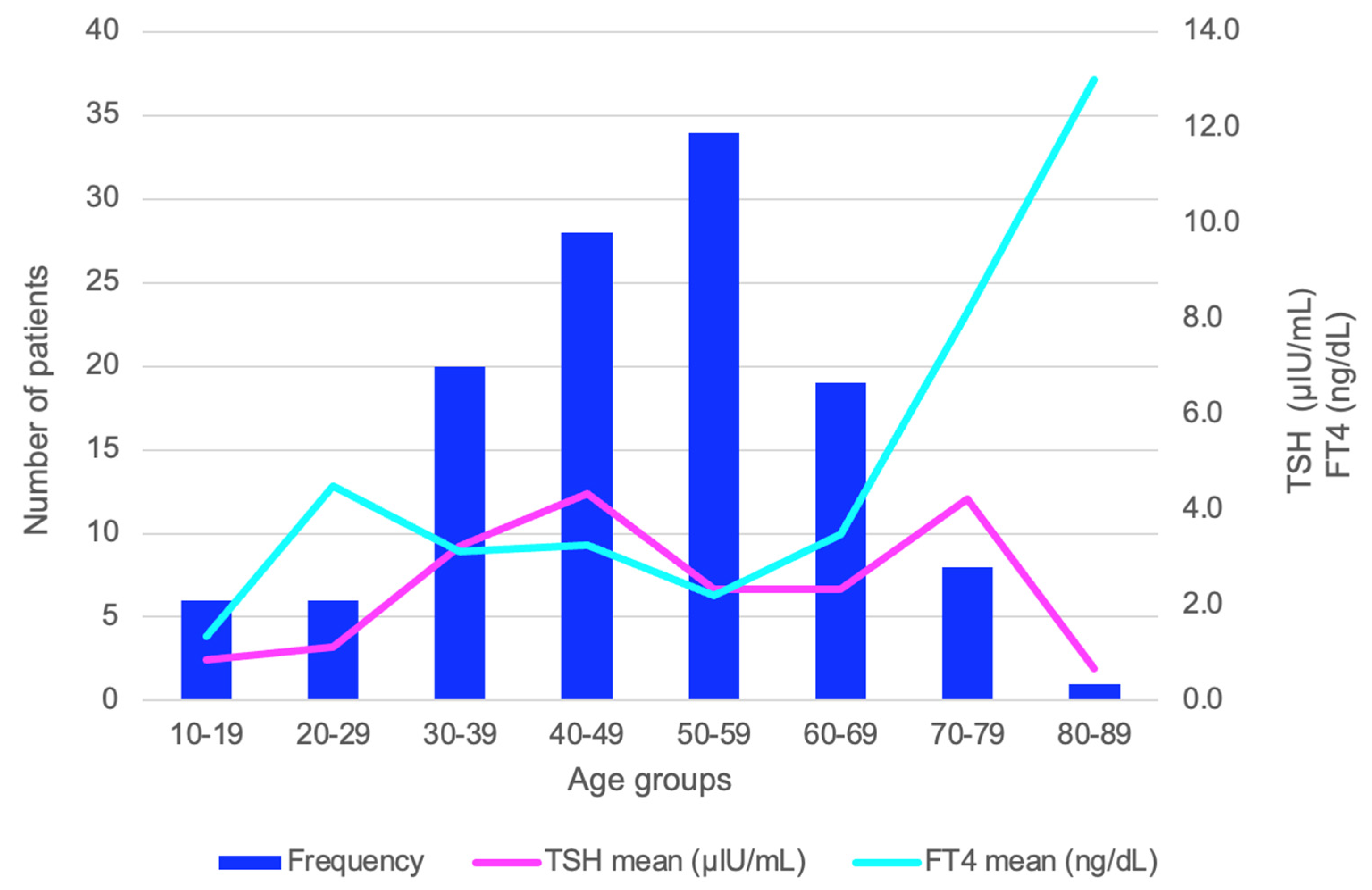
| Studied Population | Number of Patients (N) (Total Patients Within the Group) | Patients with Hypothyroidism (TSH > 4.5 μIU/mL) N (% from the Entire Sub-Group) |
|---|---|---|
| Entire studied cohort | 120 | 37 (30.8) |
| AS group | 59 | 23 (39.0) |
| AS-negative group | 61 | 14 (23.0) |
| 10–19 years | 6 | 0 (0.0) |
| 20–29 years | 6 | 1 (16.7) |
| 30–39 years | 20 | 7 (35.0) |
| 40–49 years | 27 | 11 (40.7) |
| 50–59 years | 34 | 10 (29.4) |
| 60–69 years | 18 | 7 (38.9) |
| 70–79 years | 8 | 1 (12.5) |
| 80–89 years | 1 | 0 (0.0) |
| Parameter | Total Cohort (N = 120) | AS Group (N = 59) | AS-Negative Group (N = 61) | p-Value | Normal Range |
|---|---|---|---|---|---|
| TSH (µIU/mL), M (Q1, Q3) | 2.7 (1.4, 5.4) | 2.8 (1.4, 8.2) | 2.6 (1.3, 4.4) | 0.275 | 0.4–4.5 |
| FT4 (ng/dL), M (Q1, Q3) | 1.2 (1.0, 1.6) | 1.2 (1.0, 1.6) | 1.3 (1.0, 1.5) | 0.339 | 0.89–1.76 |
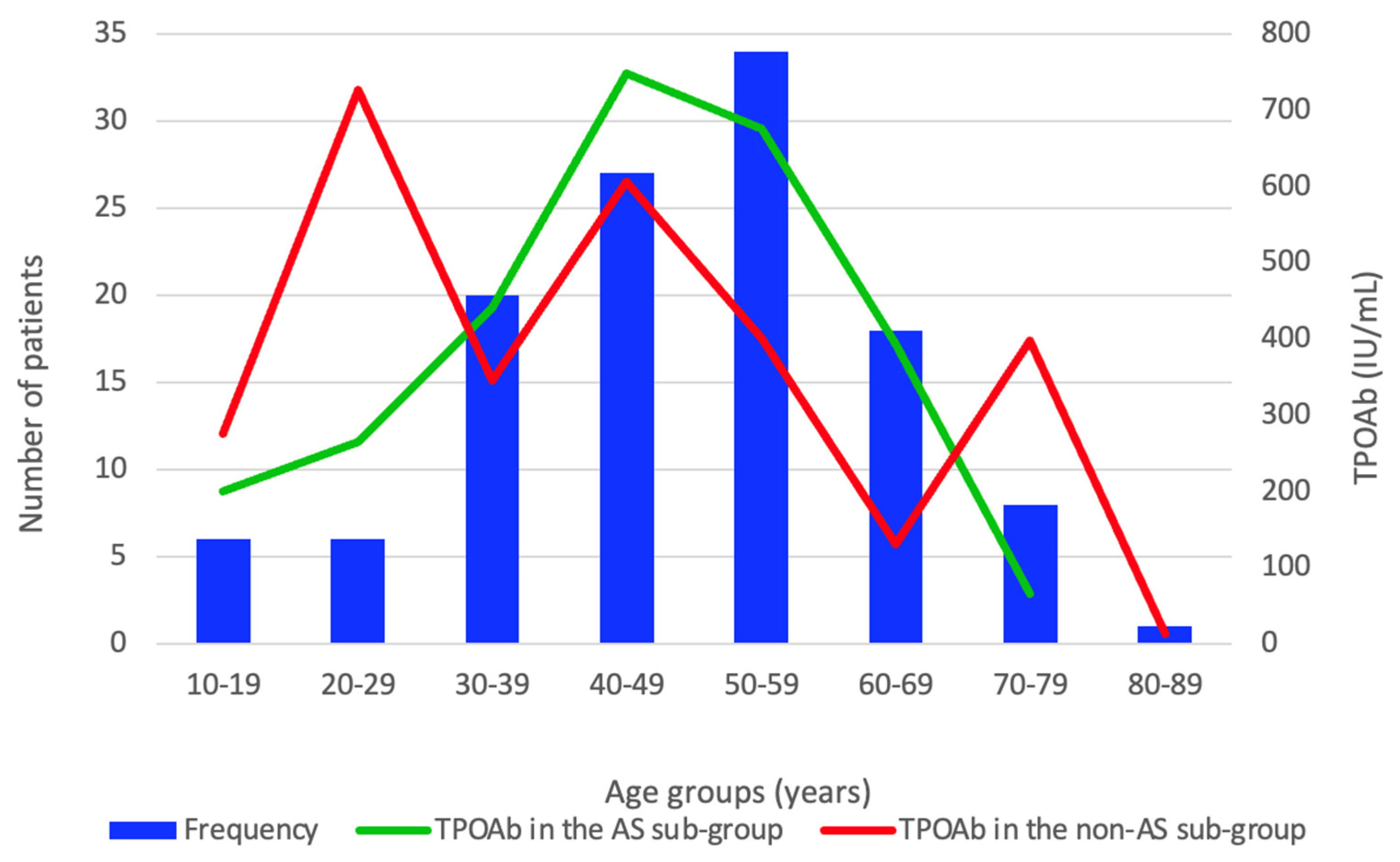
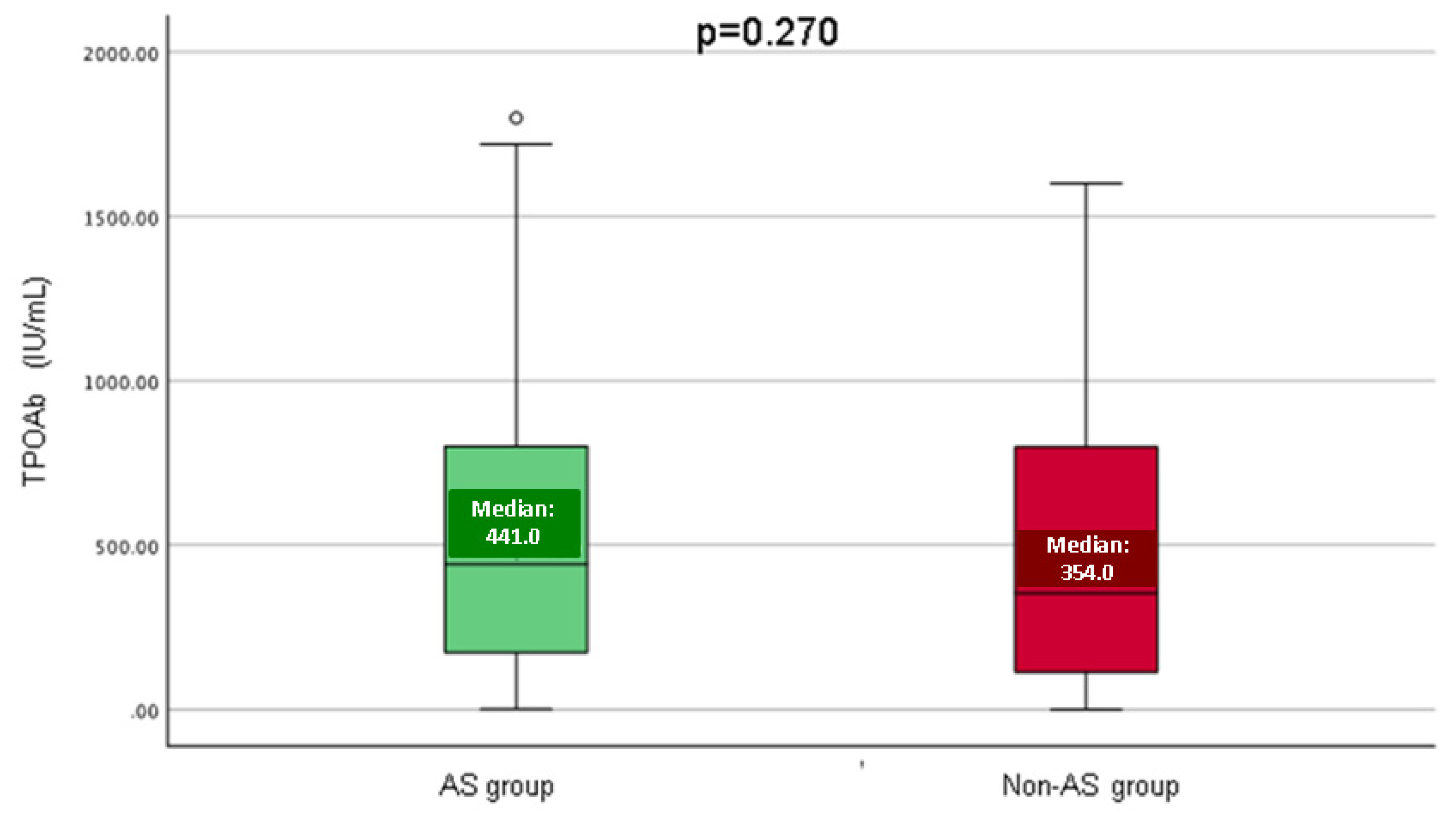
| TPOAb (IU/mL), M (Q1, Q3) | 403.5(136.0, 800.5) | 441.0 (161.5, 801.0) | 354.0 (111.9, 798.8) | 0.270 | 0–50 |
| TgAb (IU/mL), M (Q1, Q3) | 57.5 (25.7, 364.5) | 49.1 (25.0, 437.0) | 69.0 (31.8, 257.5) | 0.944 | 0–60 |
| Total cholesterol (mg/dL), M (Q1, Q3) | 201.0 (174.3, 228.0) | 201.0 (176.0, 228) | 201 (164.5, 228) | 0.442 | 0–200 |
| Triglycerides (mg/dL), M (Q1, Q3) | 94.0 (69.0, 139.8) | 111.0 (74.0, 141.0) | 87.0 (67.0, 136.5) | 0.234 | 0–150 |
| Parameter | Age (Years) | Total Cholesterol (mg/dL) | Triglycerides (mg/dL) | |
|---|---|---|---|---|
| Entire cohort (N = 120) | ||||
| TSH (µIU/mL) | Correlation coefficient | −0.141 | 0.004 | −0.078 |
| p-value | 0.125 | 0.0967 | 0.399 | |
| FT4 (pmol/L) | Correlation coefficient | 0.132 | −0.086 | 0.010 |
| p-value | 0.152 | 0.351 | 0.914 | |
| TPOAb (IU/mL) | Correlation coefficient | −0.086 | 0.141 | −0.039 |
| p-value | 0.348 | 0.125 | 0.671 | |
| TgAb (IU/mL) | Correlation coefficient | 0.039 | 0.117 | 0.145 |
| p-value | 0.672 | 0.202 | 0.113 | |
| AS group (N = 59) | ||||
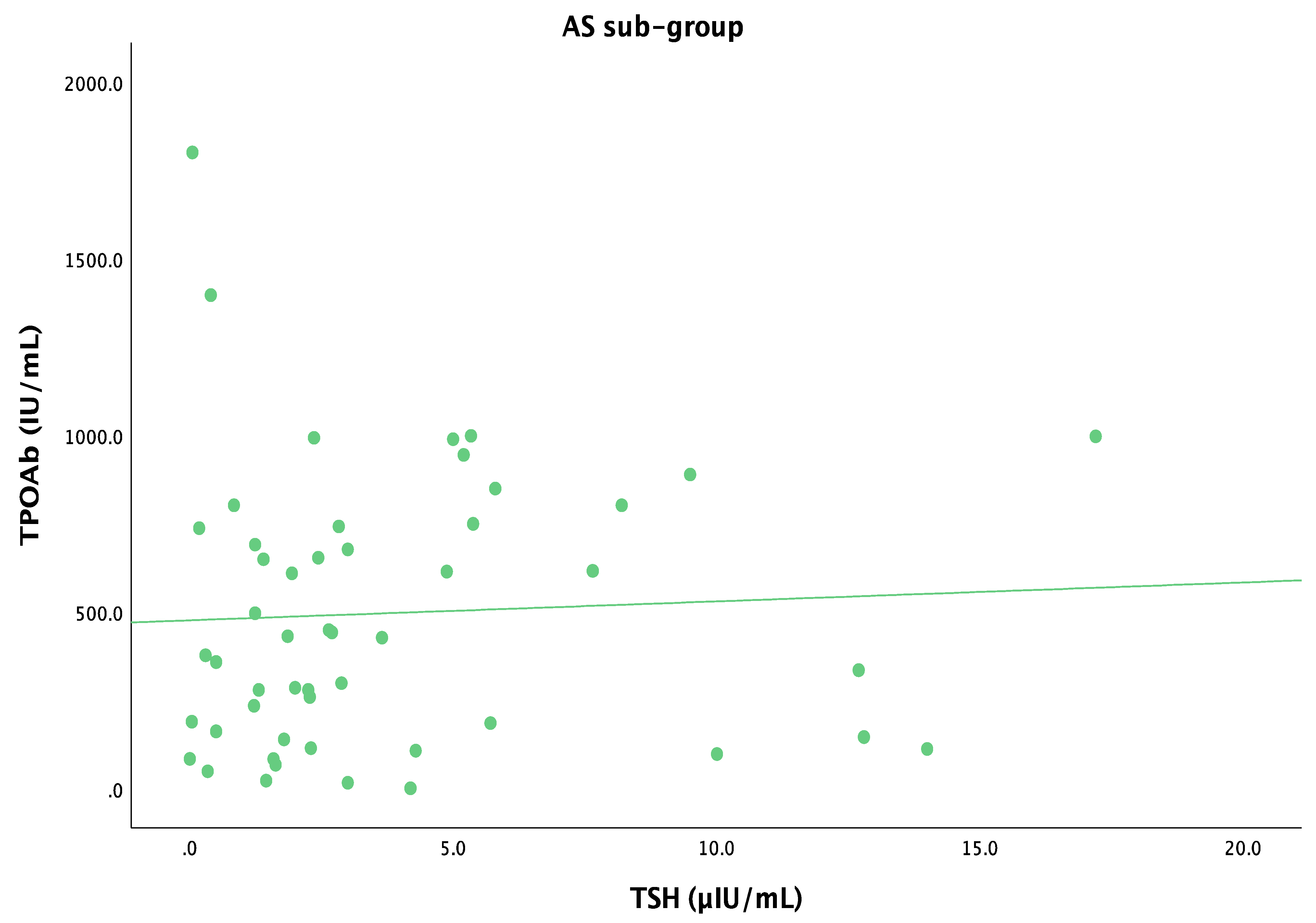
| TSH (µIU/mL) | Correlation coefficient | −0.089 | 0.033 | −0.029 |
| p-value | 0.323 | 0.709 | 0.744 | |
| FT4 (pmol/L) | Correlation coefficient | 0.098 | −0.085 | 0.060 |
| p-value | 0.282 | 0.349 | 0.508 | |
| TPOAb (IU/mL) | Correlation coefficient | −0.156 | 0.103 | −0.066 |
| p-value | 0.084 | 0.252 | 0.464 | |
| TgAb (IU/mL) | Correlation coefficient | 0.035 | 0.069 | 0.185 |
| p-value | 0.699 | 0.444 | 0.040 | |
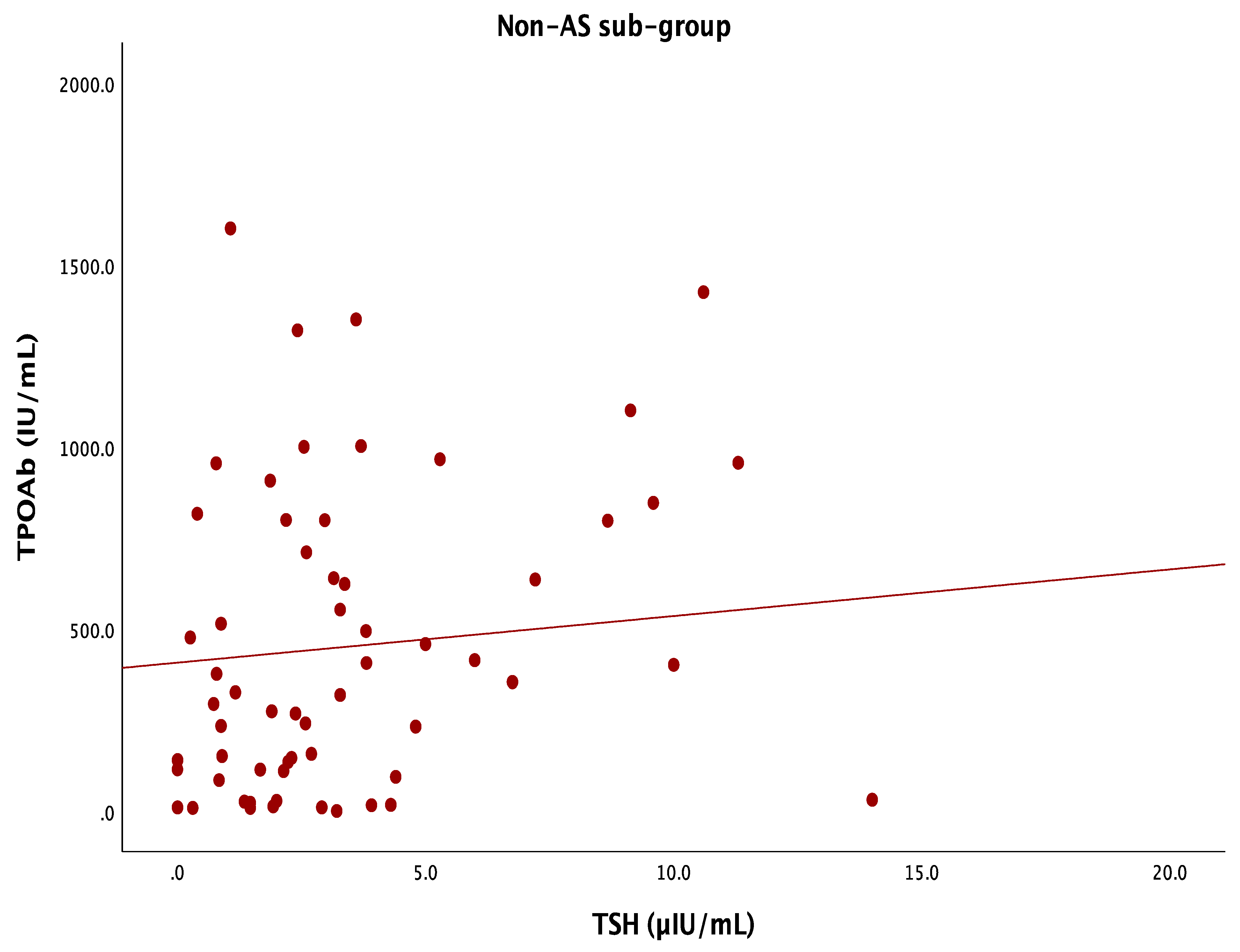
| AS-negative group (N = 61) | ||||
| TSH (µIU/mL) | Correlation coefficient | −0.129 | −0.012 | −0.075 |
| p-value | 0.147 | 0.896 | 0.397 | |
| FT4 (pmol/L) | Correlation coefficient | 0.092 | −0.039 | 0.001 |
| p-value | 0.303 | 0.663 | 0.990 | |
| TPOAb (IU/mL) | Correlation coefficient | −0.009 | 0.096 | −0.014 |
| p-value | 0.921 | 0.273 | 0.876 | |
| TgAb (IU/mL) | Correlation coefficient | 0.022 | 0.105 | 0.012 |
| p-value | 0.808 | 0.234 | 0.891 | |
| Parameter | Correlation Coefficient | p-Value | |
|---|---|---|---|
| Entire cohort (N = 120) | |||
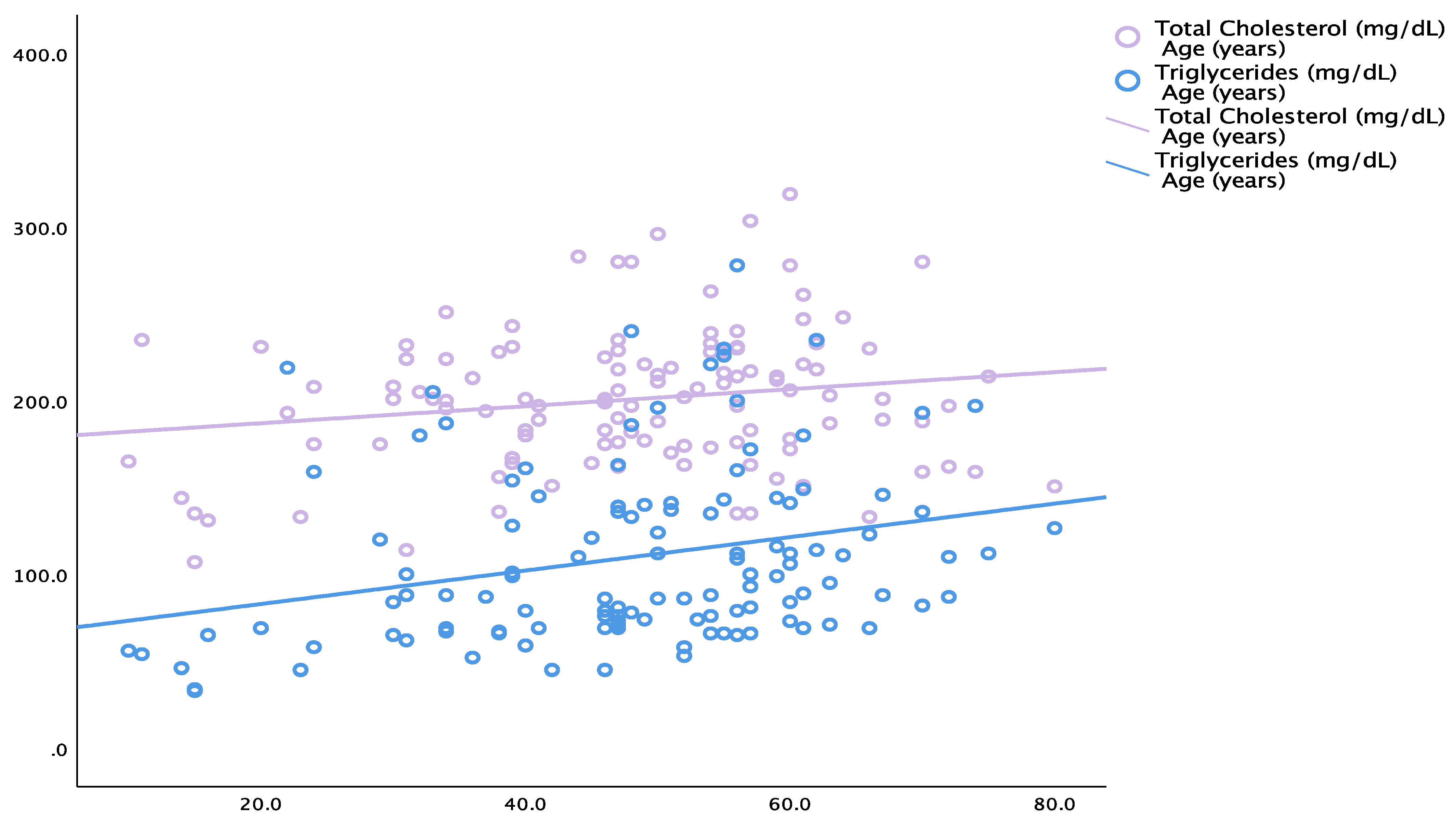
| Total cholesterol | Age | 0.180 | 0.049 |
| Triglycerides | 0.324 | <0.001 | |
| Triglycerides | Age | 0.277 | 0.002 |
| AS group (N = 59) | |||
| Total cholesterol | Age | −0.107 | 0.236 |
| Triglycerides | 0.104 | 0.249 | |
| Triglycerides | Age | 0.125 | 0.169 |
| AS-negative group (N = 61) | |||
| Total cholesterol | Age | 0.246 | 0.006 |
| Triglycerides | 0.319 | <0.001 | |
| Triglycerides | Age | 0.338 | <0.001 |
| Thyroid Ultrasound Trait | Total Studied Population (N = 120) | AS Group (N = 59) | AS-Negative Group (N = 61) | p-Value |
|---|---|---|---|---|
| Homogeneity | ||||
| Homogeneous, number (%) | 8 (6.7) | 3 (5.1) | 5 (8.2) | 0.494 |
| Inhomogeneous, number (%) | 112 (93.3) | 56 (94.9) | 56 (91.8) | |
| Echogenicity | ||||
| Hypoechoic, number (%) | 85 (70.8) | 41 (69.5) | 44 (72.1) | 0.575 |
| Isoechoic, number (%) | 31 (25.8) | 15 (25.4) | 16 (26.2) | |
| Hyperechoic, number (%) | 4 (3.3) | 3 (5.1) | 1 (1.6) | |
| Nodules ≥5 mm diameter | ||||
| Present, number (%) | 25 (20.8) | 15 (25.4) | 10 (16.4) | 0.355 |
| Absent, number (%) | 95 (79.2) | 44 (74.6) | 51 (83.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valea, A.; Costachescu, M.; Stanciu, M.; Nistor, C.; Sima, O.-C.; Carsote, M.; Nistor, T.V.I.; Tanasescu, D.; Popa, F.L.; Ciobica, M.-L. A Real-Life Study in Patients Newly Diagnosed with Autoimmune Hashimoto’s Thyroiditis: Analysis of Asthenia as Admission Complaint. Life 2024, 14, 1380. https://doi.org/10.3390/life14111380
Valea A, Costachescu M, Stanciu M, Nistor C, Sima O-C, Carsote M, Nistor TVI, Tanasescu D, Popa FL, Ciobica M-L. A Real-Life Study in Patients Newly Diagnosed with Autoimmune Hashimoto’s Thyroiditis: Analysis of Asthenia as Admission Complaint. Life. 2024; 14(11):1380. https://doi.org/10.3390/life14111380
Chicago/Turabian StyleValea, Ana, Mihai Costachescu, Mihaela Stanciu, Claudiu Nistor, Oana-Claudia Sima, Mara Carsote, Tiberiu Vasile Ioan Nistor, Denisa Tanasescu, Florina Ligia Popa, and Mihai-Lucian Ciobica. 2024. "A Real-Life Study in Patients Newly Diagnosed with Autoimmune Hashimoto’s Thyroiditis: Analysis of Asthenia as Admission Complaint" Life 14, no. 11: 1380. https://doi.org/10.3390/life14111380
APA StyleValea, A., Costachescu, M., Stanciu, M., Nistor, C., Sima, O.-C., Carsote, M., Nistor, T. V. I., Tanasescu, D., Popa, F. L., & Ciobica, M.-L. (2024). A Real-Life Study in Patients Newly Diagnosed with Autoimmune Hashimoto’s Thyroiditis: Analysis of Asthenia as Admission Complaint. Life, 14(11), 1380. https://doi.org/10.3390/life14111380









