The Effect of Szigetvár Medicinal Water on HaCaT Cells Exposed to Dithranol
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Experimental Media
2.2. XTT Cell Viability Assay
2.3. Experiment Protocol for RT-qPCR and MDA Assay
2.4. Lipid Peroxidation (MDA) Assay
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Statistical Analysis
3. Results
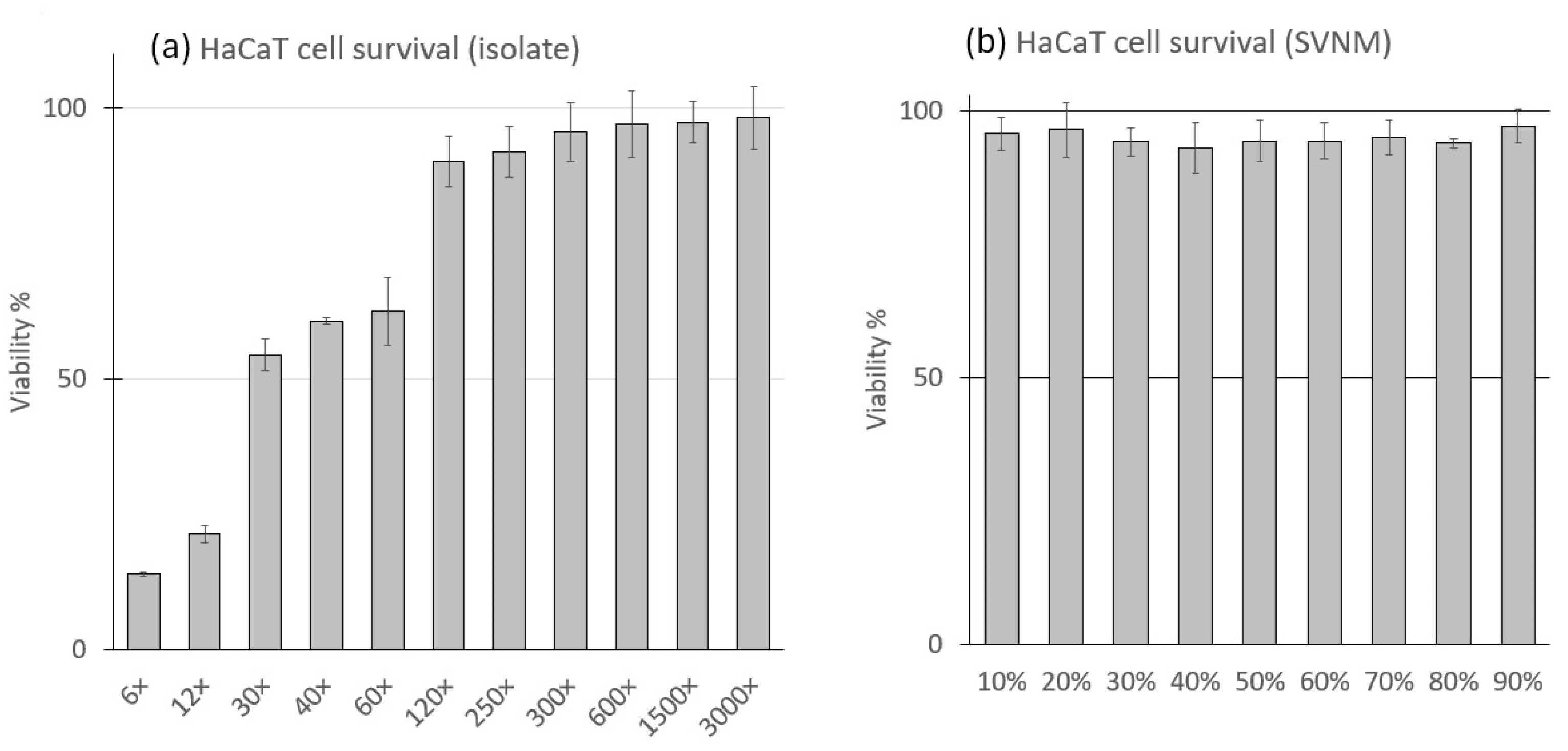
3.1. Cell Viability in SVNM and Organic Isolates
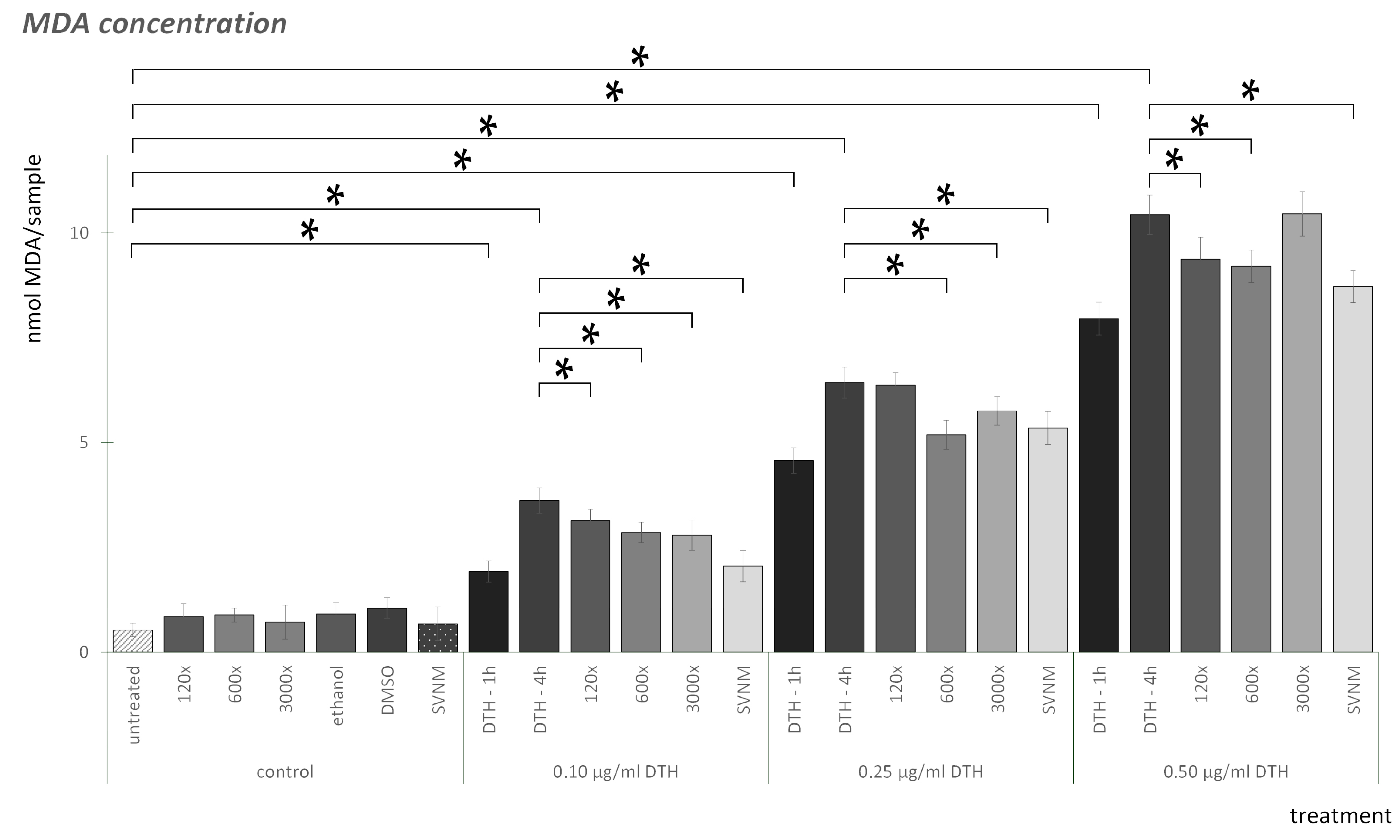
3.2. Lipid Peroxidation (MDA) Assay
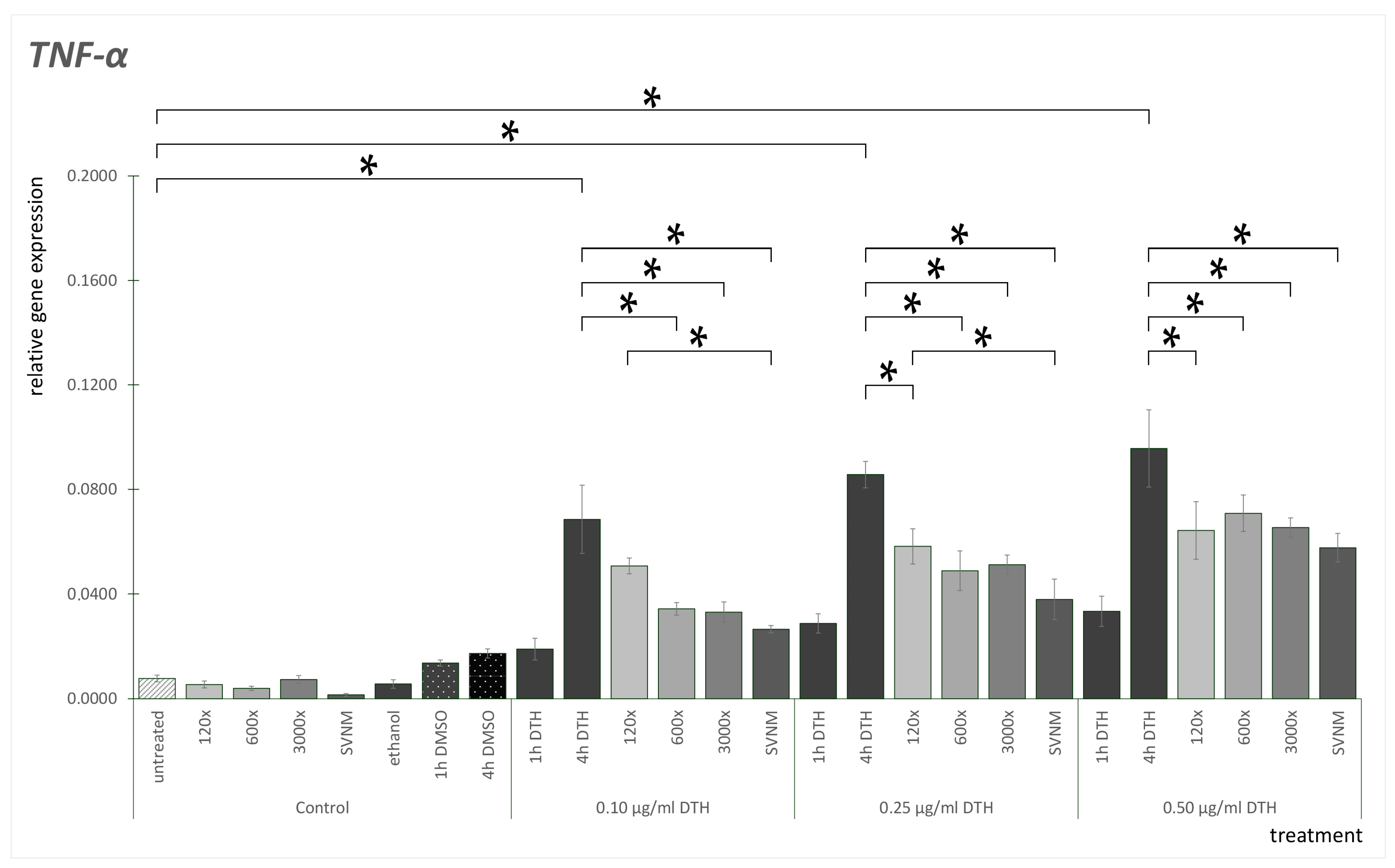
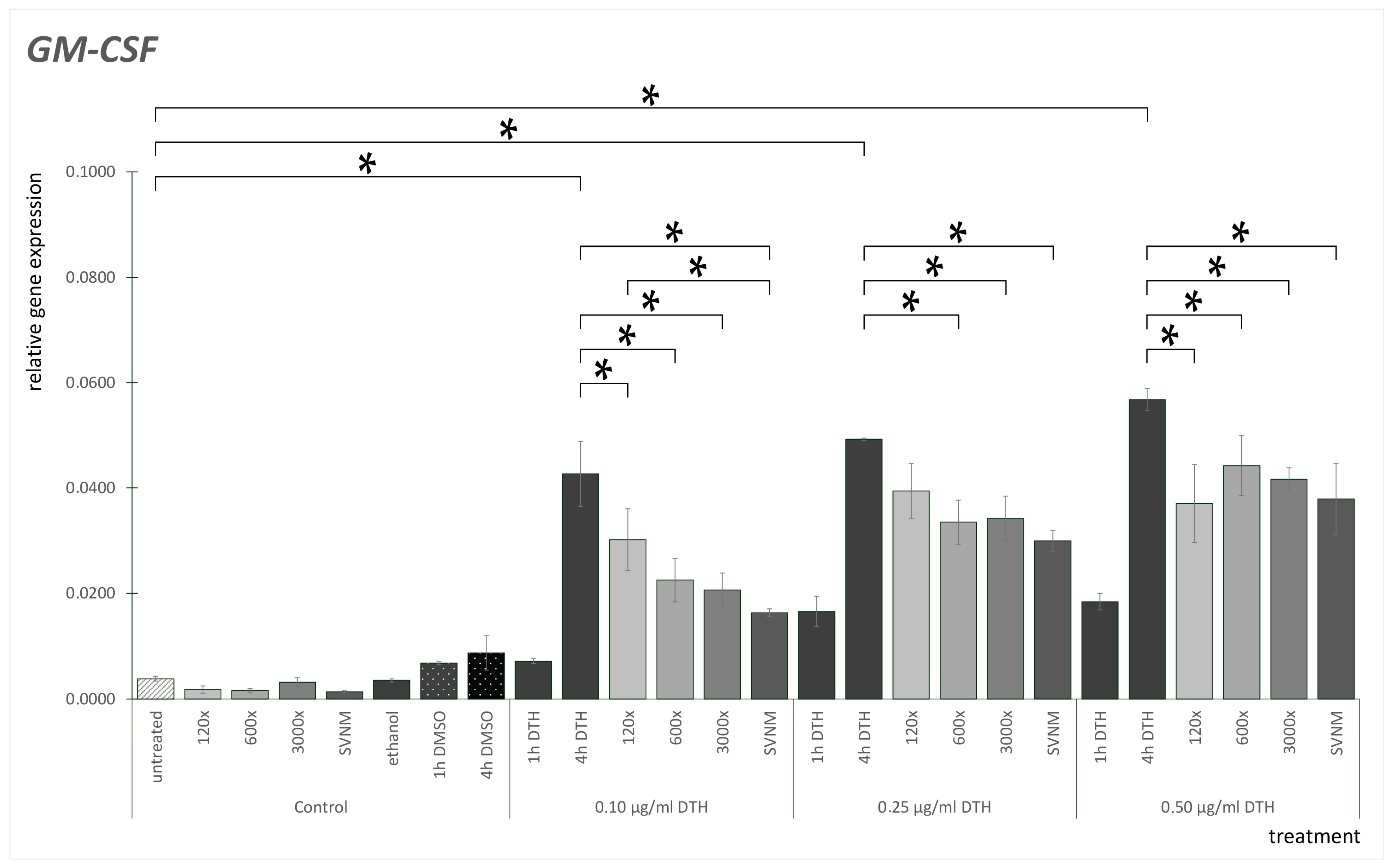
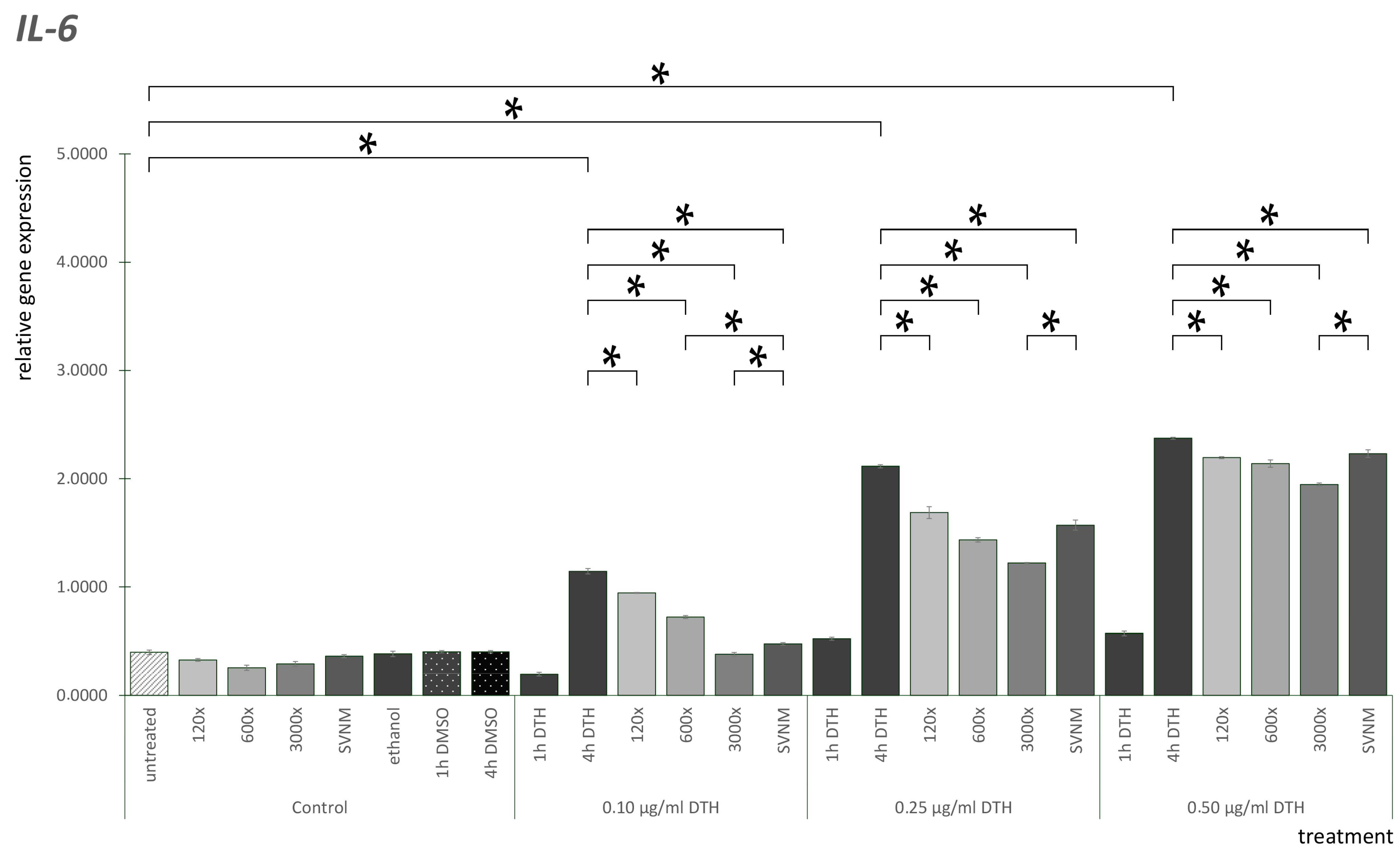
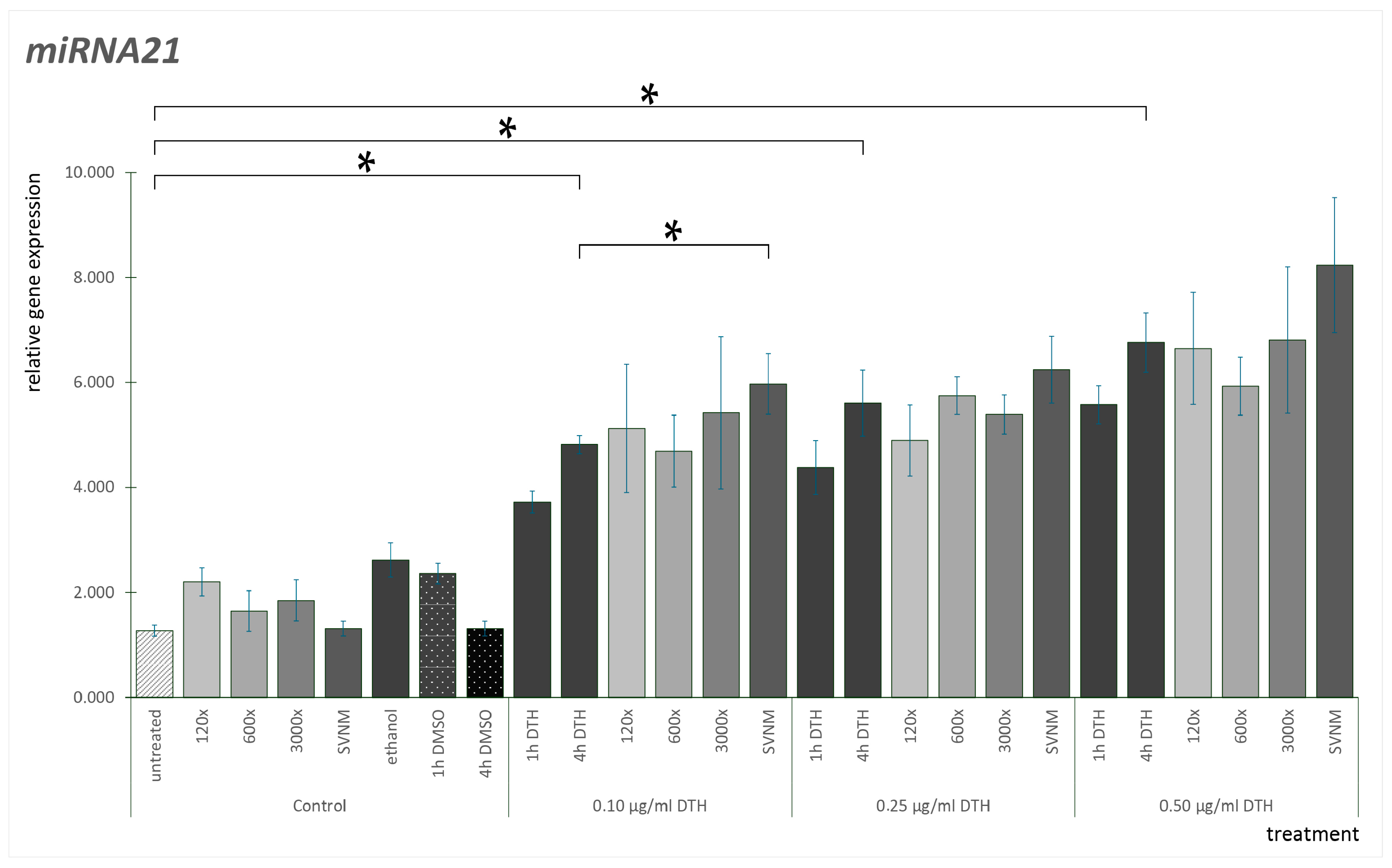
3.3. Relative Gene Expression of Selected Cytokines
4. Discussion
Effect of SVNM and Organic Isolates on the mRNA Expression of Selected Cytokines (IL-6, IL-8, TNF-α, GM-CSF)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lie, E.; Choi, M.; Wang, S.-P.; Eichenfield, L.F. Topical Management of Pediatric Psoriasis: A Review of New Developments and Existing Therapies. Pediatr. Drugs 2024, 26, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Elhabal, S.F.; Abdelaal, N.; Al-Zuhairy, S.A.S.; Mohamed Elrefai, M.F.; Khalifa, M.M.; Khasawneh, M.A.; Elsaid Hamdan, A.M.; Mohie, P.M.; Gad, R.A.; Kabil, S.L.; et al. Revolutionizing Psoriasis Topical Treatment: Enhanced Efficacy Through Ceramide/Phospholipid Composite Cerosomes Co-Delivery of Cyclosporine and Dithranol: In-Vitro, Ex-Vivo, and in-Vivo Studies. Int. J. Nanomed. 2024, 19, 1163–1187. [Google Scholar] [CrossRef] [PubMed]
- Lange, R.W.; Germolec, D.R.; Foley, J.F.; Luster, M.I. Antioxidants attenuate anthralin-induced skin inflammation in BALB/c mice: Role of specific proinflammatory cytokines. J. Leukoc. Biol. 1998, 64, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Kemény, L.; Ruzicka, T.; Braun-Falco, O. Dithranol: A review of the mechanism of action in the treatment of psoriasis vulgaris. Ski. Pharmacol. Physiol. 1990, 3, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Benezeder, T.; Painsi, C.; Patra, V.; Dey, S.; Holcmann, M.; Lange-Asschenfeldt, B.; Sibilia, M.; Wolf, P. Dithranol targets keratinocytes, their crosstalk with neutrophils and inhibits the IL-36 inflammatory loop in psoriasis. eLife 2020, 9, e56991. [Google Scholar] [CrossRef]
- Baliwag, J.; Barnes, D.H.; Johnston, A. Cytokines in psoriasis. Cytokine 2015, 73, 342–350. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nishioka, K. Alteration of the Expression of Bcl-2, Bcl-x, Bax, Fas, and Fas Ligand in the Involved Skin of Psoriasis vulgaris following Topical Anthralin Therapy. Ski. Pharmacol. Physiol. 2003, 16, 50–58. [Google Scholar] [CrossRef]
- Moini Jazani, A.; Ayati, M.H.; Nadiri, A.A.; Nasimi Doost Azgomi, R. Efficacy of hydrotherapy, spa therapy, and balneotherapy for psoriasis and atopic dermatitis: A systematic review. Int. J. Dermatol. 2023, 62, 177–189. [Google Scholar] [CrossRef]
- Péter, I.; Jagicza, A.; Ajtay, Z.; Boncz, I.; Kiss, I.; Szendi, K.; Kustán, P.; Németh, B. Balneotherapy in Psoriasis Rehabilitation. Vivo 2017, 31, 1163–1168. [Google Scholar] [CrossRef]
- Szenczi, A.; Peter, I.; Nusser, N.; Ajtay, Z.; Szendi, K.; Berenyi, K.; Horvath-Szalai, Z.; Szirmay, B.; Sumegi, A.; Hanzel, A.; et al. Is Balneotherapy Protective Against Oxidative Stress? A Pilot Study. Vivo 2023, 37, 858–861. [Google Scholar] [CrossRef]
- Bender, T.; Bálint, G.; Prohászka, Z.; Géher, P.; Tefner, I.K. Evidence-based hydro- and balneotherapy in Hungary—A systematic review and meta-analysis. Int. J. Biometeorol. 2014, 58, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Gutenbrunner, C.; Bender, T.; Cantista, P.; Karagülle, Z. A proposal for a worldwide definition of health resort medicine, balneology, medical hydrology and climatology. Int. J. Biometeorol. 2010, 54, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Bender, T.; Kalics, G.; Árvai, K.; Illés, A.; Kósa, J.P.; Tobiás, B.; Lakatos, P.; Papp, M.; Nemes, K. The Effects of Lakitelek Thermal Water and Tap Water on Skin Microbiome, a Randomized Control Pilot Study. Life 2023, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, A.; Cantarini, L.; Guidelli, G.M.; Galeazzi, M. Mechanisms of action of spa therapies in rheumatic diseases: What scientific evidence is there? Rheumatol. Int. 2011, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Varga, C. Problems with classification of spa waters used in balneology. Health 2010, 2, 1260–1263. [Google Scholar] [CrossRef]
- Baros, D.N.; Gajanin, V.S.; Gajanin, R.B.; Zrnić, B. Comparative analysis of success of psoriasis treatment with standard therapeutic modalities and balneotherapy. Med. Pregl. 2014, 67, 154–160. [Google Scholar] [CrossRef]
- Golušin, Z.; Jovanović, M.; Jeremic Gajinov, B.; Jolić, S. Balneotherapy of Psoriasis. Serbian J. Dermatol. Venerol. 2014, 6, 105–112. [Google Scholar] [CrossRef]
- Fioravanti, A.; Karagülle, M.; Bender, T.; Karagülle, M.Z. Balneotherapy in osteoarthritis: Facts, fiction and gaps in knowledge. Eur. J. Integr. Med. 2017, 9, 148–150. [Google Scholar] [CrossRef]
- Morer, C.; Michan-Doña, A.; Alvarez-Badillo, A.; Zuluaga, P.; Maraver, F. Evaluation of the Feasibility of a Two-Week Course of Aquatic Therapy and Thalassotherapy in a Mild Post-Stroke Population. Int. J. Environ. Res. Public Health 2020, 17, 8163. [Google Scholar] [CrossRef]
- Fekete, J.; Sajgó, C.; Horváth, I.; Kárpáti, Z.; Vetõ, I.; Hetényi, M. Interaction and variation of isotopic age, temperature, organic and inorganic solutes in Hungarian thermal waters. Cent. Eur. Geol. 2009, 52, 269–285. [Google Scholar] [CrossRef]
- Fekete, J.; Sajgó, C.; Kramarics, Á.; Eke, Z.; Kovács, K.; Kárpáti, Z. Aquathermolysis of humic and fulvic acids: Simulation of organic matter maturation in hot thermal waters. Org. Geochem. 2012, 53, 109–118. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Cancho-Grande, B.; Araujo-Nespereira, P.; Cid-Fernández, J.A.; Simal-Gándara, J. Occurrence of soluble organic compounds in thermal waters by ion trap mass detection. Chemosphere 2009, 75, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Szabó, I.; Varga, C. Finding possible pharmacological effects of identified organic compounds in medicinal waters (BTEX and phenolic compounds). Int. J. Biometeorol. 2020, 64, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Kompanichenko, V.N.; Poturay, V.A. Organic Matter in Hydrotherms of the Pauzhetka Field: Composition and Comparative Analysis with Other Sites. Geochem. Int. 2024, 62, 832–843. [Google Scholar] [CrossRef]
- Varga, C. Balneoprevention: New approaches. Int. J. Biometeorol. 2012, 56, 195–197. [Google Scholar] [CrossRef]
- van Rensburg, C.E. The Antiinflammatory Properties of Humic Substances: A Mini Review. Phytother. Res. 2015, 29, 791–795. [Google Scholar] [CrossRef]
- Zykova, M.V.; Schepetkin, I.A.; Belousov, M.V.; Krivoshchekov, S.V.; Logvinova, L.A.; Bratishko, K.A.; Yusubov, M.S.; Romanenko, S.V.; Quinn, M.T. Physicochemical Characterization and Antioxidant Activity of Humic Acids Isolated from Peat of Various Origins. Molecules 2018, 23, 753. [Google Scholar] [CrossRef]
- Gerencsér, G.; Szabó, I.; Szendi, K.; Hanzel, A.; Raposa, B.; Gyöngyi, Z.; Varga, C. Effects of medicinal waters on the UV-sensitivity of human keratinocytes—A comparative pilot study. Int. J. Biometeorol. 2019, 63, 1417–1423. [Google Scholar] [CrossRef]
- Hanzel, A.; Berényi, K.; Horváth, K.; Szendi, K.; Németh, B.; Varga, C. Evidence for the therapeutic effect of the organic content in Szigetvár thermal water on osteoarthritis: A double-blind, randomized, controlled clinical trial. Int. J. Biometeorol. 2019, 63, 449–458. [Google Scholar] [CrossRef]
- Szendi, K.; Gerencsér, G.; Murányi, E.; Varga, C. Mutagenic activity of peloids in the Salmonella Ames test. Appl. Clay Sci. 2012, 55, 70–74. [Google Scholar] [CrossRef]
- Varga, C.; László, M.; Gerencsér, G.; Gyöngyi, Z.; Szendi, K. Natural UV-protective organic matter in thermal water. J. Photochem. Photobiol. B 2015, 144, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, I.J.; Zewudie, G.T. Antioxidant Effect and Acute Oral Toxicity of Hot Springs. Comput. Intell. Neurosci. 2022, 2022, 4200824. [Google Scholar] [CrossRef] [PubMed]
- Hollywood, K.A.; Winder, C.L.; Dunn, W.B.; Xu, Y.; Broadhurst, D.; Griffiths, C.E.; Goodacre, R. Exploring the mode of action of dithranol therapy for psoriasis: A metabolomic analysis using HaCaT cells. Mol. Biosyst. 2015, 11, 2198–2209. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jangir, B.L.; Rao, R. A new perspective for psoriasis: Dithranol nanosponge loaded hydrogels. Appl. Surf. Sci. Adv. 2022, 12, 100347. [Google Scholar] [CrossRef]
- Lange, R.W.; Hayden, P.J.; Chignell, C.F.; Luster, M.I. Anthralin stimulates keratinocyte-derived proinflammatory cytokines via generation of reactive oxygen species. Inflamm. Res. 1998, 47, 174–181. [Google Scholar] [CrossRef]
- Wójciak, M.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z.; Ziemlewska, A.; Furman-Toczek, D.; Szczepanek, D.; Sowa, I. In Vitro Evaluation of Anti-Inflammatory and Protective Potential of an Extract from Cornus mas L. Fruit against H2O2-Induced Oxidative Stress in Human Skin Keratinocytes and Fibroblasts. Int. J. Mol. Sci. 2022, 23, 13755. [Google Scholar] [CrossRef]
- Lee, H.P.; Choi, Y.J.; Cho, K.A.; Woo, S.Y.; Yun, S.T.; Lee, J.T.; Kim, H.J.; Lee, K.H.; Kim, J.W. Effect of Spa Spring Water on Cytokine Expression in Human Keratinocyte HaCaT Cells and on Differentiation of CD4(+) T Cells. Ann. Dermatol. 2012, 24, 324–336. [Google Scholar] [CrossRef]
- Abdallah, F.; Henriet, E.; Suet, A.; Arar, A.; Clemençon, R.; Malinge, J.M.; Lecellier, G.; Baril, P.; Pichon, C. miR-21-3p/IL-22 Axes Are Major Drivers of Psoriasis Pathogenesis by Modulating Keratinocytes Proliferation-Survival Balance and Inflammatory Response. Cells 2021, 10, 2547. [Google Scholar] [CrossRef]
- Ebrahimi, S.O.; Reiisi, S.; Shareef, S. miRNAs, oxidative stress, and cancer: A comprehensive and updated review. J. Cell. Physiol. 2020, 235, 8812–8825. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Guo, S.L.; Fan, K.J.; Li, J.; Wang, Y.L.; Teng, Y.; Yang, X. miR-21 promotes keratinocyte migration and re-epithelialization during wound healing. Int. J. Biol. Sci. 2011, 7, 685–690. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Portalès, P.; Ariès, M.F.; Licu, D.; Pinton, J.; Hernandez-Pion, C.; Gall, Y.; Dupuy, P.; Charveron, M.; Clot, J. Immunomodulation induced by Avène spring water on Th1- and Th2-dependent cytokine production in healthy subjects and atopic dermatitis patients. Ski. Pharmacol. Physiol. 2001, 14, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Baz, K.; Cimen, M.Y.; Kokturk, A.; Yazici, A.C.; Eskandari, G.; Ikizoglu, G.; Api, H.; Atik, U. Oxidant/antioxidant status in patients with psoriasis. Yonsei Med. J. 2003, 44, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Wiegrebe, W.; Younes, M. Formation of active oxygen species by dithranol, III. Dithranol, active oxygen species and lipid peroxidation in vivo. Arch. Pharm. 1987, 320, 59–66. [Google Scholar] [CrossRef]
- Braga, P.C.; Sambataro, G.; Dal Sasso, M.; Culici, M.; Alfieri, M.; Nappi, G. Antioxidant effect of sulphurous thermal water on human neutrophil bursts: Chemiluminescence evaluation. Respiration 2008, 75, 193–201. [Google Scholar] [CrossRef]
- Joly, F.; Branka, J.-E.; Lefeuvre, L. Thermal Water from Uriage-les-Bains Exerts DNA Protection, Induction of Catalase Activity and Claudin-6 Expression on UV Irradiated Human Skin in Addition to Its Own Antioxidant Properties. J. Cosmet. Dermatol. Sci. Appl. 2014, 4, 99–106. [Google Scholar] [CrossRef][Green Version]
- Prandelli, C.; Parola, C.; Buizza, L.; Delbarba, A.; Marziano, M.; Salvi, V.; Zacchi, V.; Memo, M.; Sozzani, S.; Calza, S.; et al. Sulphurous thermal water increases the release of the anti-inflammatory cytokine IL-10 and modulates antioxidant enzyme activity. Int. J. Immunopathol. Pharmacol. 2013, 26, 633–646. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Keet, L.; Magcwebeba, T.; Abel, S.; Louw, A.; Gelderblom, W.; Lilly, M. Modulation of UVB-induced oxidative stress and inflammation in skin keratinocytes (HaCaT) utilising unfermented rooibos and honeybush aqueous extracts. J. Photochem. Photobiol. 2024, 22, 100242. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Zhou, P.L.; Yin, X.Y.; Wang, A.X.; Wang, D.H.; Yang, Y.; Liu, Q. Circulating inflammatory cytokines and psoriasis risk: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0293327. [Google Scholar] [CrossRef] [PubMed]
- Zöller, N.; Valesky, E.; Hofmann, M.; Bereiter-Hahn, J.; Bernd, A.; Kaufmann, R.; Meissner, M.; Kippenberger, S. Impact of Different Spa Waters on Inflammation Parameters in Human Keratinocyte HaCaT Cells. Ann. Dermatol. 2015, 27, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Mourelle, M.L.; Segura, J.; Ayats, J.; Vitale, M.; López, A. Potential Benefits of a Cosmetic Ingredient Combining Thermal Spring Water and Diatom Algae Extract. Cosmetics 2024, 11, 62. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Balato, A.; Enerbäck, C.; Sabat, R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet 2021, 397, 754–766. [Google Scholar] [CrossRef]
- Boutet, M.-A.; Nerviani, A.; Gallo Afflitto, G.; Pitzalis, C. Role of the IL-23/IL-17 Axis in Psoriasis and Psoriatic Arthritis: The Clinical Importance of Its Divergence in Skin and Joints. Int. J. Mol. Sci. 2018, 19, 530. [Google Scholar] [CrossRef]
- Potestio, L.; Martora, F.; Lauletta, G.; Vallone, Y.; Battista, T.; Megna, M. The Role of Interleukin 23/17 Axis in Psoriasis Management: A Comprehensive Review of Clinical Trials. Clin. Cosmet. Investig. Dermatol. 2024, 17, 829–842. [Google Scholar] [CrossRef]
- Menter, A.; Krueger, G.G.; Paek, S.Y.; Kivelevitch, D.; Adamopoulos, I.E.; Langley, R.G. Interleukin-17 and Interleukin-23: A Narrative Review of Mechanisms of Action in Psoriasis and Associated Comorbidities. Dermatol. Ther. 2021, 11, 385–400. [Google Scholar] [CrossRef]
- Hawkes, J.E.; Yan, B.Y.; Chan, T.C.; Krueger, J.G. Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis. J. Immunol. 2018, 201, 1605–1613. [Google Scholar] [CrossRef]
- Lee, Y.B.; Lee, J.Y.; Lee, H.J.; Yun, S.T.; Lee, J.T.; Kim, H.J.; Yu, D.S.; Woo, S.Y.; Kim, J.W. Immunomodulatory effects of balneotherapy with hae-un-dae thermal water on imiquimod-induced psoriasis-like murine model. Ann. Dermatol. 2014, 26, 221–230. [Google Scholar] [CrossRef]
- Eliasse, Y.; Galliano, M.F.; Redoules, D.; Espinosa, E. Effect of thermal spring water on human dendritic cell inflammatory response. J. Inflamm. Res. 2019, 12, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Jung, B.Y.; Lee, S.Y.; Yu, D.S.; Woo, S.Y.; Yun, S.T.; Lee, J.T.; Kim, J.W.; Lee, Y.B. NaHCO(3)- and NaCl-Type Hot Springs Enhance the Secretion of Inflammatory Cytokine Induced by Polyinosinic-Polycytidylic Acid in HaCaT Cells. Ann. Dermatol. 2021, 33, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Ratti, M.; Lampis, A.; Ghidini, M.; Salati, M.; Mirchev, M.B.; Valeri, N.; Hahne, J.C. MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target. Oncol. 2020, 15, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, A.; Ghosh, A. Oxidative Stress Associated Non-coding RNAs in Pathogenesis of Urologic Cancers; Prognostic and Therapeutic Importance. In Handbook of Oxidative Stress in Cancer: Therapeutic Aspects; Chakraborti, S., Ed.; Springer: Singapore, 2021; pp. 1–19. [Google Scholar]
- Jiang, X.; Shi, R.; Ma, R.; Tang, X.; Gong, Y.; Yu, Z.; Shi, Y. The role of microRNA in psoriasis: A review. Exp. Dermatol. 2023, 32, 1598–1612. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Becker Buscaglia, L.E.; Barker, J.R.; Li, Y. MicroRNAs in NF-kappaB signaling. J. Mol. Cell Biol. 2011, 3, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Li, L.; Zhao, J.; Sun, Y.; Yang, H. Effects of Trimetazidine on PDCD4/NF-κB/TNF-α Pathway in Coronary Microembolization. Cell. Physiol. Biochem. 2017, 42, 753–760. [Google Scholar] [CrossRef]
- Xie, J.; Wu, W.; Zheng, L.; Lin, X.; Tai, Y.; Wang, Y.; Wang, L. Roles of MicroRNA-21 in Skin Wound Healing: A Comprehensive Review. Front. Pharmacol. 2022, 13, 828627. [Google Scholar] [CrossRef]


| Dilution | DMEM Added to 100 μL of Isolate (mL) |
|---|---|
| 6× | 0.5 |
| 12× | 1.1 |
| 30× | 2.4 |
| 40× | 3.9 |
| 60× | 5.9 |
| 120× | 11.9 |
| 250× | 24.9 |
| 300× | 29.9 |
| 600× | 59.9 |
| 1500× | 149.9 |
| 3000× | 299.9 |
| Treatment | Controls (3 h) | |
|---|---|---|
| #1 | 120× | |
| #2 | 600× | |
| #3 | 3000× | |
| #4 | SVNM | |
| #5 | vehicle controls (ethanol–3 concentrations) | |
| #6 | vehicle controls (DMSO–3 concentrations) | |
| #7 | negative control | |
| dithranol treatment (µg/mL−1 h) | post dithranol treatment (3 h) | |
| #8 | 0.1 | - |
| #9 | 0.1 | DMEM |
| #10 | 0.1 | 120× |
| #11 | 0.1 | 600× |
| #12 | 0.1 | 3000× |
| #13 | 0.1 | SVNM |
| #14 | 0.25 | - |
| #15 | 0.25 | DMEM |
| #16 | 0.25 | 120× |
| #17 | 0.25 | 600× |
| #18 | 0.25 | 3000× |
| #19 | 0.25 | SVNM |
| #20 | 0.5 | - |
| #21 | 0.5 | DMEM |
| #22 | 0.5 | 120× |
| #23 | 0.5 | 6000× |
| #24 | 0.5 | 3000× |
| #25 | 0.5 | SVNM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, I.; Szenczi, Á.; Zand, A.; Varjas, T.; Varga, C. The Effect of Szigetvár Medicinal Water on HaCaT Cells Exposed to Dithranol. Life 2024, 14, 1318. https://doi.org/10.3390/life14101318
Szabó I, Szenczi Á, Zand A, Varjas T, Varga C. The Effect of Szigetvár Medicinal Water on HaCaT Cells Exposed to Dithranol. Life. 2024; 14(10):1318. https://doi.org/10.3390/life14101318
Chicago/Turabian StyleSzabó, István, Ágnes Szenczi, Afshin Zand, Tímea Varjas, and Csaba Varga. 2024. "The Effect of Szigetvár Medicinal Water on HaCaT Cells Exposed to Dithranol" Life 14, no. 10: 1318. https://doi.org/10.3390/life14101318
APA StyleSzabó, I., Szenczi, Á., Zand, A., Varjas, T., & Varga, C. (2024). The Effect of Szigetvár Medicinal Water on HaCaT Cells Exposed to Dithranol. Life, 14(10), 1318. https://doi.org/10.3390/life14101318







