State-of-the-Art in Skin Fluorescent Photography for Cosmetic and Skincare Research: From Molecular Spectra to AI Image Analysis
Abstract
1. Introduction
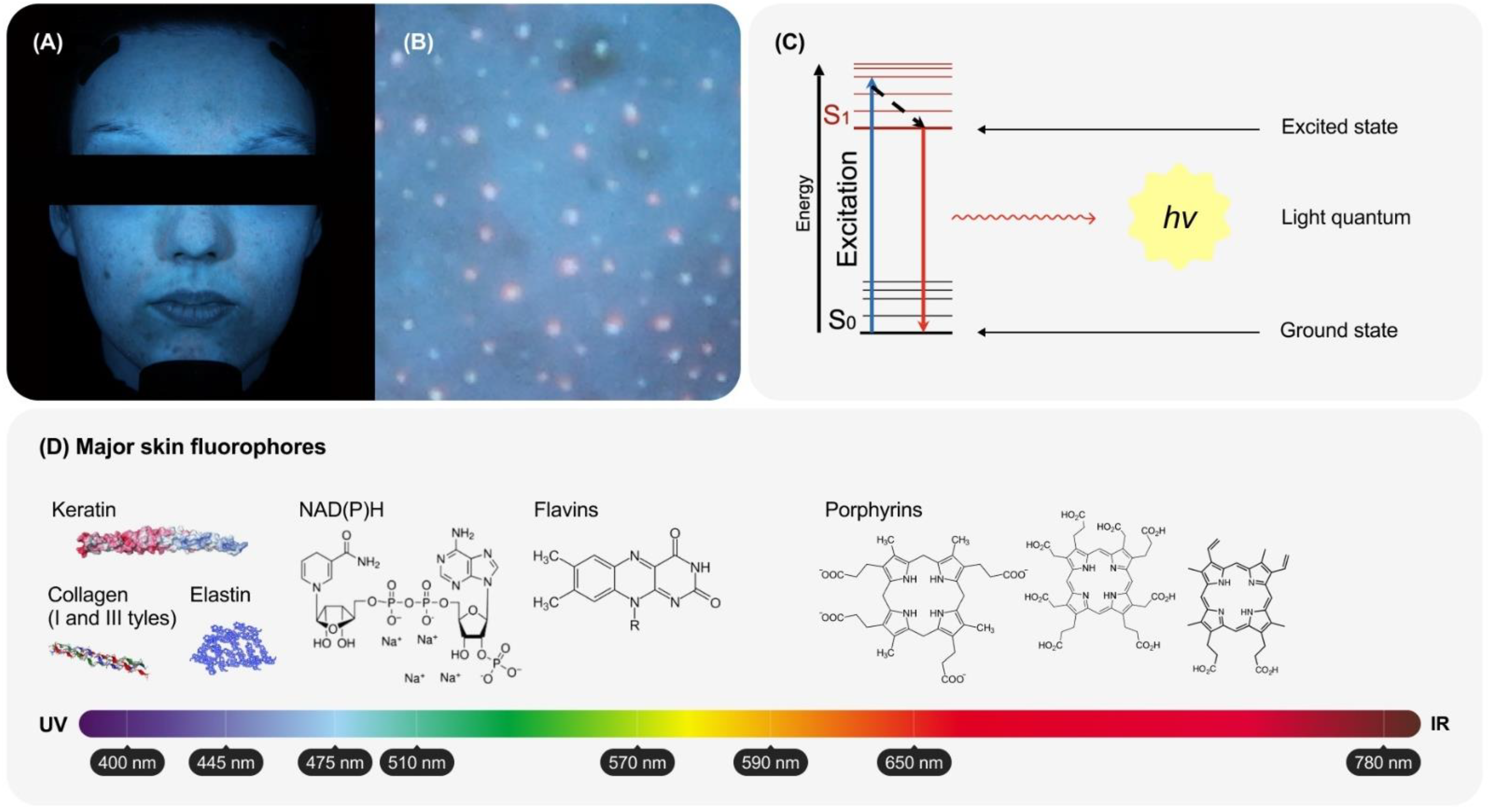
2. Biophysical Basis of Skin Fluorescence
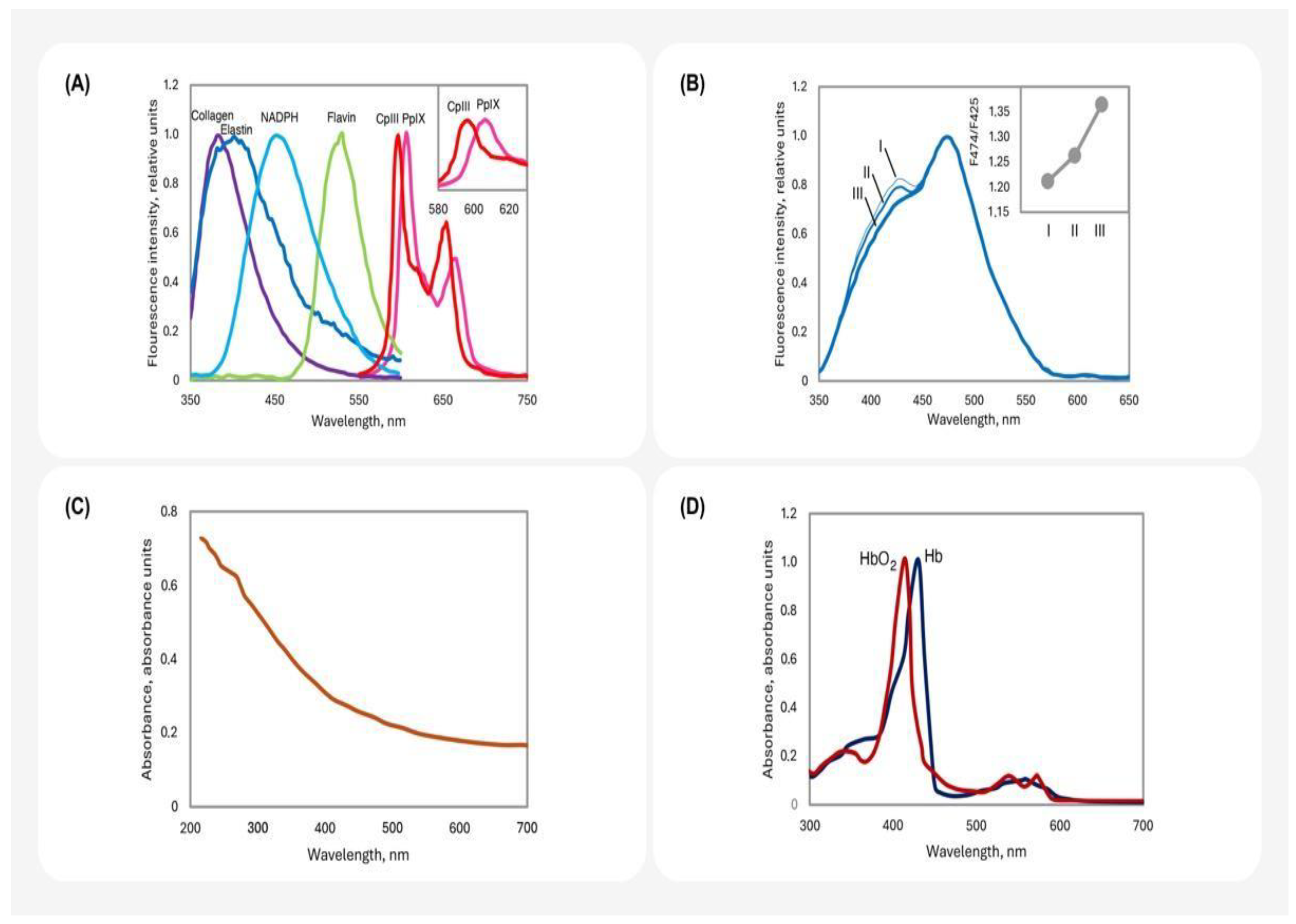
2.1. The Main Sources of Fluorescence in the Skin
2.2. The Effects of Light Absorbance and Refraction on Skin Fluorescence
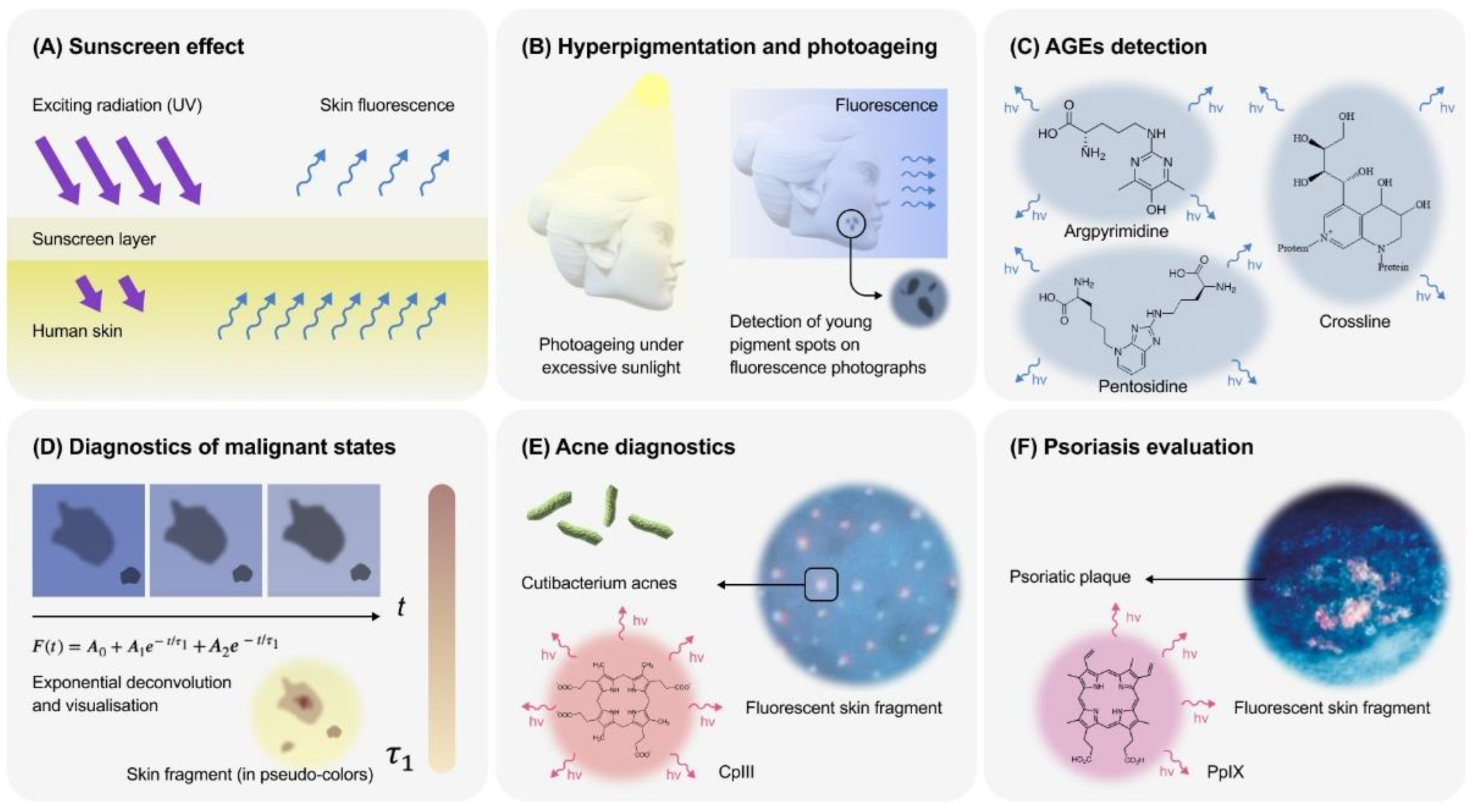
3. The Main Applications of Skin Fluorescence
3.1. Assessment of Optical Screening
3.2. Hyperpigmentation and Photoageing
3.3. Age, AGEs and Skin Fluorescence
3.4. Skin’s Malignant States
3.5. Follicular Fluorescence and Acne
3.6. Psoriasis
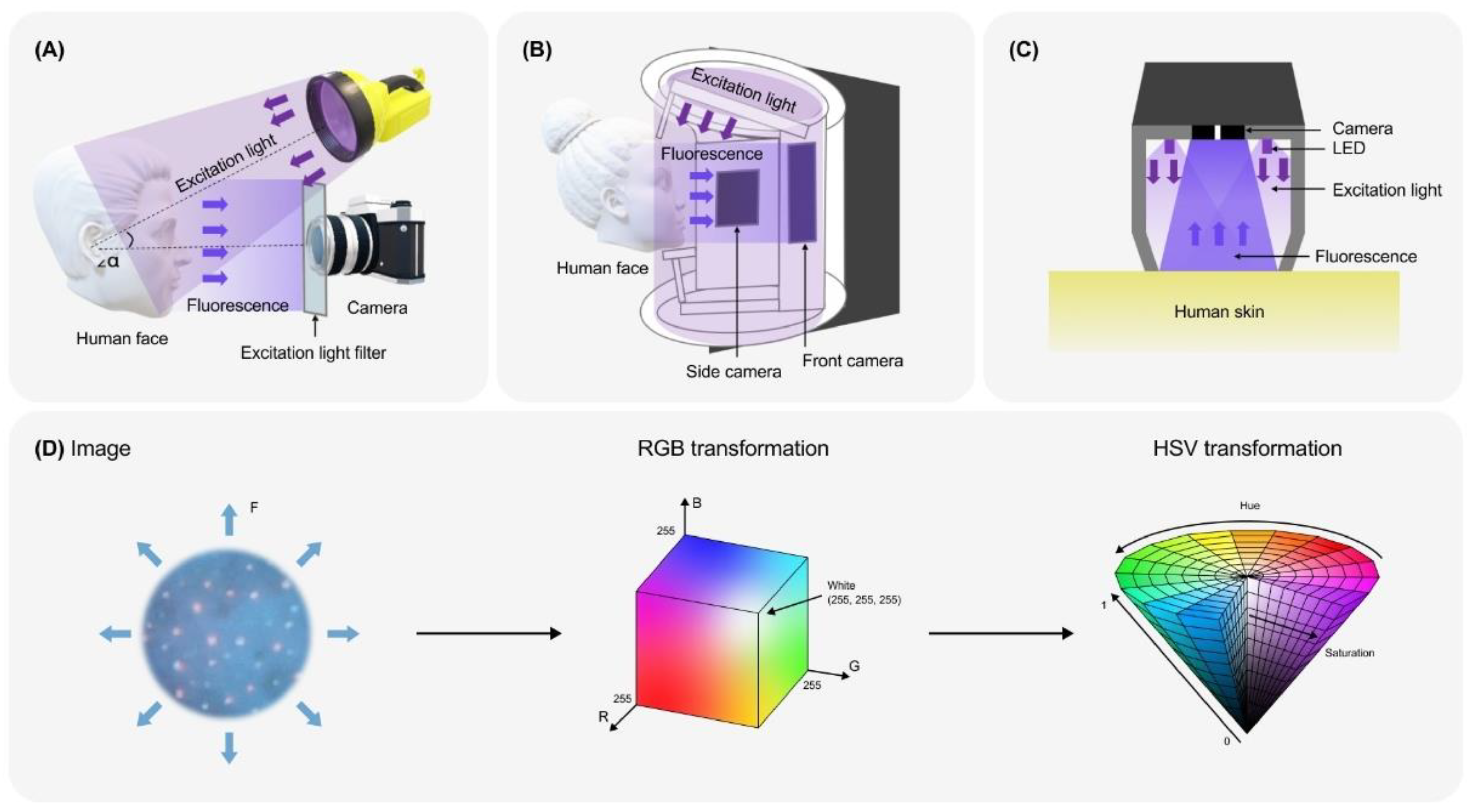
4. Devices and Registration Principles
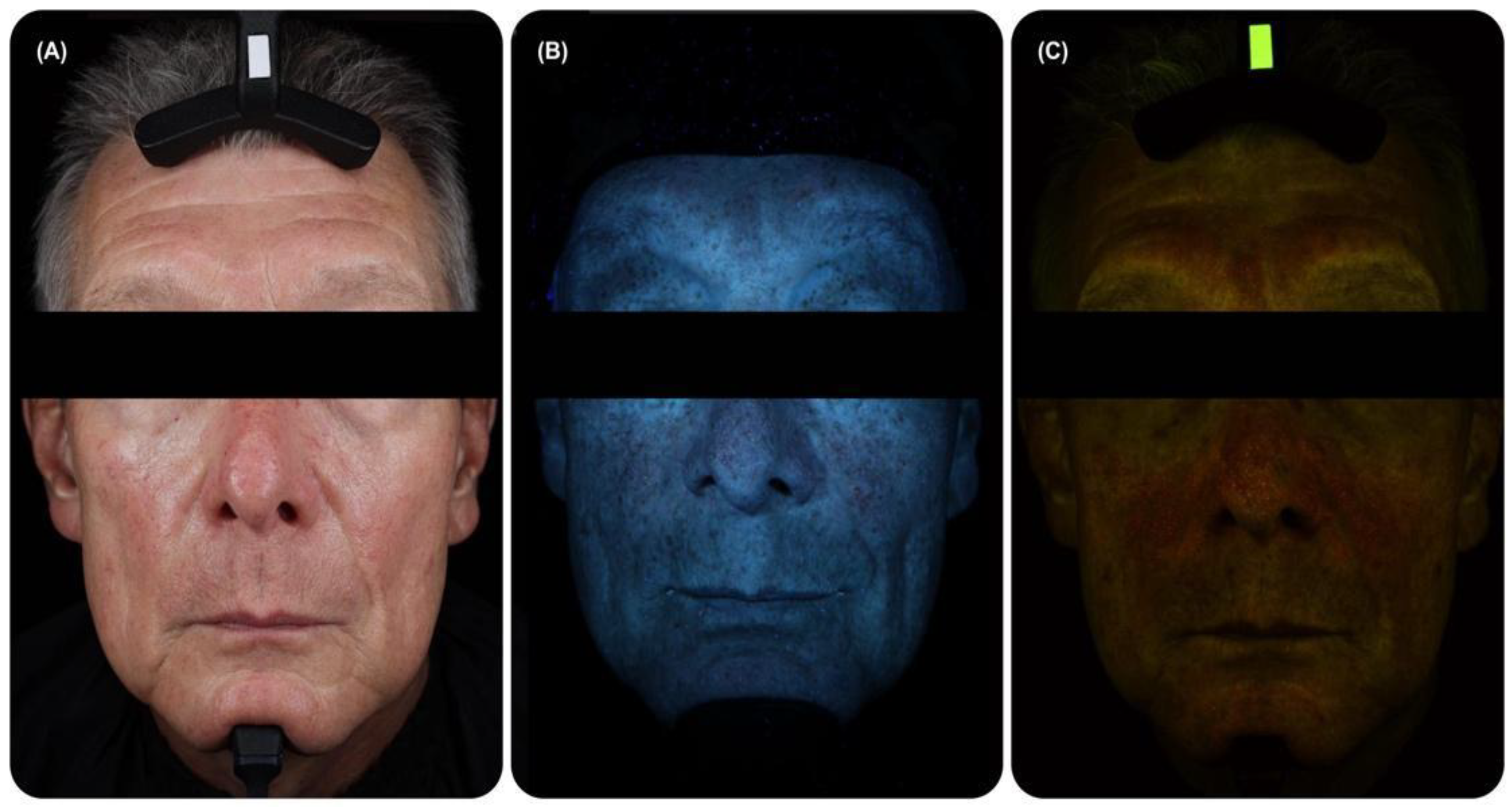
4.1. General Principles of FP Registration
4.2. Standardised Registration Systems
4.3. Towards the IoT Technologies for FP Registration
5. Data Analysis with Traditional Computer Vision Approaches
6. Analysis of Skin Fluorescence Images by AI Algorithms
6.1. Possibilities of the Use AI for the Analysis of FP
6.2. Possible Legal and Ethical Limitations of the Use of AI in Skin Fluorescent Imaging
7. FP as an Alternative or Complement to Bright-Field Imaging
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szepetiuk, G.; Piérard-Franchimont, C.; Quatresooz, P.; Piérard, G.E. Physico-biological foundation of skin fluorescence—Review. Pathol. Biol. 2012, 60, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Sinichkin, Y.P.; Utz, S.R.; Mavliutov, A.H.; Pilipenko, H.A. In vivo fluorescence spectroscopy of the human skin: Experiments and models. J. Biomed. Opt. 1998, 3, 201–211. [Google Scholar] [CrossRef]
- Zeng, H.; MacAulay, C.; Palcic, B.; McLean, D.I. Spectroscopic and microscopic characteristics of human skin autofluorescence emission. Photochem. Photobiol. 1995, 61, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Drakaki, E.; Borisova, E.; Makropoulou, M.; Avramov, L.; Serafetinides, A.A.; Angelov, I. Laser induced autofluorescence studies of animal skin used in modeling of human cutaneous tissue spectroscopic measurements. Skin Res. Technol. 2007, 13, 350–359. [Google Scholar] [CrossRef]
- Giovannacci, I.; Magnoni, C.; Vescovi, P.; Painelli, A.; Tarentini, E.; Meleti, M. Which are the main fluorophores in skin and oral mucosa? A review with emphasis on clinical applications of tissue autofluorescence. Arch. Oral Biol. 2019, 105, 89–98. [Google Scholar] [CrossRef]
- Koenig, K.; Schneckenburger, H. Laser-induced autofluorescence for medical diagnosis. J. Fluoresc. 1994, 4, 17–40. [Google Scholar] [CrossRef]
- König, K.; Rueck, A.C.; Schneckenburger, H. Fluorescence detection and photodynamic activity of endogenous protoporphyrin in human skin. Opt. Eng. 1992, 31, 1470–1475. [Google Scholar] [CrossRef]
- Ortiz, A.E.; Ahluwalia, J.; Anderson, R.R.; Franco, W.; Jiang, S.I.B. Autofluorescence Excitation Imaging of Nonmelanoma Skin Cancer for Margin Assessment Before Mohs Micrographic Surgery: A Pilot Study. Dermatol. Surg. 2024, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Spigulis, J. Biophotonic Technologies for Non-Invasive Assessment of Skin Condition and Blood Microcirculation. Latv. J. Phys. Tech. Sci. 2012, 49, 63–80. [Google Scholar]
- Spigulis, J.; Lihachev, A.; Gailite, L.; Erts, R. Novel Laser Technologies for Human Skin In-Vivo Assessment. In Proceedings of the Advanced Laser Technologies 2007, Siofok, Hungary, 5 June 2008; Volume 7022, pp. 214–219. [Google Scholar]
- Spigulis, J.; Lihachev, A.; Erts, R. Imaging of Laser-Excited Tissue Autofluorescence Bleaching Rates. Appl. Opt. 2009, 48, D163–D168. [Google Scholar] [CrossRef]
- Lihachev, A.; Rozniece, K.; Lesins, J.; Spigulis, J. Photobleaching Measurements of Pigmented and Vascular Skin Lesions: Results of a Clinical Trial. In Proceedings of the European Conference on Biomedical Optics, Munich, Germany, 22–27 May 2011; p. 80872F. [Google Scholar]
- Varikasuvu, S.R.; Varshney, S.; Sulekar, H. Skin Autofluorescence as a Novel and Noninvasive Technology for Advanced Glycation End Products in Diabetic Foot Ulcers: A Systematic Review and Meta-Analysis. Adv. Skin Wound Care 2021, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.N.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Simple Non-Invasive Assessment of Advanced Glycation Endproduct Accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef]
- van Waateringe, R.P.; Slagter, S.N.; van Beek, A.P.; van der Klauw, M.M.; van Vliet-Ostaptchouk, J.V.; Graaff, R.; Paterson, A.D.; Lutgers, H.L.; Wolffenbuttel, B.H. Skin Autofluorescence, a Non-Invasive Biomarker for Advanced Glycation End Products, Is Associated with the Metabolic Syndrome and Its Individual Components. Diabetol. Metabol. Syndr. 2017, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- De Vos, L.C.; Noordzij, M.J.; Mulder, D.J.; Smit, A.J.; Lutgers, H.L.; Dullaart, R.P.; Kamphuisen, P.W.; Zeebregts, C.J.; Lefrandt, J.D. Skin Autofluorescence as a Measure of Advanced Glycation End Products Deposition Is Elevated in Peripheral Artery Disease. Arter. Thromb. Vasc. Biol. 2013, 33, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, I.M.; van de Zande, S.C.; Westra, J.; Zwerver, J.; Smit, A.J.; Mulder, D.J. The AGE Reader: A Non-Invasive Method to Assess Long-Term Tissue Damage. Methods 2022, 203, 533–541. [Google Scholar] [CrossRef]
- Hull, E.L.; Ediger, M.N.; Unione, A.H.T.; Deemer, E.K.; Stroman, M.L.; Baynes, J.W. Noninvasive, Optical Detection of Diabetes: Model Studies with Porcine Skin. Opt. Express 2004, 12, 4496–4510. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R.; Sun, W.; Gao, X.; Strauch, C.; Lachin, J.M.; Cleary, P.A.; Genuth, S.; The DCCT/EDIC Research Group; Monnier, V.M. Skin Collagen Fluorophore LW-1 Versus Skin Fluorescence as Markers for the Long-term Progression of Subclinical Macrovascular Disease in Type 1 Diabetes. Cardiovasc. Diabetol. 2016, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- den Dekker, M.A.M.; Zwiers, M.; van den Heuvel, E.R.; de Vos, L.C.; Smit, A.J.; Zeebregts, C.J.; Oudkerk, M.; Vliegenthart, R.; Lefrandt, J.D.; Mulder, D.J. Skin Autofluorescence, a Non-invasive Marker for AGE Accumulation, Is Associated with the Degree of Atherosclerosis. PLoS ONE 2013, 8, e83084. [Google Scholar] [CrossRef] [PubMed]
- Bommer, S. Hautuntersuchungen im gefilterten Quarzlicht. Klin. Wochenschr. 1927, 6, 1142–1144. [Google Scholar] [CrossRef]
- Kollias, N.; Zonios, G.; Stamatas, G.N. Fluorescence spectroscopy of skin. Vib. Spectrosc. 2002, 28, 17–23. [Google Scholar] [CrossRef]
- Kollias, N.; Stamatas, G.N. Optical Non-invasive Approaches to Diagnosis of Skin Diseases. J. Investig. Dermatol. Symp. Proc. 2002, 7, 64–75. [Google Scholar] [CrossRef]
- Stamatas, G.N.; Kollias, N. Modern Technology for Imaging and Evaluation of Acne Lesions. In Pathogenesis and Treatment of Acne and Rosacea; Zouboulis, C., Katsambas, A., Kligman, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Chapter 45; pp. 331–340. [Google Scholar]
- Aspres, N.; Egerton, I.B.; Lim, A.C.; Shumack, S.P. Imaging the skin. Aust. J. Dermatol. 2003, 44, 19–27. [Google Scholar] [CrossRef]
- Galkina, E.M.; Utz, S.R. Fluorescent diagnostics in dermatology (review). Saratov J. Med. Sci. Res. 2013, 9, 566–572. [Google Scholar]
- Datta, R.; Heaster, T.M.; Heaster, T.M.; Sharick, J.T.; Gillette, A.A.; Gillette, A.A.; Skala, M.C.; Skala, M.C. Fluorescence Lifetime Imaging Microscopy: Fundamentals and Advances in Instrumentation, Analysis, and Applications. J. Biomed. Opt. 2020, 25, 071203. [Google Scholar] [CrossRef]
- Bazin, R.; Flament, F.; Colonna, A.; Le Harzic, R.; Bückle, R.; Piot, B.; Laizé, F.; Kaatz, M.; König, K.; Fluhr, J.W. Clinical study on the effects of a cosmetic product on dermal extracellular matrix components using a high-resolution multiphoton tomograph. Ski. Res. Technol. 2010, 16, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Wizenty, J.; Schumann, T.; Theil, D.; Stockmann, M.; Pratschke, J.; Tacke, F.; Aigner, F.; Wuensch, T. Recent advances and the potential for clinical use of autofluorescence detection of extra-ophthalmic tissues. Molecules 2020, 25, 2095. [Google Scholar] [CrossRef]
- Cornelius, C.E., III; Ludwig, G.D. Red fluorescence of comedones: Production of porphyrins by Corynebacterium acnes. J. Investig. Dermatol. 1967, 49, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Deyl, Z.; Šulcová, H.; Praus, R.; Goldman, J.N. A fluorescent compound in collagen and its relation to the age of the animal. Exp. Gerontol. 1970, 5, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Deyl, Z.; Praus, R.; Šulcová, H.; Goldman, J.N. Fluorescence of collagen—Properties of tyrosine residues and another fluorescent element in calf skin collagen. FEBS Lett. 1969, 5, 187–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vančíková, O.; Deyl, Z. Disappearance of tyrosine residues in collagen with age. Suggestion of a possible reaction mechanism. Exp. Gerontol. 1974, 9, 123–130. [Google Scholar] [CrossRef]
- Liutkeviciute-Navickiene, J.; Mordas, A.; Rutkovskiene, L.; Bloznelyte-Plesniene, L. Skin and mucosal fluorescence diagnosis with different light sources. Eur. J. Dermatol. 2009, 19, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Rizova, E.; Kligman, A. New photographic techniques for clinical evaluation of acne. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Bargo, P.R.; Doukas, A.; González, S.; Menon, G.; Pappas, A.; Ruvolo Jr, E.C.; Stamatas, G.N. The Kollias legacy: Skin autofluorescence and beyond. Exp. Dermatol. 2017, 26, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Lucchina, L.C.; Kollias, N.; Gillies, R.; Phillips, S.B.; Muccini, J.A.; Stiller, M.J.; Trancik, R.J.; Drake, L.A. Fluorescence photography in the evaluation of acne. J. Am. Acad. Dermatol. 1996, 35, 58–63. [Google Scholar] [CrossRef]
- Kollias, N.; Gillies, R.; Cohén-Goihman, C.; Phillips, S.B.; Muccini, J.A.; Stiller, M.J.; Drake, L.A. Fluorescence photography in the evaluation of hyperpigmentation in photodamaged skin. J. Am. Acad. Dermatol. 1997, 36, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Peng, Q.; Moan, J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem. Photobiol. Sci. 2007, 6, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Pietkiewicz, P.; Navarrete-Dechent, C.; Togawa, Y.; Szlązak, P.; Salwowska, N.; Marghoob, A.A.; Marghoob, A.A.; Leszczyńska-Pietkiewicz, A.; Errichetti, E. Applications of Ultraviolet and Sub-ultraviolet Dermatoscopy in Neoplastic and Non-neoplastic Dermatoses: A Systematic Review. Dermatol. Ther. 2024, 14, 361–390. [Google Scholar] [CrossRef]
- Pagnoni, A.; Kligman, A.M.; Kollias, N.; Goldberg, S.; Stoudemayera, T. Digital fluorescence photography can assess the suppressive effect of benzoyl peroxide on Propionibacterium acnes. J. Am. Acad. Dermatol. 1999, 41, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Talebi, Z.; Kord Afshari, G.; Ahmad Nasrollahi, S.; Firooz, A.; Ghovvati, M.; Samadi, A.; Karimi, M.; Kolahdooz, S. Potential of Trachyspermum ammi (ajwain) gel for treatment of facial acne vulgaris: A pilot study with skin biophysical profile assessment and red fluorescence photography. Res. J. Pharmacogn. 2020, 7, 61–69. [Google Scholar]
- Liu, L.; Yang, Q.; Zhang, M.; Wu, Z.; Xue, P. Fluorescence lifetime imaging microscopy and its applications in skin cancer diagnosis. J. Innov. Opt. Health Sci. 2019, 12, 1930004. [Google Scholar] [CrossRef]
- Hollands, G.J.; Usher-Smith, J.A.; Hasan, R.; Alexander, F.; Clarke, N.; Griffin, S.J. Visualising health risks with medical imaging for changing recipients’ health behaviours and risk factors: Systematic review with meta-analysis. PLoS Med. 2022, 19, e1003920. [Google Scholar] [CrossRef] [PubMed]
- Baig, I.T.; Nguyen, Q.B.D.; Jahan-Tigh, R.R.; Migden, M.R. Digital photography for the dermatologist. Clin. Dermatol. 2023, 41, 171–177. [Google Scholar] [CrossRef]
- Mojeski, J.A.; Almashali, M.; Jowdy, P.; Fitzgerald, M.E.; Brady, K.L.; Zeitouni, N.C.; Colegio, O.R.; Paragh, G. Ultraviolet imaging in dermatology. Photodiagn. Photodyn. Ther. 2020, 30, 101743. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, I.A.; Danilova, M.K.; Sasin, M.E.; Belik, V.P.; Golyshev, D.P.; Vasyutinskii, O.S. Determination of fluorescence quantum yields and decay times of NADH and FAD in water–alcohol mixtures: The analysis of radiative and nonradiative relaxation pathways. J. Photochem. Photobiol. A: Chem. 2023, 436, 114388. [Google Scholar] [CrossRef]
- Gillies, R.; Zonios, G.; Anderson, R.R.; Kollias, N. Fluorescence excitation spectroscopy provides information about human skin in vivo. J. Investig. Dermatol. 2000, 115, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Zhu, G.; Wang, Q.; Ni, J.; Liu, L.; Zhang, J.; Zhang, T.; Li, Z.; Wang, X.; et al. Noninvasive detection of diabetes mellitus based on skin fluorescence and diffuse reflectance spectroscopy. J. Biophotonics 2023, 17, e202300098. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Pu, Y.; Yang, Y.; Alfano, R.R. Biomarkers spectral subspace for cancer detection. J. Biomed. Opt. 2012, 17, 107005. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.C.A.; Patterson, M.S.; Hayward, J.E.; Fang, Q. Time-resolved fluorescence in photodynamic therapy. Photonics 2014, 1, 530–564. [Google Scholar] [CrossRef]
- Huang, W.; Liu, Q.; Zhu, E.Y.; Shindi, A.A.F.; Li, Y.Q. Rapid simultaneous determination of protoporphyrin IX, uroporphyrin III and coproporphyrin III in human whole blood by non-linear variable-angle synchronous fluorescence technique coupled with partial least squares. Talanta 2010, 82, 1516–1520. [Google Scholar] [CrossRef]
- Mbonyiryivuze, A.; Omollo, I.; Ngom, B.D.; Mwakikunga, B.; Dhlamini, S.M.; Park, E.; Maaza, M. Natural dye sensitizer for Grätzel cells: Sepia melanin. Phys. Mater. Chem. 2015, 3, 1. [Google Scholar]
- Dervieux, E.; Bodinier, Q.; Uhring, W.; Théron, M. Measuring hemoglobin spectra: Searching for carbamino-hemoglobin. J. Biomed. Opt. 2020, 25, 105001. [Google Scholar] [CrossRef]
- Blacker, T.S.; Mann, Z.F.; Gale, J.E.; Ziegler, M.; Bain, A.J.; Szabadkai, G.; Duchen, M.R. Separating NADH and NADPH fluorescence in live cells and tissues using FLIM. Nat. Commun. 2014, 5, 3936. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.J.W.G.; Hoek, A.V. The fluorescence decay of reduced nicotinamides in aqueous solution after excitation with a UV-mode locked Ar ion laser. Photochem. Photobiol. 1981, 33, 35–40. [Google Scholar] [CrossRef]
- Chorvat, D., Jr.; Chorvatova, A. Multi-wavelength fluorescence lifetime spectroscopy: A new approach to the study of endogenous fluorescence in living cells and tissues. Laser Phys. Lett. 2009, 6, 175–193. [Google Scholar] [CrossRef]
- Islam, M.S.; Honma, M.; Nakabayashi, T.; Kinjo, M.; Ohta, N. pH dependence of the fluorescence lifetime of FAD in solution and in cells. Int. J. Mol. Sci. 2013, 14, 1952–1963. [Google Scholar] [CrossRef]
- Mienaltowski, M.J.; Birk, D.E. Structure, Physiology, and Biochemistry of Collagens. In Progress in Heritable Soft Connective Tissue Diseases; Springer: Dordrecht, The Netherlands, 2014; pp. 5–29. [Google Scholar]
- Mithieux, S.M.; Weiss, A.S. Elastin. Adv. Protein Chem. 2005, 70, 437–461. [Google Scholar] [PubMed]
- Tanzer, M.L. Cross-Linking of Collagen: Endogenous aldehydes in collagen react in several ways to form a variety of unique covalent cross-links. Science 1973, 180, 561–566. [Google Scholar] [CrossRef]
- Robins, S.P. Biochemistry and functional significance of collagen cross-linking. Biochem. Soc. Trans. 2007, 35, 849–852. [Google Scholar] [CrossRef] [PubMed]
- La Bella, F.; Thornhill, D.P. Effects of UV irradiation in Human and Bovine Collagen and Elastin. In Proceedings of the Third Canadian Conference on Research in the Rheumatic Disorders, Toronto, ON, Canada, 25–27 February 1965; pp. 25–27. [Google Scholar]
- Lutz, V.; Sattler, M.; Gallinat, S.; Wenck, H.; Poertner, R.; Fischer, F. Impact of collagen crosslinking on the second harmonic generation signal and the fluorescence lifetime of collagen autofluorescence. Skin Res. Technol. 2012, 18, 168–179. [Google Scholar] [CrossRef]
- Muir, R.; Forbes, S.; Birch, D.J.; Vyshemirsky, V.; Rolinski, O.J. Collagen glycation detected by its intrinsic fluorescence. J. Phys. Chem. B 2021, 125, 11058–11066. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Portalatin, N.; Alfonso-Garcia, A.; Liu, J.C.; Marcu, L.; Panitch, A. Physical, biomechanical, and optical characterization of collagen and elastin blend hydrogels. Ann. Biomed. Eng. 2021, 48, 2924–2935. [Google Scholar] [CrossRef]
- Shu, M.; Kuo, S.; Wang, Y.; Jiang, Y.; Liu, Y.T.; Gallo, R.L.; Huang, C.M. Porphyrin metabolisms in human skin commensal Propionibacterium acnes bacteria: Potential application to monitor human radiation risk. Curr. Med. Chem. 2013, 20, 562–568. [Google Scholar] [PubMed]
- Lavallee, D.K.; Norelius, J.T. Evaluation of tetraphenylporphinatozinc (II) as a stable standard for porphyrin determinations by fluorescence spectrometry. Anal. Chem. 1977, 49, 1453–1456. [Google Scholar] [CrossRef]
- Bykhovsky, V.Y.; Zaitseva, N.I.; Mironov, A.F.; Osin, N.S.; Pecherskikh, E.V.; Rumyantseva, V.D.; Sukhin, G.M. Coproporphyrins, uroporphyrins, and their metal Complexes: Biosynthesis and application to immune analysis and diagnostic methods. Appl. Biochem. Microbiol. 2001, 37, 561–568. [Google Scholar] [CrossRef]
- Seo, I.; Tseng, S.H.; Cula, G.O.; Bargo, P.R.; Kollias, N. Fluorescence Spectroscopy for Endogenous Porphyrins in Human Facial Skin. In Photonic Therapeutics and Diagnostics V; SPIE: Bellingham, WA, USA, 2009; Volume 7161, pp. 10–15. [Google Scholar]
- Xu, D.T.; Yan, J.N.; Liu, W.; Hou, X.X.; Zheng, Y.; Jiang, W.W.; Ju, Q.; Zouboulis, C.C.; Wang, X.L. Is human sebum the source of skin follicular ultraviolet-induced red fluorescence? A cellular to histological study. Dermatology 2018, 234, 43–50. [Google Scholar] [CrossRef]
- Lozovaya, G.I.; Masinovsky, Z.; Sivash, A.A. Protoporphyrin IX as a possible ancient photosensitizer: Spectral and photochemical studies. Orig. Life Evol. Biosph. 1990, 20, 321–330. [Google Scholar] [CrossRef]
- Stratonnikov, A.A.; Polikarpov, V.S.; Loschenov, V.B. Photobleaching of endogenous fluorochroms in tissues in vivo during laser irradiation. In Saratov Fall Meeting 2000: Optical Technologies in Biophysics and Medicine II; SPIE: Bellingham, WA, USA, 2000; Volume 4241, pp. 13–24. [Google Scholar]
- Schechter, A.N. Hemoglobin research and the origins of molecular medicine. Blood. J. Am. Soc. Hematol. 2008, 112, 3927–3938. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J.; Tsukamoto, K. Enzymatic control of pigmentation in mammals. FASEB J. 1991, 5, 2902–2909. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K.; Sarna, T. Photodegradation of eumelanin and pheomelanin and its pathophysiological implications. Photochem. Photobiol. 2018, 94, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Płonka, P.; Grabacka, M. Melanin synthesis in microorganisms—Biotechnological and medical aspects. Acta Biochim. Pol. 2006, 53, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.H.; Bjerring, P. Noninvasive computerized analysis of skin chromophores in vivo by reflectance spectroscopy. Photodermatol. Photoimmunol. Photomed. 1990, 7, 249–257. [Google Scholar] [PubMed]
- Dremin, V.V.; Dunaev, A.V. How the melanin concentration in the skin affects the fluorescence-spectroscopy signal formation. J. Opt. Technol. 2016, 83, 43–48. [Google Scholar] [CrossRef]
- Georgievskaya, A.; Tlyachev, T.; Danko, D.; Chekanov, K.; Corstjens, H. How artificial intelligence adopts human biases: The case of cosmetic skincare industry. AI Ethics 2023, 1–11. [Google Scholar] [CrossRef]
- Chekanov, K. Diversity and distribution of carotenogenic algae in Europe: A review. Mar. Drugs 2003, 21, 108. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, A.; Chekanov, K.; Zaytsev, P.; Bakhareva, D.; Gorelova, O.; Kochkin, D.; Lobakova, E. Sunscreen effect exerted by secondary carotenoids and mycosporine-like amino acids in the aeroterrestrial chlorophyte Coelastrella rubescens under high light and UV-A irradiation. Plants 2021, 10, 2601. [Google Scholar] [CrossRef]
- Sen, S.; Mallick, N. Mycosporine-like amino acids: Algal metabolites shaping the safety and sustainability profiles of commercial sunscreens. Algal Res. 2021, 58, 102425. [Google Scholar] [CrossRef]
- Solovchenko, A.; Chekanov, K. Production of carotenoids using microalgae cultivated in photobioreactors. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Springer: Dordrecht, The Netherlands, 2014; pp. 63–91. [Google Scholar]
- Utz, S.R.; Knuschke, P.; Sinichkin, Y.P. In vivo evaluation of sunscreens by spectroscopic methods. Skin Res. Technol. 1996, 2, 114–121. [Google Scholar] [CrossRef] [PubMed]
- US Food & Drug Administration. Sun Protection Factor (SPF). Available online: https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/sun-protection-factor-spf (accessed on 31 August 2024).
- Springsteen, A.; Yurek, R.; Frazier, M.; Carr, K.F. In Vitro Measurement of Sun Protection Factor of Sunscreens by Diffuse Transmittance. Anal. Chim. Acta 1999, 380, 155–164. [Google Scholar] [CrossRef]
- Bissonnette, R.; Zeng, H.; McLean, D.I.; Schreiber, W.E.; Lui, H. Psoriatic Plaques Exhibit Red Autofluorescence That Is Due to Protoporphyrin IX. J. Investig. Dermatol. 1998, 111, 586–591. [Google Scholar] [CrossRef][Green Version]
- Messaraa, C.; Walsh, M.; Hurley, S.; Robertson, N.; O’Connor, C.; Doyle, L.; Mansfield, A.; McNamee, D. A novel UV-fluorescence approach to assess the long wear efficacy of foundations. Skin Res. Technol. 2021, 27, 758–765. [Google Scholar] [CrossRef]
- Hermanns, J.F.; Petit, L.; Martalo, O.; Piérard-Franchimont, C.; Cauwenbergh, G.; Pierard, G.E. Unravelling the patterns of subclinical pheomelanin-enriched facial hyperpigmentation: Effect of depigmenting agents. Dermatology 2000, 201, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2022, 138, 1462–1470. [Google Scholar] [CrossRef]
- Odetti, P.R.; Borgoglio, A.; Rolandi, R. Age-related increase of collagen fluorescence in human subcutaneous tissue. Metabolism 1992, 41, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.D.; Gillies, R.; Brancaleon, L.; Kollias, N. Aging and Effects of Ultraviolet A Exposure May Be Quantified by Fluorescence Excitation Spectroscopy In Vivo. J. Investig. Dermatol. 2001, 116, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Veasey, J.V.; Miguel, B.A.; Bedrikow, R.B.; Mota, R.D.C., Jr.; Buarque, V. Wood’s lamp in dermatology: Applications in the daily practice. Surg. Cosmet. Dermatol. 2017, 9, 324–326. [Google Scholar] [CrossRef]
- Errichetti, E.; Pietkiewicz, P.; Bhat, Y.J.; Salwowska, N.; Szlązak, P.; Stinco, G. Diagnostic accuracy of ultravioletinduced fluorescence dermoscopy in non-neoplastic dermatoses (general dermatology): A multicentric retrospective comparative study. J. Eur. Acad. Dermatol. Venereol. 2024. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Singh, R.B.A.M.; Barden, A.; Mori, T.; Beilin, L. Advanced Glycation End-Products: A Review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Kuzan, A. Toxicity of advanced glycation end products (review). Biomed. Rep. 2021, 14, 46. [Google Scholar] [CrossRef]
- Obayashi, H.; Nakano, K.; Shigeta, H.; Yamaguchi, M.; Yoshimori, K.; Fukui, M.; Fujii, M.; Kitagawa, Y.; Nakamura, N.; Nakamura, K.; et al. Formation of Crossline as a Fluorescent Advanced Glycation End Product in Vitro and in Vivo. Biochem. Biophys. Res. Commun. 1996, 226, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, N.; Bijukumar, G.; Karmakar, N.; Anand, S.; Misra, A. Autofluorescence Characterization of Advanced Glycation End Products of Hemoglobin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 163–170. [Google Scholar] [CrossRef]
- Schmitt, A.; Schmitt, J.; Münch, G.; Gasic-Milencovic, J. Characterization of Advanced Glycation End Products for Biochemical Studies: Side Chain Modifications and Fluorescence Characteristics. Anal. Biochem. 2005, 338, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Shimizu, M.; Miura, J.; Matsuda, Y.; Kubo, M.; Hashimoto, M.; Hashimoto, M.; Aoki, T.; Takeshige, F.; Araki, T. Decrease in fluorescence lifetime by glycation of collagen and its application in determining advanced glycation end-products in human dentin. Biomed. Opt. Express 2015, 6, 1844–1856. [Google Scholar] [CrossRef]
- Beisswenger, P.J.; Howell, S.; Mackenzie, T.; Corstjens, H.; Muizzuddin, N.; Matsui, M.S. Two Fluorescent Wavelengths, 440ex/520em nm and 370ex/440em nm, Reflect Advanced Glycation and Oxidation End Products in Human Skin without Diabetes. Diabetes Technol. Ther. 2012, 14, 285–292. [Google Scholar] [CrossRef]
- Almengló, C.; Rodriguez-Ruiz, E.; Alvarez, E.; López-Lago, A.; González-Juanatey, J.R.; Garcia-Allut, J.L. Minimal invasive fluorescence methods to quantify advanced glycation end products (AGEs) in skin and plasma of humans. Methods 2022, 203, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.; Favilla, R.; Strömberg, T. Assessment of advanced glycated end product accumulation in skin using auto fluorescence multispectral imaging. Comput. Biol. Med. 2017, 85, 106–111. [Google Scholar] [CrossRef]
- Sterenborg, H.J.C.M.; Motamedi, M.; Wagner, R.F.; Duvic, M.; Thomsen, S.; Jacques, S.L. In vivo fluorescence spectroscopy and imaging of human skin tumours. Lasers Med. Sci. 1994, 9, 191–201. [Google Scholar] [CrossRef]
- Scherer, D.; Kumar, R. Genetics of pigmentation in skin cancer—A review. Mutat. Res. Rev. Mutat. Res. 2010, 705, 141–153. [Google Scholar] [CrossRef]
- Piérard, G.E.; Hermanns-Lê, T.; Piérard, S.L.; Dewalque, L.; Charlier, C.; Piérard-Franchimont, C.; Delvenne, P. In vivo skin fluorescence imaging in young Caucasian adults with early malignant melanomas. Clin. Cosmet. Investig. Dermatol. 2014, 22, 225–230. [Google Scholar] [CrossRef]
- Martens, G.; Gunzel, E.P. Method of detecting anomalies of the skin, more particularly melanomae, and apparatus for carrying out the method. U.S. Patent No. 5,363,854, November 15, 1994. [Google Scholar]
- Streckfus, C.F. Exocrinology: A Textbook and Atlas of the Exocrine Cells, Glands, and Organs; Springer Nature: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Bonkovsky, H.L.; Guo, J.T.; Hou, W.; Li, T.; Narang, T.; Thapar, M. Porphyrin and Heme Metabolism and the Porphyrias. Compr. Physiol. 2013, 3, 365–401. [Google Scholar] [PubMed]
- Zouboulis, C. C. Acne and Sebaceous Gland Function. Clin. Dermatol. 2004, 22, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Romiti, R.; Schaller, M.; Jacob, K.; Plewig, G. High-Performance Liquid Chromatography Analysis of Porphyrins in Propionibacterium acnes. Arch. Dermatol. Res. 2000, 292, 320–322. [Google Scholar] [CrossRef]
- McDowell, A.; McLaughlin, J.; Layton, A.M. Is Cutibacterium (Previously Propionibacterium) acnes a Potential Pathogenic Factor in the Aetiology of the Skin Disease Progressive Macular Hypomelanosis? J. Eur. Acad. Dermatol. Venereol. 2021, 35, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Melø, T.B.; Johnsson, M. In Vivo Porphyrin Fluorescence from Propionibacterium acnes: A Characterization of the Fluorescing Pigments. Dermatology 1982, 164, 167–174. [Google Scholar] [CrossRef]
- Borelli, C.; Merk, K.; Schaller, M.; Jacob, K.; Vogeser, M.; Weindl, G.; Berger, U.; Plewig, G. In Vivo Porphyrin Production by P. acnes in Untreated Acne Patients and Its Modulation by Acne Treatment. Acta Derm.-Venereol. 2006, 86, 316–319. [Google Scholar]
- Patwardhan, S.V.; Richter, C.; Vogt, A.; Blume-Peytavi, U.; Canfield, D.; Kottner, J. Measuring Acne Using Coproporphyrin III, Protoporphyrin IX, and Lesion-Specific Inflammation: An Exploratory Study. Arch. Dermatol. Res. 2017, 309, 159–167. [Google Scholar] [CrossRef]
- Sauermann, G.; Ebens, B.; Hoppe, U. The Analysis of Facial Comedones by Porphyrin Fluorescence and Image Analysis. J. Toxicol. 1990, 8, 369–386. [Google Scholar]
- Yeung, C.K.; Shek, S.Y.; Bjerring, P.; Yu, C.S.; Kono, T.; Chan, H.H. A Comparative Study of Intense Pulsed Light Alone and Its Combination with Photodynamic Therapy for the Treatment of Facial Acne in Asian Skin. Lasers Surg. Med. 2007, 39, 1–6. [Google Scholar] [CrossRef]
- Han, B.; Jung, B.; Nelson, J.S.; Choi, E.H. Analysis of Facial Sebum Distribution Using a Digital Fluorescent Imaging System. J. Biomed. Opt. 2007, 12, 014006. [Google Scholar] [CrossRef]
- Morss-Walton, P.C.; McGee, J.S.; Rosales Santillan, M.; Kimball, R.; Cukras, A.; Patwardhan, S.V.; Kimball, A.B. Yin and Yang of Skin Microbiota in “Swimmer Acne”. Exp. Dermatol. 2022, 31, 899–905. [Google Scholar] [CrossRef] [PubMed]
- McGinley, K.J.; Webster, G.F.; Leyden, J.J. Facial Follicular Porphyrin Fluorescence: Correlation with Age and Density of Propionibacterium acnes. Br. J. Dermatol. 1980, 102, 437–441. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, T.Y.; Bae, S.J.; Kang, U.; Ro, Y.S. The Clinical Usefulness of Portable Digital Skin Fluorescence Equipment in Acne Patients. Korean J. Dermatol. 2008, 46, 889–895. [Google Scholar]
- Lee, W.L.; Shalita, A.R.; Poh-Fitzpatrick, M.B. Comparative Studies of Porphyrin Production in Propionibacterium acnes and Propionibacterium granulosum. J. Bacteriol. 1978, 133, 811–815. [Google Scholar] [CrossRef]
- Youn, S.W.; Kim, J.H.; Lee, J.E.; Kim, S.O.; Park, K.C. The Facial Red Fluorescence of Ultraviolet Photography: Is This Color Due to Propionibacterium acnes or the Unknown Content of Secreted Sebum? Skin Res. Technol. 2009, 15, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Choi, J.W.; Park, K.C.; Youn, S.W. Ultraviolet-Induced Red Fluorescence of Patients with Acne Reflects Regional Casual Sebum Level and Acne Lesion Distribution: Qualitative and Quantitative Analyses of Facial Fluorescence. Br. J. Dermatol. 2012, 166, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Okoro, E.O.; Bulus, N.G.; Zouboulis, C.C. Study of Facial Sebum Levels and Follicular Red Fluorescence in Patients with Acne Vulgaris in Nigeria. Dermatology 2016, 232, 156–161. [Google Scholar] [CrossRef]
- Dobrev, H. Fluorescence Diagnostic Imaging in Patients with Acne. Photodermatol. Photoimmunol. Photomed. 2010, 26, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.M.; Horswill, A.R. Staphylococcus epidermidis—Skin Friend or Foe? PLoS Pathog. 2020, 16, e1009026. [Google Scholar] [CrossRef] [PubMed]
- Claudel, J.P.; Auffret, N.; Leccia, M.T.; Poli, F.; Corvec, S.; Dréno, B. Staphylococcus epidermidis: A Potential New Player in the Physiopathology of Acne? Dermatology 2019, 235, 287–294. [Google Scholar] [CrossRef]
- Wu, Y.; Akimoto, M.; Igarashi, H.; Shibagaki, Y.; Tanaka, T. Quantitative Assessment of Age-Dependent Changes in Porphyrins from Fluorescence Images of Ultraviolet Photography by Image Processing. Photodiagnosis Photodyn. Ther. 2021, 35, 102388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, X.; Mao, Y.; Xu, Z.; Sun, Y.; Mei, X.; Shi, W. Variation of Biophysical Parameters of the Skin with Age, Gender, and Lifestyles. J. Cosmet. Dermatol. 2021, 20, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Heerfordt, I.M.; Lerche, C.M.; Wulf, H.C. Trends in Erythrocyte Protoporphyrin IX Concentration by Age, Sex and Season Among Patients with Erythropoietic Protoporphyria—20 Years of Follow-Up. Photodiagnosis Photodyn. Ther. 2020, 32, 101928. [Google Scholar] [CrossRef] [PubMed]
- Polo, C.F.; Frisardi, A.L.; Resnik, E.R.; Schoua, A.E.; Batlle, A.M. Factors influencing fluorescence spectra of free porphyrins. Clin. Chem. 1988, 34, 757–760. [Google Scholar] [CrossRef]
- Son, T.; Han, B.; Jung, B.; Nelson, J.S. Fluorescent Image Analysis for Evaluating the Condition of Facial Sebaceous Follicles. Skin Res. Technol. 2008, 14, 201–207. [Google Scholar] [CrossRef][Green Version]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Structural Implications on Color, Fluorescence, and Antiradical Activity in Betalains. Planta 2010, 232, 449–460. [Google Scholar] [CrossRef]
- Herpens, A.; Schagen, S.; Scheede, S.; Kristof, B. Evaluation of Comedogenic Activity by Skin Fluorescence Imaging Analysis (Skin Analyzing Fluorescence Imaging Recorder). In Bioengineering of the Skin; CRC Press: Boca Raton, FI, USA, 2007; pp. 447–458. [Google Scholar]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Y.T.; Zhang, L.; Zheng, J.; Sroka, R.; Wang, H.W.; Wang, X.L. Protoporphyrin IX Fluorescence as Potential Indicator of Psoriasis Severity and Progression. Photodiagn. Photodyn. Ther. 2017, 19, 304–307. [Google Scholar] [CrossRef]
- Pietkiewicz, P.; Navarrete-Dechent, C.; Mayisoğlu, H.; Jolly, G.; Kutlu, Ö.; Errichetti, E. Pink-Red Fluorescence Observed in Ultraviolet-Induced Fluorescence Dermoscopy of Psoriatic Plaques. Dermatol. Pract. Concept. 2023, 13, e2023243. [Google Scholar] [CrossRef]
- Schmitt, J.; Wozel, G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatol. 2005, 210, 194–199. [Google Scholar] [CrossRef]
- Kikuchi, I.; Inoue, S.; Idemori, M.; Uchimura, H. Reflection Ultraviolet Photography as Surface Photography of the Skin. J. Dermatol. 1983, 10, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Uhoda, E.; Piérard-Franchimont, C.; Petit, L.; Piérard, G.E. The Conundrum of Skin Pores in Dermocosmetology. Dermatology 2004, 210, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Peris Fajarnés, G.; Moncho Santonja, M.; Defez García, B.; Lengua Lengua, I. Segmentation Methods for Acne Vulgaris Images: Proposal of a New Methodology Applied to Fluorescence Images. Skin Res. Technol. 2020, 26, 734–739. [Google Scholar] [CrossRef]
- Cline, R.W.; Leduc, P. Portable system for detecting skin abnormalities based on characteristic autofluorescence. U.S. Patent No. 6,603,552, August 5, 2003. [Google Scholar]
- Merola, K.; Kollias, N.; Pote, J.S.; Payonk, G. Method of promoting skin care products. US Patent No. 6,961,517, November 1, 2005. [Google Scholar]
- Merola, K.; Kollias, N.; Pote, J.S.; Payonk, G. Method of promoting skin care products. US Patent No. 6,922,523, July 26, 2005. [Google Scholar]
- Kollias, N.; Merola, K.; Pote, J.; Payonk, G. Imaging apparatus and methods for capturing and analyzing digital images of the skin. US Patent Application No. 20,080,079,843, April 3, 2008. [Google Scholar]
- Kollias, N.; Merola, K.; Pote, J.S.; Payonk, G. Apparatus for and method of taking and viewing images of the skin. US Patent No. 7,738,032, June 15, 2010. [Google Scholar]
- Demirli, R.; Hillebrand, G.G. Method and Apparatus for Simulation of Dacial Skin Aging and De-Aging. US Patent No. 8,290,257, 16 October 2012. [Google Scholar]
- VISIA-CR User Guide. Available online: https://www.esthetec.fr/wa_files/VISIA-CR-User-Guide.pdf (accessed on 3 August 2024).
- OBSERV 520. Sylton Diagnostic Systems. Available online: https://us.sylton.com/product/observ-520x-face-analysis/ (accessed on 3 August 2024).
- Lumsail BS-3800 Built-In 20 Million Pixel 8 Different Spectrums 3D Skin Analyzer. Available online: https://www.lumsail.com/product/lumsail-bs-3800-built-in-20-million-pixel-8-different-spectrums-3d-skin-analyzer/ (accessed on 3 August 2024).
- Van der Beek, N. From Practice to Theory: Computational Studies on Fluorescence Detection and Laser Therapy in Dermatology. Ph.D. Thesis, University of Wales Trinity Saint David, Lampeter, UK, 2017. [Google Scholar]
- Visiopor® PP 34N—Monitoring Acne-Lesionsby Skin Fluorescence. Available online: https://www.courage-khazaka.com/images/Downloads/Brochures/Wissenschaftlich/Brochure_Visiopor.pdf (accessed on 3 August 2024).
- Information and Operating Instruction. Visioscan® VC 98 and the Software SELS (Surface Evaluation of the Living Skin). Available online: https://www.cosmeticsonline.com.br/produtos/arquivos/A42_instructions_vc98_sels_fw.pdf (accessed on 3 August 2024).
- Tobar, M.D.P.B.; Clemann, S.; Hagens, R.; Pagel-Wolff, S.; Hoppe, S.; Behm, P.; Engelhard, F.; Langhals, M.; Gallinat, S.; Zhavoronkov, A.; et al. Skinly: A Novel Handheld IoT Device for Validating Biophysical Skin Characteristics. Skin Res. Technol. 2024, 30, e13613. [Google Scholar] [CrossRef] [PubMed]
- Dermlte.com: Lumio 2. Available online: https://dermlite.com/products/lumio-2?_pos=1&_psq=lumio&_ss=e&_v=1.0 (accessed on 3 August 2024).
- Canfield VOSIOMED Dermoscopy. Optima 3-in-1 Magnifier Brochure. Available online: https://www.canfieldsci.com/common/docs/products/47/brochures/OptimaBrochure.pdf (accessed on 3 August 2024).
- DermLite. Available online: https://dermlite.com (accessed on 3 August 2024).
- プロ仕様の肌・頭皮・髪測定を1台で実現. Achieve professional-grade measurement of skin, scalp, and hair with a single device. Available online: https://www.sma-ski.com/#shiyo (accessed on 3 August 2024).
- Khongsuwan, M.; Kiattisin, S.; Wongseree, W.; Leelasantitham, A. Counting Number of Points for Acne Vulgaris Using UV Fluorescence and Image Processing. In Proceedings of the 4th Biomedical Engineering International Conference, Chiang Mai, Thailand, 29–31 January 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 142–146. [Google Scholar]
- Goldsberry, A.; Hanke, C.W.; Hanke, K.E. VISIA System: A Possible Tool in the Cosmetic Practice. J. Drugs Dermatol. 2014, 13, 1312–1314. [Google Scholar]
- Chen, X.; Lu, Q.; Chen, C.; Jiang, G. Recent Developments in Dermoscopy for Dermatology. J. Cosmet. Dermatol. 2021, 20, 1611–1617. [Google Scholar] [CrossRef]
- Deda, L.C.; Goldberg, R.H.; Jamerson, T.A.; Lee, I.; Tejasvi, T. Dermoscopy Practice Guidelines for Use in Telemedicine. NPJ Digit. Med. 2022, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Erdem, O.; Göktay, F. Increasing the Distance between the Dermoscope and the Surgical Site during Intraoperative Dermoscopy. Skin Appendage Disor. 2024, 10, 239–242. [Google Scholar] [CrossRef]
- Pietkiewicz, P.; Navarette-Dechent, C. Scabies mite is bright green under UV dermatoscopy. Dermatol. Pract. Concept. 2023, 13, e2023135. [Google Scholar] [CrossRef]
- Korecka, K.; Polańska, A.; Dańczak-Pazdrowska, A.; Navarrete-Dechent, C. Assessing field cancerization and actinic keratosis using ultraviolet-induced fluorescence dermatoscopy after the application of 5-aminolevulinic acid—An observational study. Photodiagnosis Photodyn. Ther. 2024, 46, 104056. [Google Scholar] [CrossRef] [PubMed]
- Burger, W.; Burge, M.J. Digital Image Processing: An Algorithmic Introduction; Springer Nature: Berlin, Germany, 2022. [Google Scholar]
- May, A.; Bhaumik, S.; Gambhir, S.S.; Zhan, C.; Yazdanfar, S. Whole-Body, Real-Time Preclinical Imaging of Quantum Dot Fluorescence with Time-Gated Detection. J. Biomed. Opt. 2009, 14, 060504. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.S.; Chen, T.S.; Chung, P.C. A Fast Algorithm for Multilevel Thresholding. J. Inf. Sci. Eng. 2001, 17, 713–727. [Google Scholar]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep Learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Deepa, R.; ALMahadin, G.; Sivasamy, A. Early Detection of Skin Cancer Using AI: Deciphering Dermatology Images for Melanoma Detection. AIP Adv. 2024, 14, 045001. [Google Scholar] [CrossRef]
- Strzelecki, M.; Kociołek, M.; Strąkowska, M.; Kozłowski, M.; Grzybowski, A.; Szczypiński, P.M. Artificial Intelligence in the Detection of Skin Cancer: State of the Art. Clin. Dermatol. 2024, 42, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Furriel, B.C.; Oliveira, B.D.; Prôa, R.; Paiva, J.Q.; Loureiro, R.M.; Calixto, W.P.; Giavina-Bianchi, M. Artificial Intelligence for Skin Cancer Detection and Classification in Clinical Environments: A Systematic Review. Front. Med. 2024, 10, 1305954. [Google Scholar] [CrossRef]
- Rong, G.; Mendez, A.; Assi, E.B.; Zhao, B.; Sawan, M. Artificial Intelligence in Healthcare: Review and Prediction Case Studies. Engineering 2020, 6, 291–301. [Google Scholar] [CrossRef]
- Georgievskaya, A.; Danko, D.; Baxter, R.A.; Corstjens, H.; Tlyachev, T. Artificial Intelligence Approaches for Skin Anti-aging and Skin Resilience Research. In Artificial Intelligence for Healthy Longevity; Springer International Publishing: Cham, Switzerland, 2023; pp. 189–214. [Google Scholar]
- Georgievskaya, A.; Tlyachev, T.; Kiselev, K.; Hillebrand, G.; Chekanov, K.; Danko, D.; Majmudar, G. Predicting Human Chronological Age via AI Analysis of Dorsal Hand Versus Facial Images: A Study in a Cohort of Indian Females. Exp. Dermatol. 2024, 33, e15045. [Google Scholar] [CrossRef]
- Gohil, Z.M.; Desai, M.B. Revolutionizing Dermatology: A Comprehensive Survey of AI-Enhanced Early Skin Cancer Diagnosis. Arch. Comput. Methods Eng. 2024. [Google Scholar] [CrossRef]
- Miyamoto, K.; Dissanayake, B.; Omotezako, T.; Takemura, M.; Tsuji, G.; Furue, M. Daily Fluctuation of Facial Pore Area, Roughness and Redness among Young Japanese Women; Beneficial Effects of Galactomyces Ferment Filtrate Containing Antioxidative Skin Care Formula. J. Clin. Med. 2021, 10, 2502. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Song, M.H. High Performing Facial Skin Problem Diagnosis with Enhanced Mask R-CNN and Super Resolution GAN. Appl. Sci. 2023, 13, 989. [Google Scholar] [CrossRef]
- Bobrov, E.; Georgievskaya, A.; Kiselev, K.; Sevastopolsky, A.; Zhavoronkov, A.; Gurov, S.; Rudakov, K.; Tobar, M.d.P.B.; Jaspers, S.; Clemann, S. PhotoAgeClock: Deep Learning Algorithms for Development of Non-Invasive Visual Biomarkers of Aging. Aging 2018, 10, 3249. [Google Scholar] [CrossRef] [PubMed]
- Alamdari, N.; Tavakolian, K.; Alhashim, M.; Fazel-Rezai, R. Detection and Classification of Acne Lesions in Acne Patients: A Mobile Application. In Proceedings of the 2016 IEEE International Conference on Electro Information Technology (EIT), Grand Forks, ND, USA, 19–21 May 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 739–743. [Google Scholar]
- Khan, J.; Malik, A.S.; Kamel, N.; Dass, S.C.; Affandi, A.M. Segmentation of Acne Lesion Using Fuzzy C-Means Technique with Intelligent Selection of the Desired Cluster. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 3077–3080. [Google Scholar]
- Chang, C.Y.; Liao, H.Y. Automatic Facial Spots and Acnes Detection System. J. Cosmet. Dermatol. Sci. Appl. 2013, 3, 28. [Google Scholar] [CrossRef]
- Malik, A.S.; Humayun, J.; Kamel, N.; Yap, F.B. Novel Techniques for Enhancement and Segmentation of Acne Vulgaris Lesions. Skin Res. Technol. 2014, 20, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Huynh, Q.T.; Nguyen, P.H.; Le, H.X.; Ngo, L.T.; Trinh, N.-T.; Tran, M.T.-T.; Nguyen, H.T.; Vu, N.T.; Nguyen, A.T.; Suda, K.; et al. Automatic Acne Object Detection and Acne Severity Grading Using Smartphone Images and Artificial Intelligence. Diagnostics 2022, 12, 1879. [Google Scholar] [CrossRef] [PubMed]
- The International Skin Collaboration. Available online: https://www.isic-archive.com/ (accessed on 3 September 2024).
- Tschandl, P.; Rosendahl, C.; Kittler, H. The HAM10000 Dataset, a Large Collection of Multi-Source Dermatoscopic Images of Common Pigmented Skin Lesions. Sci. Data 2018, 5, 180161. [Google Scholar] [CrossRef]
- Giotis, I.; Molders, N.; Land, S.; Biehl, M.; Jonkman, M.F.; Petkov, N. MED-NODE: A Computer-Assisted Melanoma Diagnosis System Using Non-Dermoscopic Images. Expert Syst. Appl. 2015, 42, 6578–6585. [Google Scholar] [CrossRef]
| Device | Manufacturer | Excitation | Detection | Reference |
|---|---|---|---|---|
| Clinical imaging systems | ||||
| VISIA-CR (Narrow band model) | Canfield Scientific, Inc., Parsippany, NJ, USA | Xenon flash lightning with blue light filter | Red/Green/Blue | [152] |
| VISIA-CR (UV-A model) | Canfield Scientific, Inc., Parsippany, NJ, USA | UV-A (peak at 365 nm) | VIS | [152] |
| OBSERV 520 | Sylton Diagnostic Systems, Son, The Netherlands | “Simulated Wood’s”/“True UV” | VIS | [153] |
| BS-3800 Skin Analyzer | Lumsail Industrial Inc., Shanghai, China | 365/405 nm | VIS | [154] |
| DyaDerm Expert | Biocam GmbH, Regensburg, Germany | 409 nm | VIS | [155] |
| Dermoscopic systems | ||||
| Visiopor PP 34 N | Courage & Khazaka, Cologne, Germany | 16 LEDs: 375–385 | c.a. >500 nm | [156] |
| Visioscan VC 98 | Courage & Khazaka, Cologne, Germany | 380–395 nm, peak: 387 nm | VIS | [157] |
| Skinly | Beiersdorf AG, Hamburg, Germany | 4 LEDs: 385 nm | VIS | [158] |
| Lumio 2 | DermLite, Aliso Viejo, CA, USA | 365/385/405 nm/ “Wood mode” | “OptiClip”: 405 nm long pass | [159] |
| Optima 3-in-1 | Canfield Scientific GmbH, Bielefeld, Germany | 365 nm/“Wood’s light” | VIS | [160] |
| DermLite DL 5 | DermLite Aliso Viejo CA USA | 4 LEDs: Peak: 365 nm | VIS | [161] |
| Smart Skin Care (スマートスキンケア) | IT Access (アイティアクセス), Yokohama, Japan | 8 LEDs: 340–405 nm, peak near 377 nm | VIS/315 nm long pass | [131,162] |
| Reference | Image Source | Approach | Accuracy | n |
|---|---|---|---|---|
| [145] | Facial images | k-means clustering | 4.0–11.2% a | 54 |
| [163] | Facial images | Extended maxima transform | 83.75% b | 10 |
| [135] | Facial images | Otsu’s segmentation | 0.947 c | 29 |
| [131] | Dermoscope | Analysis of colours in HSV space | 71% b | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chekanov, K.; Danko, D.; Tlyachev, T.; Kiselev, K.; Hagens, R.; Georgievskaya, A. State-of-the-Art in Skin Fluorescent Photography for Cosmetic and Skincare Research: From Molecular Spectra to AI Image Analysis. Life 2024, 14, 1271. https://doi.org/10.3390/life14101271
Chekanov K, Danko D, Tlyachev T, Kiselev K, Hagens R, Georgievskaya A. State-of-the-Art in Skin Fluorescent Photography for Cosmetic and Skincare Research: From Molecular Spectra to AI Image Analysis. Life. 2024; 14(10):1271. https://doi.org/10.3390/life14101271
Chicago/Turabian StyleChekanov, Konstantin, Daniil Danko, Timur Tlyachev, Konstantin Kiselev, Ralf Hagens, and Anastasia Georgievskaya. 2024. "State-of-the-Art in Skin Fluorescent Photography for Cosmetic and Skincare Research: From Molecular Spectra to AI Image Analysis" Life 14, no. 10: 1271. https://doi.org/10.3390/life14101271
APA StyleChekanov, K., Danko, D., Tlyachev, T., Kiselev, K., Hagens, R., & Georgievskaya, A. (2024). State-of-the-Art in Skin Fluorescent Photography for Cosmetic and Skincare Research: From Molecular Spectra to AI Image Analysis. Life, 14(10), 1271. https://doi.org/10.3390/life14101271






