Treatment Patterns for and Characteristics of Headache in Children and Adolescents Aged 6–17 Years in Japan: A Retrospective Cross-Sectional and Longitudinal Analysis of Health Insurance Claims Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study 1 (Cross-Sectional Study): Prescription Pattern for Headache Patients
2.2. Study 2 (longitudinal Study): Overprescription during the 2 Years from the Initial Diagnosis
2.3. Statistical Analysis
2.4. Ethics
3. Results
3.1. Study 1 (Cross-Sectional Study): Prescription Pattern for Headache Patients
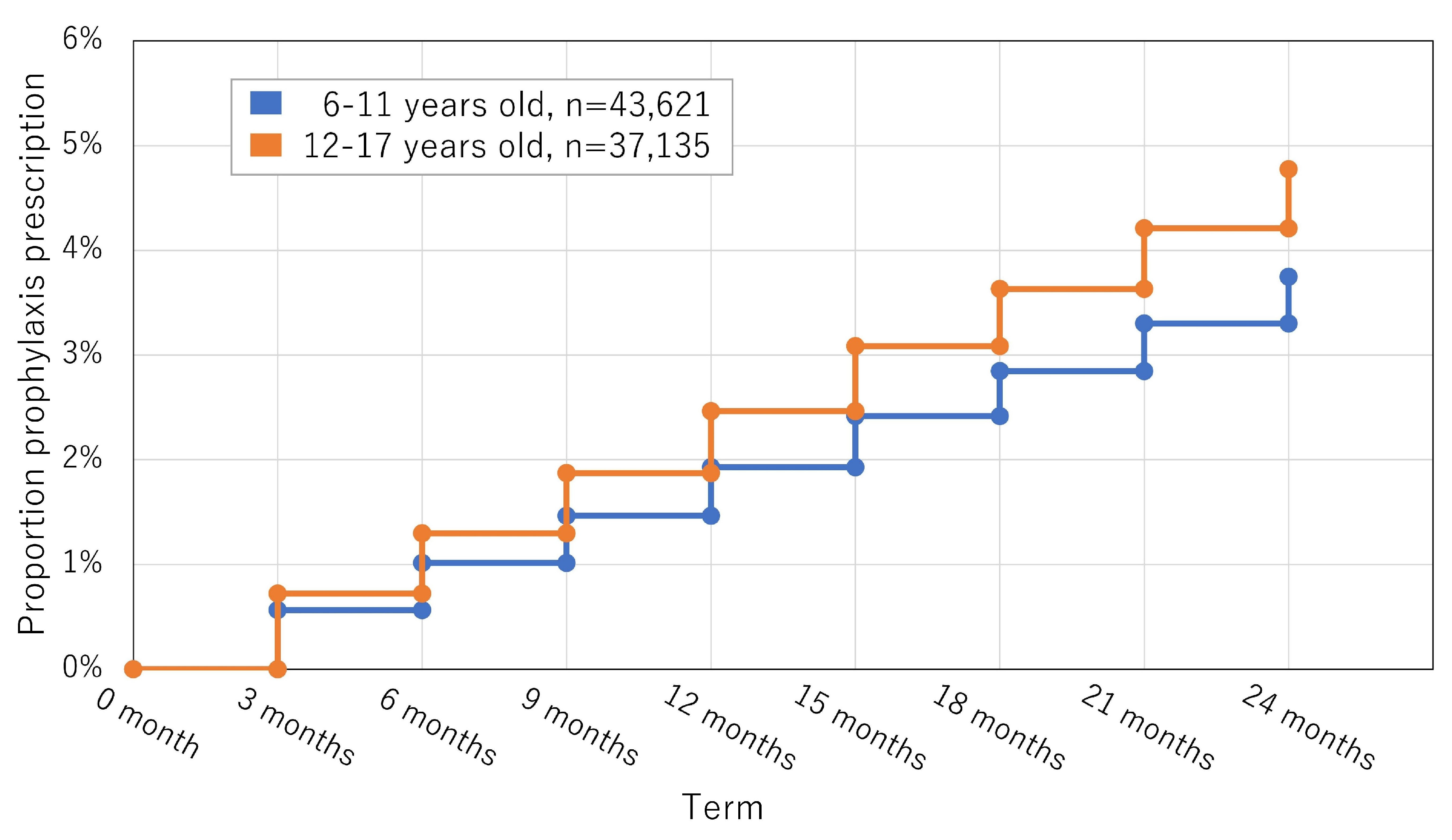
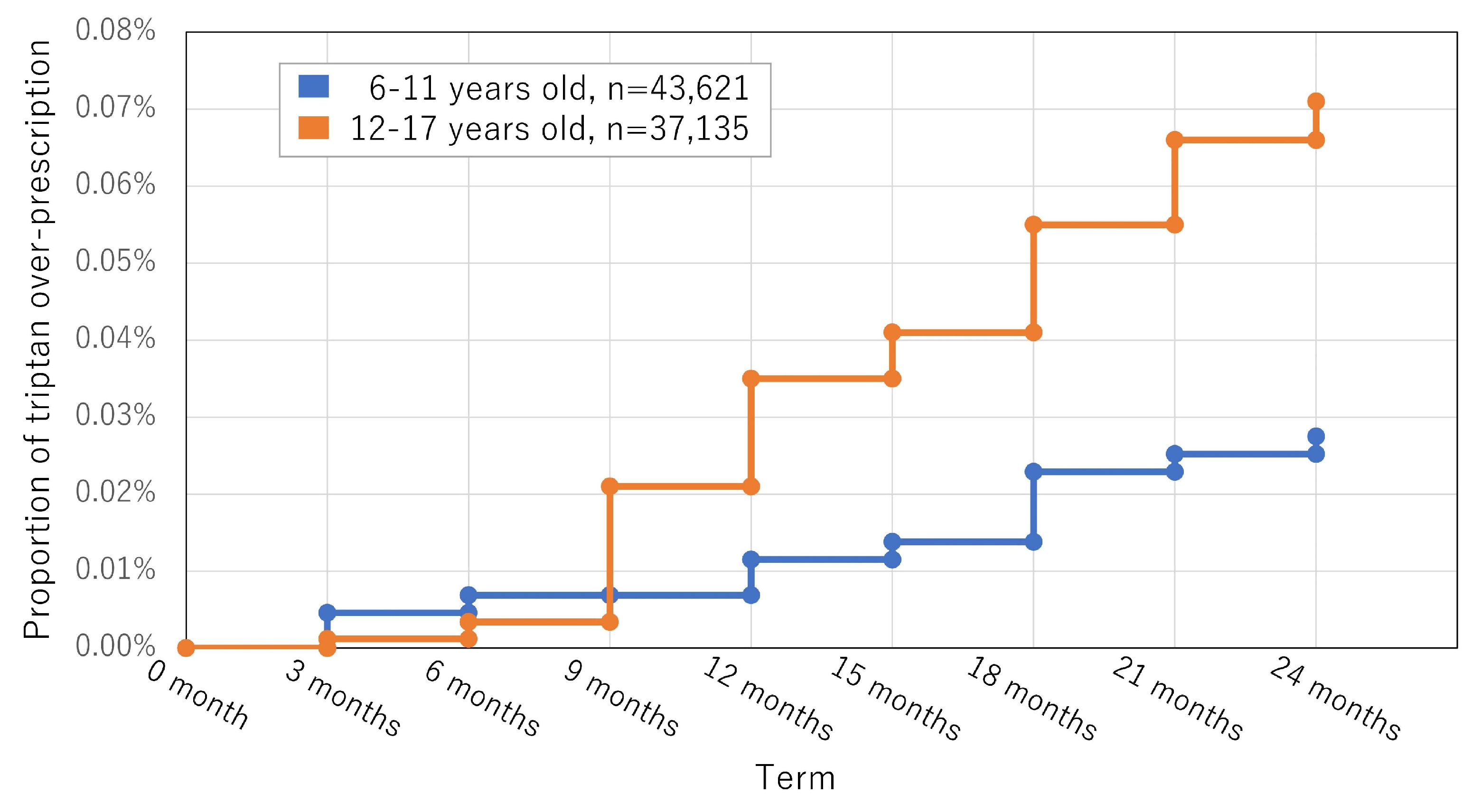
3.2. Study 2 (Longitudinal Study): Overprescription during the Study Period
4. Discussion
4.1. Investigation of Health Insurance Claim Databases of Adult Headache Disorders
4.2. Investigation of Health Insurance Claim Databases for Headache Disorders among Children and Adolescents
4.3. Headache, Migraine, and MOH Prevalence in Children and Adolescents
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CGRP | calcitonin-gene-related peptide |
| CI | confidence interval |
| ICD-10 | International Statistical Classification of Diseases and Related Health Problems-10 |
| ICHD-3 | International Classification of Headache Disorders, 3rd edition |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| OTC | over-the-counter |
| TTH | tension-type headache |
References
- Sakai, F.; Igarashi, H. Prevalence of migraine in Japan: A nationwide survey. Cephalalgia 1997, 17, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, T.; Ishizaki, K.; Fukuhara, Y.; Ijiri, T.; Kusumi, M.; Wakutani, Y.; Mori, M.; Kawashima, M.; Kowa, H.; Adachi, Y.; et al. Population-based door-to-door survey of migraine in Japan: The Daisen Study. Headache: J. Head. Face Pain. 2004, 44, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, M.; Yamagishi, C.; Matsumori, Y.; Koh, A.; Kawamura, S.; Kashiwagi, K.; Kito, T.; Entani, A.; Yamamoto, T.; Ikeda, T.; et al. Questionnaire-based survey on the prevalence of medication-overuse headache in Japanese one city-Itoigawa study. Neurol. Sci. 2022, 43, 3811–3822. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, T.; Wan, Q.; Zhang, Y.; Komori, M.; Stretton, S.; Rajan, N.; Treuer, T.; Ueda, K. Prevalence, burden, and clinical management of migraine in China, Japan, and South Korea: A comprehensive review of the literature. J. Headache Pain 2019, 20, 111. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M. Burden of migraine: What should we say more? Neurol. Sci. 2015, 36, 1–3. [Google Scholar] [CrossRef]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangervari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Agosti, R. Migraine burden of disease: From the patient’s experience to a socio-economic view. Headache J. Head. Face Pain. 2018, 58, 17–32. [Google Scholar] [CrossRef]
- Kikui, S.; Chen, Y.; Todaka, H.; Asao, K.; Adachi, K.; Takeshima, T. Burden of migraine among Japanese patients: A cross-sectional National Health and Wellness Survey. J. Headache Pain 2020, 21, 110. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Constantin, L.; Ebel-Bitoun, C.; Turudic, I.I.; Hitier, S.; Amand-Bourdon, C.; Stewart, A. Multinational descriptive analysis of the real-world burden of headache using the Migraine Buddy application. Eur. J. Neurol. 2021, 28, 4184–4193. [Google Scholar] [CrossRef]
- Ford, J.H.; Jackson, J.; Milligan, G.; Cotton, S.; Ahl, J.; Aurora, S. A Real-World Analysis of Migraine: A Cross-Sectional Study of Disease Burden and Treatment Patterns. Headache 2017, 57, 1532–1544. [Google Scholar] [CrossRef]
- Wöber-Bingöl, Ç.; Wöber, C.; Uluduz, D.; Uygunoğlu, U.; Aslan, T.S.; Kernmayer, M.; Zesch, H.-E.; Gerges, N.T.A.; Wagner, G.; Siva, A.; et al. The global burden of headache in children and adolescents—Developing a questionnaire and methodology for a global study. J. Headache Pain 2014, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Genc, D.; Vaičienė-Magistris, N.; Zaborskis, A.; Şaşmaz, T.; Tunç, A.Y.; Uluduz, D.; Wöber, C.; Wöber-Bingöl, Ç.; Steiner, T.J. The burden attributable to headache disorders in children and adolescents in Lithuania: Estimates from a national schools-based study. J. Headache Pain 2021, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, Y.; Ueda, K.; Komori, M.; Zagar, A.J.; Kim, Y.; Jaffe, D.H.; Takeshima, T.; Hirata, K. Burden of Migraine in Japan: Results of the ObserVational Survey of the Epidemiology, tReatment, and Care Of MigrainE (OVERCOME [Japan]) Study. Neurol. Ther. 2022, 11, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, M.; Matsumori, Y.; Kawahara, J.; Yamagishi, C.; Koh, A.; Kawamura, S.; Kashiwagi, K.; Kito, T.; Oguri, M.; Mizuno, S.; et al. School-based online survey on chronic headache, migraine, and medication-overuse headache prevalence among children and adolescents in Japanese one city-Itoigawa Benizuwaigani study. Clin. Neurol. Neurosurg. 2023, 226, 107610. [Google Scholar] [CrossRef] [PubMed]
- Philipp, J.; Zeiler, M.; Wöber, C.; Wagner, G.; Karwautz, A.F.K.; Steiner, T.J.; Wöber-Bingöl, Ç. Prevalence and burden of headache in children and adolescents in Austria—A nationwide study in a representative sample of pupils aged 10–18 years. J. Headache Pain 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Headache Clinical Practice Guideline Development Committee. Clinical Practice Guideline for Headache Disorders 2021 (Japanese); Igaku-Shoin: Tokyo, Japan, 2021. [Google Scholar]
- Katsuki, M.; Kashiwagi, K.; Kawamura, S.; Koh, A. The efficacy of Japanese herbal kampo medicine as an acute and prophylactic medication to treat chronic daily headache and medication overuse headache: -Single arm retrospective study. Cureus 2022, 14, e25419. [Google Scholar] [CrossRef]
- Katsuki, M.; Kawahara, J.; Matsumori, Y.; Yamagishi, C.; Koh, A.; Kawamura, S.; Kashiwagi, K.; Kito, T.; Entani, A.; Yamamoto, T.; et al. Questionnaire-based survey during COVID-19 vaccination on the prevalence of elderly’s migraine, chronic daily headache, and medication-overuse headache in one Japanese city—Itoigawa Hisui Study. J. Clin. Med. 2022, 11, 4707. [Google Scholar] [CrossRef]
- Roxas, A.; Quiles, L.E.; Wang, S.-J. Delivery of care for migraine in the Asian Oceanian region: A cross-sectional study. Cephalalgia 2021, 41, 1348–1358. [Google Scholar] [CrossRef]
- Woldeamanuel, Y.W. Headache in resource-Limited settings. Curr. Pain. Headache Rep. 2017, 21, 51. [Google Scholar] [CrossRef]
- Katsarava, Z.; Mania, M.; Lampl, C.; Herberhold, J.; Steiner, T.J. Poor medical care for people with migraine in Europe—Evidence from the Eurolight study. J. Headache Pain 2018, 19, 10. [Google Scholar] [CrossRef]
- Lipton, R.B.; Nicholson, R.A.; Reed, M.L.; Araujo, A.B.; Jaffe, D.H.; Faries, D.E.; Buse, D.C.; Shapiro, R.E.; Ashina, S.; Cambron-Mellott, M.J.; et al. Diagnosis, consultation, treatment, and impact of migraine in the US: Results of the OVERCOME (US) study. Headache J. Head Face Pain 2022, 62, 122–140. [Google Scholar] [CrossRef] [PubMed]
- Dekker, F.; Wiendels, N.J.; De Valk, V.; Van Der Vliet, C.; Neven, A.K.; Assendelft, W.J.J.; Ferrari, M.D. Triptan overuse in the Dutch general population: A nationwide pharmaco-epidemiology database analysis in 6.7 million people. Cephalalgia 2011, 31, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Huber, C.A.; Agosti, R.; Näpflin, M.; Blozik, E. Treatment patterns in patients using triptan and prophylactic medication: An analysis of clinical practice prior to the introduction of CGRP antagonists. J. Pain Res. 2019, 12, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Zebenholzer, K.; Gall, W.; Wöber, C. Use and overuse of triptans in Austria—A survey based on nationwide healthcare claims data. J. Headache Pain 2018, 19, 34 . [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Adornetto, A.; Rombolà, L.; Naturale, M.D.; De Francesco, A.E.; Esposito, S.; Zito, M.; Morrone, L.A.; Bagetta, G.; Tonin, P.; et al. Pattern of triptans use: A retrospective prescription study in Calabria, Italy. Neural Regen. Res. 2020, 15, 1340–1343. [Google Scholar] [PubMed]
- Da Cas, R.; Nigro, A.; Terrazzino, S.; Sances, G.; Viana, M.; Tassorelli, C.; Nappi, G.; Cargnin, S.; Pisterna, A.; Traversa, G.; et al. Triptan use in Italy: Insights from administrative databases. Cephalalgia 2015, 35, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, D.; Donnet, A.; Pradel, V.; Sciortino, V.; Allaria-Lapierre, V.; Lantéri-Minet, M.; Micallef, J. Triptans use and overuse: A pharmacoepidemiology study from the French health insurance system database covering 4.1 million people. Cephalalgia 2015, 35, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.L.; Davis, K.L.; Lenz, R.A.; Sakai, F.; Xue, F. Treatment patterns and characteristics of patients with migraine in Japan: A retrospective analysis of health insurance claims data. Cephalalgia 2019, 39, 1518–1534. [Google Scholar] [CrossRef]
- Sakai, F.; Hirata, K.; Igarashi, H.; Takeshima, T.; Nakayama, T.; Sano, H.; Kondo, H.; Shibasaki, T.; Koga, N. A study to investigate the prevalence of headache disorders and migraine among people registered in a health insurance association in Japan. J. Headache Pain 2022, 23, 70. [Google Scholar] [CrossRef]
- Hirata, K.; Sano, H.; Kondo, H.; Shibasaki, Y.; Koga, N. Clinical characteristics, medication use, and impact of primary headache on daily activities: An observational study using linked online survey and medical claims data in Japan. BMC Neurol. 2023, 23, 80. [Google Scholar] [CrossRef]
- Obermeier, V.; Murawski, M.; Heinen, F.; Landgraf, M.N.; Straube, A.; Von Kries, R.; Ruscheweyh, R. Total health insurance costs in children with a migraine diagnosis compared to a control group. J. Headache Pain 2021, 22, 140. [Google Scholar] [CrossRef] [PubMed]
- Law, E.F.; Palermo, T.M.; Zhou, C.; Groenewald, C.B. Economic Impact of Headache and Psychiatric Comorbidities on Healthcare Expenditures Among Children in the United States: A Retrospective Cross-Sectional Study. Headache 2019, 59, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, M.; Matsumori, Y.; Kawahara, J.; Yamagishi, C.; Koh, A.; Kawamura, S.; Kawashiwagi, K.; Kito, T.; Oguri, M.; Mizuno, S.; et al. Headache education by leaflet distribution during COVID-19 vaccination and school-based on-demand e-learning: Itoigawa Geopark Headache Awareness Campaign. Headache J. Head Face Pain 2023, 63, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, T.; Ueda, K.; Komori, M.; Zagar, A.Z.; Kim, Y.; Jaffe, D.H.; Matsumori, Y.; Hirata, K. Potential unmet needs in acute treatment of migraine in Japan: Results of the OVERCOME (Japan) study. Adv. Ther. 2022, 39, 5176–5190. [Google Scholar] [CrossRef] [PubMed]
- Sakai, F.; Igarashi, H.; Yokoyama, M.; De Dahaem, O.B.; Kato, H.; Azuma, Y.; Koh, R.; Phillips, H.; Singh, N.; Craven, A.; et al. Diagnosis, knowledge, perception, and productivity impact of headache education and clinical evaluation program in the workplace at an information technology company of more than 70,000 employees. Cephalalgia 2023, 43, 3331024231165682. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Sakai, F.; Miyake, H.; Sone, T.; Sato, M.; Tanabe, S.; Azuma, Y.; Dodick, D.W. Disability, quality of life, productivity impairment and employer costs of migraine in the workplace. J. Headache Pain 2021, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Wöber-Bingöl, C. Epidemiology of migraine and headache in children and adolescents. Curr. Pain. Headache Rep. 2013, 17, 341. [Google Scholar] [CrossRef]
- Zewde, Y.Z.; Zebenigus, M.; Demissie, H.; Tekle-Haimanot, R.; Uluduz, D.; Şaşmaz, T.; Bozdag, F.; Steiner, T.J. The prevalence of headache disorders in children and adolescents in Ethiopia: A schools-based study. J. Headache Pain 2020, 21, 108. [Google Scholar] [CrossRef]
- Luvsannorov, O.; Anisbayar, T.; Davaasuren, M.; Baatar, O.; Batmagnai, K.; Tumurbaatar, K.; Enkhbaatar, S.; Uluduz, D.; Şaşmaz, T.; Solmaz, E.T.; et al. The prevalence of headache disorders in children and adolescents in Mongolia: A nationwide schools-based study. J. Headache Pain 2020, 21, 107. [Google Scholar] [CrossRef]
- Genc, D.; Vaičienė-Magistris, N.; Zaborskis, A.; Şaşmaz, T.; Tunç, A.Y.; Uluduz, D.; Steiner, T.J. The prevalence of headache disorders in children and adolescents in Lithuania: A schools-based study. J. Headache Pain 2020, 21, 73. [Google Scholar] [CrossRef]
- Kawatu, N.; Wa Somwe, S.; Ciccone, O.; Mukanzu, M.; Uluduz, D.; Şaşmaz, T.; Yalçın, B.N.B.; Wöber, C.; Steiner, T.J. The prevalence of primary headache disorders in children and adolescents in Zambia: A schools-based study. J. Headache Pain 2022, 23, 118. [Google Scholar] [CrossRef] [PubMed]
- Wöber, C.; Wöber-Bingöl, Ç.; Uluduz, D.; Aslan, T.S.; Uygunoglu, U.; Tüfekçi, A.; Alp, S.I.; Duman, T.; Sürgün, F.; Emir, G.K.; et al. Undifferentiated headache: Broadening the approach to headache in children and adolescents, with supporting evidence from a nationwide school-based cross-sectional survey in Turkey. J. Headache Pain 2018, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Togha, M.; Rafiee, P.; Ghorbani, Z.; Khosravi, A.; Şaşmaz, T.; Kale, D.A.; Uluduz, D.; Steiner, J.T. The prevalence of headache disorders in children and adolescents in Iran: A schools-based study. Cephalalgia 2022, 42, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, M.; Shimazu, T.; Kikui, S.; Danno, D.; Miyahara, J.; Takeshima, R.; Takeshima, E.; Shimazu, Y.; Nakashima, T.; Matsuo, M.; et al. Developing an artificial intelligence-based headache diagnostic model and its utility for non-specialists’ diagnostic accuracy. Cephalalgia 2023, 43, 033310242311569. [Google Scholar] [CrossRef] [PubMed]
- Cohen, F. The role of artificial intelligence in headache medicine: Potential and peril. Headache 2023, 63, 694–696. [Google Scholar] [CrossRef]
- Sasaki, S.; Katsuki, M.; Kawahara, J.; Yamagishi, C.; Koh, A.; Kawamura, S.; Kashiwagi, K.; Ikeda, T.; Goto, T.; Kaneko, K.; et al. Developing an artificial intelligence-based pediatric and adolescent migraine diagnostic model. Cureus 2023, 15, e44415. [Google Scholar] [CrossRef]
- Eli Lilly and Company. A Study of Galcanezumab (LY2951742) in Participants 6 to 17 Years of Age with Episodic Migraine (REBUILD-1). ClinicalTrials.gov, NCT03432286. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03432286 (accessed on 10 October 2023).
- Szperka, C.L.; VanderPluym, J.; Orr, S.L.; Oakley, C.B.; Qubty, W.; Patniyot, I.; Lagman-Bartolome, A.M.; Morris, C.; Gautreaux, J.; Victorio, M.C.; et al. Recommendations on the use of anti-CGRP monoclonal antibodies in children and adolescents. Headache 2018, 58, 1658–1669. [Google Scholar] [CrossRef]
- Ishii, R.; Schwedt, T.J.; Trivedi, M.; Dumkrieger, G.; Cortex, M.M.; Brennan, K.C.; Digre, K.; Dodick, D.W. Mild traumatic brain injury affects the features of migraine. J. Headache Pain 2021, 22, 80. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Matsushige, T.; Kawano, R.; Yoshiyama, M.; Hara, T.; Kobayashi, S.; Ono, C.; Sakamoto, S.; Horie, N. Headache characteristics to screen for cervicocerebral artery dissection in patients with acute onset unusual headache. Headache 2023, 63, 283–289. [Google Scholar] [CrossRef]
- Kano, Y.; Inui, S.; Oguri, T.; Kato, H.; Yuasa, H.; Morimot, S.; Sakurai, K. Utility of T2-weighted high-resolution vessel wall imaging for the diagnosis of isolated posterior inferior cerebellar artery dissection at acute and early subacute stages. J. Neurol. Sci. 2020, 411, 116693. [Google Scholar] [CrossRef]
- Fukazawa, R.; Ishii, R.; Higashimoto, Y.; Hanya, M.; Shimizu, Y.; Shinomoto, M.; Fujii, A.; Mizuno, T. Zinc administration favorably affects prophylactic therapy-refractory migraine attacks: A case series. Intern. Med. 2023, 6, 2111–2123. [Google Scholar] [CrossRef]
- Hepp, Z.; Dodick, D.W.; Varon, S.F.; Chia, J.; Matthew, N.; Gillard, P.; Hansen, R.N.; Devine, E.B. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: A retrospective claims analysis. Cephalalgia 2017, 37, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Hepp, Z.; Dodick, D.W.; Varon, S.F.; Gillard, P.; Hansen, R.N.; Devine, E.B. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia 2015, 35, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Woolley, J.M.; Bonafede, M.M.; Maiese, B.A.; Lenz, R.A. Migraine prophylaxis and acute treatment patterns among commercially insured patients in the United States. Headache 2017, 57, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.W.; Szépligeti, S.K.; Xue, F.; Pedersen, L.; Sørensen, H.T.; Ehrenstein, V. Patterns of initial migraine treatment in Denmark: A population-based study. Pharmacoepidemiol. Drug Saf. 2019, 28, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Korolainen, M.A.; Kurki, S.; Lassenius, M.I.; Toppila, I.; Costa-Scharplatz, M.; Purmonen, T.; Nissilä, M. Burden of migraine in Finland: Health care resource use, sick-leaves and comorbidities in occupational health care. J. Headache Pain 2019, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Perrone, V.; Veronesi, C.; Giacomini, E.; Andretta, M.; Dell’Orco, S.; De Sarro, G.; Lena, F.; Menti, A.M.; Naclerio, M.; Ritrovato, D.; et al. Treatment patterns, health resource consumption, and costs of patients with migraine in an Italian real-world setting. Curr. Med. Res. Opin. 2020, 36, 1991–1998. [Google Scholar] [CrossRef]
- Ojo, A.; Zhang, S.; Bleibdrey, N.; Zimskind, D.; Keshvanni, N.; Chalmers, R. Persistence and switching patterns of migraine prophylactic medications in Canada: A retrospective claims analysis comparing adherence and evaluating the economic burden of illness. J. Pharm. Pharm. Sci. 2023, 25, 402–417. [Google Scholar] [CrossRef]
- Ihara, K.; Ohtani, S.; Watanabe, N.; Takahashi, N.; Miyazaki, N.; Ishizuchi, K.; Hori, S.; Takemura, R.; Nakahara, J.; Takizawa, T. Predicting response to CGRP-monoclonal antibodies in patients with migraine in Japan: A single-centre retrospective observational study. J. Headache Pain 2023, 24, 23. [Google Scholar] [CrossRef]
- Katsuki, M.; Kashiwagi, K.; Kawamura, S.; Koh, A. Monoclonal antibodies against the calcitonin gene-related peptide and its receptor in Japanese adolescents with migraines. Cureus 2023, 15, e33689. [Google Scholar] [CrossRef]
- Tsai, M.; Nery, E.S.M.; Kerr, L.; Khanna, R.; Komori, M.; Dennehy, E.B.; Wilbraham, D.; Winner, P. Pharmacokinetics, safety, and tolerability of lasmiditan in pediatric patients with migraine. Clin. Pharmacokinet. 2021, 60, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Loh, N.R.; Whitehouse, W.P.; Howells, R. What is new in migraine management in children and young people? Arch. Dis. Child. 2022, 107, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall | %/SD | A w/o P | %/SD | T w/o P | %/SD | A w/P | %/SD | T w/P | %/SD |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients number | 624 | 100% | 607 | 97.28% | 4 | 0.64% | 17 | 2.72% | 1 | 0.16% |
| Number of triptan overprescriptions (%) | 1 | 0.11% | 1 | 0.17% | 1 | 25.00% | 0 | 0% | 0 | 0% |
| Age (mean, SD) | 8.83 | 1.67 | 8.81 | 1.67 | 10.25 | 0.25 | 9.47 | 1.67 | 10 | - |
| Sex: Female | 314 | 50.32% | 305 | 50.25% | 3 | 25.00% | 9 | 52.94% | 1 | 100% |
| Acute treatment | ||||||||||
| Combination NSAIDs | 1 | 0.16% | 1 | 0.17% | - | - | 0 | 0% | - | - |
| Single NSAIDs | 618 | 99.04% | 602 | 99.18% | - | - | 16 | 94.12% | - | - |
| Triptans | 3 | 0.48% | 2 | 0.33% | 2 | 50.00% | 1 | 5.88% | 1 | 100% |
| Single NSAIDs and triptans | 2 | 0.32% | 2 | 0.33% | 2 | 50.00% | 0 | 0% | - | - |
| Prophylactic treatment | 17 | 2.72% | - | - | - | - | 17 | 100% | 1 | 100% |
| Calcium-channel blockers (lomerizine) | 1 | 0.16% | - | - | - | - | 1 | 5.88% | - | - |
| Antidepressants (amitriptyline) | 1 | 0.16% | - | - | - | - | 1 | 5.88% | - | - |
| Japanese herbal Kampo medicine | 14 | 2.24% | - | - | - | - | 14 | 82.35% | 1 | 100% |
| Anticonvulsants and Kampo medicine | 1 | 0.16% | - | - | - | - | 1 | 5.88% | - | - |
| Characteristics | Overall | %/SD | A w/o P | %/SD | T w/o P | %/SD | A w/P | %/SD | T w/P | %/SD |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients number | 900 | 100% | 824 | 91.56% | 63 | 7.00% | 76 | 8.44% | 28 | 3.11% |
| Number of combination NSAID overprescriptions (%) | 6 | 0.67% | 5 | 0.61% | 0 | 0.00% | 0 | 0.00% | 1 | 3.57% |
| Number of triptan overprescriptions (%) | 10 | 1.11% | 5 | 0.61% | 5 | 7.94% | 5 | 6.58% | 5 | 17.86% |
| Age (mean, SD) | 14.25 | 1.66 | 14.20 | 1.65 | 14.16 | 1.63 | 14.76 | 1.65 | 15.11 | 1.63 |
| Sex: Female | 479 | 53.22% | 428 | 51.94% | 39 | 61.91% | 51 | 67.11% | 20 | 71.43% |
| Acute treatment | ||||||||||
| Combination NSAIDs | 13 | 1.44% | 10 | 1.21% | - | - | 3 | 3.95% | - | - |
| Single NSAIDs | 793 | 88.11% | 749 | 90.90% | - | - | 44 | 57.90% | - | - |
| Triptans | 55 | 6.11% | 42 | 5.10% | 42 | 66.67% | 13 | 17.11% | 13 | 46.43% |
| Combination NSAIDs and single NSAIDs | 3 | 0.33% | 2 | 0.24% | - | - | 1 | 1.32% | - | - |
| Single NSAIDs and triptans | 31 | 3.44% | 18 | 2.18% | 18 | 28.57% | 13 | 17.11% | 13 | 46.43% |
| All 3 types | 5 | 0.56% | 3 | 0.36% | 3 | 4.76% | 2 | 2.63% | 2 | 7.14% |
| Prophylactic treatment | 76 | 8.44% | - | - | - | - | 76 | 100% | 100% | |
| Calcium-channel blockers (lomerizine) | 17 | 1.89% | - | - | - | - | 17 | 22.37% | 10 | 35.71% |
| Beta-blockers (propranolol) | 1 | 0.11% | - | - | - | - | 1 | 1.32% | 1 | 3.57% |
| Anticonvulsants (valproic acid) | 15 | 1.67% | - | - | - | - | 15 | 19.74% | 5 | 17.67% |
| Antidepressants (amitriptyline) | 3 | 0.33% | - | - | - | - | 3 | 3.95% | 3 | 10.71% |
| Japanese herbal Kampo medicine | 27 | 3.00% | - | - | - | - | 27 | 35.53% | 1 | 3.57% |
| Combination of 2 types | 12 | 1.33% | - | - | - | - | 12 | 15.79% | 28.57% | |
| Combination of 4 types | 1 | 0.11% | - | - | - | - | 1 | 1.32% | 0% |
| Characteristics | Overall | %/SD | 0–11 tbl/90 d | %/SD | 12–29 tbl/90 d | %/SD | 30–44 tbl/90 d | %/SD | 45– tbl/90 d | %/SD |
|---|---|---|---|---|---|---|---|---|---|---|
| n (all = 1524) | 96 | 100% | 64 | 66.67% | 21 | 21.88% | 9 | 9.38% | 2 | 2.08% |
| Number of tbl/90 d | 22.77 | 21.58 | 6.78 | 2.85 | 19571 | 5.56 | 35.75 | 5.06 | 62.50 | - |
| Age (mean, SD) | 14.23 | 1.80 | 14.14 | 1.72 | 14.43 | 1.71 | 14.44 | 1.77 | 14.00 | - |
| Sex: Female | 60 | 62.50% | 38 | 59.38% | 11 | 52.38% | 9 | 100% | 2 | 100% |
| Acute treatment | ||||||||||
| Triptans | 58 | 60.42% | 40 | 62.50% | 14 | 66.67% | 3 | 33.33% | 1 | 50% |
| Single NSAIDs and triptans | 33 | 34.38% | 21 | 32.81% | 5 | 23.81% | 6 | 66.67% | 1 | 50% |
| All 3 types | 5 | 5.21% | 3 | 4.69% | 2 | 9.52% | 0 | 0% | 0 | 0% |
| Prophylactic treatment | 29 | 30.21% | 9 | 14.06% | 15 | 71.43% | 4 | 44.44% | 1 | 50% |
| Calcium-channel blockers (lomerizine) | 10 | 10.42% | 4 | 6.25% | 4 | 19.05% | 1 | 11.11% | 1 | 50% |
| Beta-blockers (propranolol) | 1 | 1.04% | 1 | 1.56% | 0 | 0% | 0 | 0% | 0 | 0% |
| Anticonvulsants (valproic acid) | 5 | 5.21% | 1 | 1.56% | 4 | 19.05% | 0 | 0% | 0 | 0% |
| Antidepressants (amitriptyline) | 3 | 3.13% | 1 | 1.56% | 0 | 0% | 2 | 22.22% | 0 | 0% |
| Japanese herbal Kampo medicine | 2 | 2.08% | 2 | 3.13% | 0 | 0% | 0 | 0% | 0 | 0% |
| Combination of 2 types | 8 | 8.33% | 0 | 0% | 7 | 33.33% | 1 | 11.11% | 0 | 0% |
| Variables | 1–3 m | 4–6 m | 7–9 m | 10–12 m | 13–15 m | 16–18 m | 19–21 m | 22–24 m |
|---|---|---|---|---|---|---|---|---|
| No prescription | 86.70% | 89.95% | 88.70% | 87.98% | 88.05% | 88.18% | 86.63% | 86.58% |
| Acute treatment | 12.92% | 9.68% | 10.90% | 11.55% | 11.43% | 11.28% | 12.78% | 12.79% |
| Single NSAIDs | 12.88% | 9.65% | 10.84% | 11.50% | 11.37% | 11.19% | 12.68% | 12.69% |
| Combination NSAIDs | 0.02% | 0.01% | 0.02% | 0.01% | 0.01% | 0.03% | 0.03% | 0.03% |
| Triptans | 0.05% | 0.05% | 0.06% | 0.05% | 0.08% | 0.11% | 0.13% | 0.14% |
| Combination NSAIDs ≥ 30 tbl/90 d | 0.01% | 0% | 0% | 0% | 0% | 0% | 0.01% | 0% |
| Triptans ≥ 30 tbl/90 d | 0.01% | 0% | 0% | 0.01% | 0% | 0.01% | 0.01% | 0% |
| Both acute and prophylactic treatments | 0.16% | 0.17% | 0.20% | 0.18% | 0.26% | 0.23% | 0.24% | 0.31% |
| Prophylactic treatment | 0.54% | 0.53% | 0.61% | 0.64% | 0.78% | 0.77% | 0.83% | 0.94% |
| Calcium-channel blockers (lomerizine) | 0.01% | 0.01% | 0.01% | 0.02% | 0.03% | 0.03% | 0.04% | 0.06% |
| Beta-blockers (propranolol) | 0.01% | 0.01% | 0.00% | 0.01% | 0.01% | 0.02% | 0.01% | 0.02% |
| Anticonvulsants (valproic acid) | 0.03% | 0.07% | 0.10% | 0.11% | 0.15% | 0.19% | 0.23% | 0.26% |
| Antidepressants (amitriptyline) | 0.02% | 0.02% | 0.02% | 0.02% | 0.03% | 0.03% | 0.03% | 0.04% |
| Japanese herbal Kampo medicine | 0.48% | 0.43% | 0.49% | 0.49% | 0.58% | 0.51% | 0.54% | 0.59% |
| Variables | 1–3 m | 4–6 m | 7–9 m | 10–12 m | 13–15 m | 16–18 m | 19–21 m | 22–24 m |
|---|---|---|---|---|---|---|---|---|
| No prescription | 87.91% | 92.17% | 91.25% | 90.87% | 90.91% | 90.89% | 90.56% | 90.47% |
| Acute treatment | 11.66% | 7.38% | 8.20% | 8.54% | 8.39% | 8.46% | 8.69% | 8.74% |
| Single NSAIDs | 11.32% | 7.13% | 7.90% | 8.21% | 8.03% | 8.04% | 8.26% | 8.35% |
| Combination NSAIDs | 0.14% | 0.09% | 0.08% | 0.09% | 0.11% | 0.11% | 0.14% | 0.12% |
| Triptans | 0.33% | 0.28% | 0.38% | 0.36% | 0.41% | 0.44% | 0.48% | 0.47% |
| Combination NSAIDs ≥ 30 tbl/90 d | 0.02% | 0.01% | 0.01% | 0.01% | 0.02% | 0.03% | 0.02% | 0.02% |
| Triptans ≥ 30 tbl/90 d | 0.00% | 0.00% | 0.02% | 0.02% | 0.02% | 0.02% | 0.02% | 0.01% |
| Both acute and prophylactic treatments | 0.32% | 0.27% | 0.32% | 0.41% | 0.37% | 0.47% | 0.47% | 0.52% |
| Prophylactic treatment | 0.75% | 0.71% | 0.88% | 1.00% | 1.07% | 1.12% | 1.23% | 1.31% |
| Calcium-channel blockers (lomerizine) | 0.06% | 0.08% | 0.14% | 0.16% | 0.15% | 0.17% | 0.18% | 0.24% |
| Beta-blockers (propranolol) | 0.01% | 0.02% | 0.03% | 0.04% | 0.05% | 0.05% | 0.04% | 0.05% |
| Anticonvulsants (valproic acid) | 0.08% | 0.13% | 0.19% | 0.24% | 0.26% | 0.32% | 0.37% | 0.39% |
| Antidepressants (amitriptyline) | 0.02% | 0.02% | 0.04% | 0.07% | 0.09% | 0.09% | 0.08% | 0.08% |
| Calcium-channel blockers (verapamil) | 0% | 0% | 0% | 0.01% | 0.01% | 0.01% | 0.01% | 0.01% |
| Japanese herbal Kampo medicine | 0.60% | 0.50% | 0.52% | 0.54% | 0.58% | 0.57% | 0.62% | 0.64% |
| 6–11-Year-Olds, n = 43,621, Event = 12 (0.0275%) | B | SE | Wald | p-Value | Odds | 95%CI (Lower) | (Upper) |
| Female | 0.04 | 0.68 | 0.00 | 0.960 | 1.04 | 0.26 | 3.90 |
| Age (y.o.) | 0.40 | 0.22 | 3.15 | 0.076 | 1.49 | 0.96 | 2.30 |
| Presence of a prescription of single NSAID during the first 90 days | −0.27 | 1.06 | 0.06 | 0.803 | 0.77 | 0.10 | 6.15 |
| Combination NSAIDs during the first 90 days (tbl/90 d) | −5.86 | 14,794.23 | 0.00 | 0.999 | 0.00 | 0.00 | Inf. |
| Triptans during the first 90 days (tbl/90 d) | 0.46 | 0.06 | 63.94 | <0.001 | 1.58 | 1.41 | 1.77 |
| Presence of a prophylactic treatment prescription during the first 90 days | −12.83 | 674.21 | 0.00 | 0.985 | 0.00 | 0.00 | Inf. |
| 12–17-Year-Olds, n = 37,135, Event = 32 (0.0862%) | B | SE | Wald | p-Value | Odds | 95%CI (Lower) | (Upper) |
| Female | 0.60 | 0.45 | 1.81 | 0.178 | 1.82 | 0.76 | 4.38 |
| Age (y.o.) | −1.47 | 51.40 | 0.00 | 0.98 | 0.23 | 0.00 | 1.30 × 1043 |
| Presence of a prescription of a single NSAID during the first 90 days | 0.16 | 0.03 | 40.79 | <0.001 | 1.17 | 1.12 | 1.23 |
| Combination NSAIDs during the first 90 days (tbl/90 d) | 2.39 | 0.58 | 17.07 | <0.001 | 10.87 | 3.50 | 33.70 |
| Triptans during the first 90 days (tbl/90 d) | 0.48 | 0.40 | 1.47 | 0.225 | 1.62 | 0.74 | 3.51 |
| Presence of a prophylactic treatment prescription during the first 90 days | −0.02 | 0.11 | 0.05 | 0.824 | 0.98 | 0.79 | 1.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsuki, M.; Matsumori, Y.; Ichihara, T.; Yamada, Y.; Kawamura, S.; Kashiwagi, K.; Koh, A.; Goto, T.; Kaneko, K.; Wada, N.; et al. Treatment Patterns for and Characteristics of Headache in Children and Adolescents Aged 6–17 Years in Japan: A Retrospective Cross-Sectional and Longitudinal Analysis of Health Insurance Claims Data. Life 2024, 14, 96. https://doi.org/10.3390/life14010096
Katsuki M, Matsumori Y, Ichihara T, Yamada Y, Kawamura S, Kashiwagi K, Koh A, Goto T, Kaneko K, Wada N, et al. Treatment Patterns for and Characteristics of Headache in Children and Adolescents Aged 6–17 Years in Japan: A Retrospective Cross-Sectional and Longitudinal Analysis of Health Insurance Claims Data. Life. 2024; 14(1):96. https://doi.org/10.3390/life14010096
Chicago/Turabian StyleKatsuki, Masahito, Yasuhiko Matsumori, Taisuke Ichihara, Yuya Yamada, Shin Kawamura, Kenta Kashiwagi, Akihito Koh, Tetsuya Goto, Kazuma Kaneko, Naomichi Wada, and et al. 2024. "Treatment Patterns for and Characteristics of Headache in Children and Adolescents Aged 6–17 Years in Japan: A Retrospective Cross-Sectional and Longitudinal Analysis of Health Insurance Claims Data" Life 14, no. 1: 96. https://doi.org/10.3390/life14010096
APA StyleKatsuki, M., Matsumori, Y., Ichihara, T., Yamada, Y., Kawamura, S., Kashiwagi, K., Koh, A., Goto, T., Kaneko, K., Wada, N., & Yamagishi, F. (2024). Treatment Patterns for and Characteristics of Headache in Children and Adolescents Aged 6–17 Years in Japan: A Retrospective Cross-Sectional and Longitudinal Analysis of Health Insurance Claims Data. Life, 14(1), 96. https://doi.org/10.3390/life14010096








