Selection of a Digitalis purpurea Cell Line with Improved Bioconversion Capacity of Hydroquinone into Arbutin
Abstract
1. Introduction
2. Materials and Methods
2.1. Digitalis Purpurea Cell Cultures
2.2. Hydroquinone Feeding Experiments
2.3. Carbon Source Experiments
2.4. Cell Growth
2.5. Arbutin Extraction and Quantification
2.6. Experimental Design
2.7. Statistical Analysis
3. Results
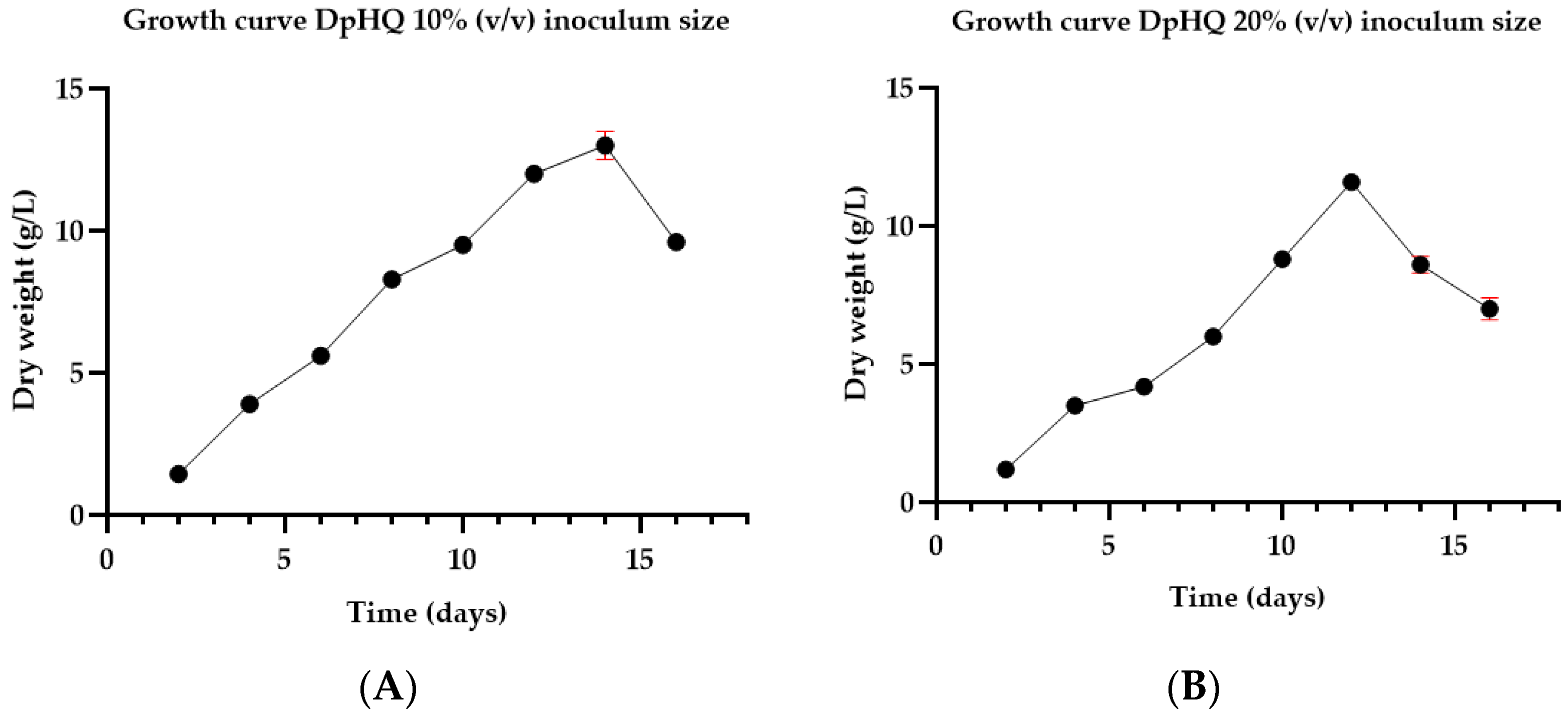
3.1. Growth Profile
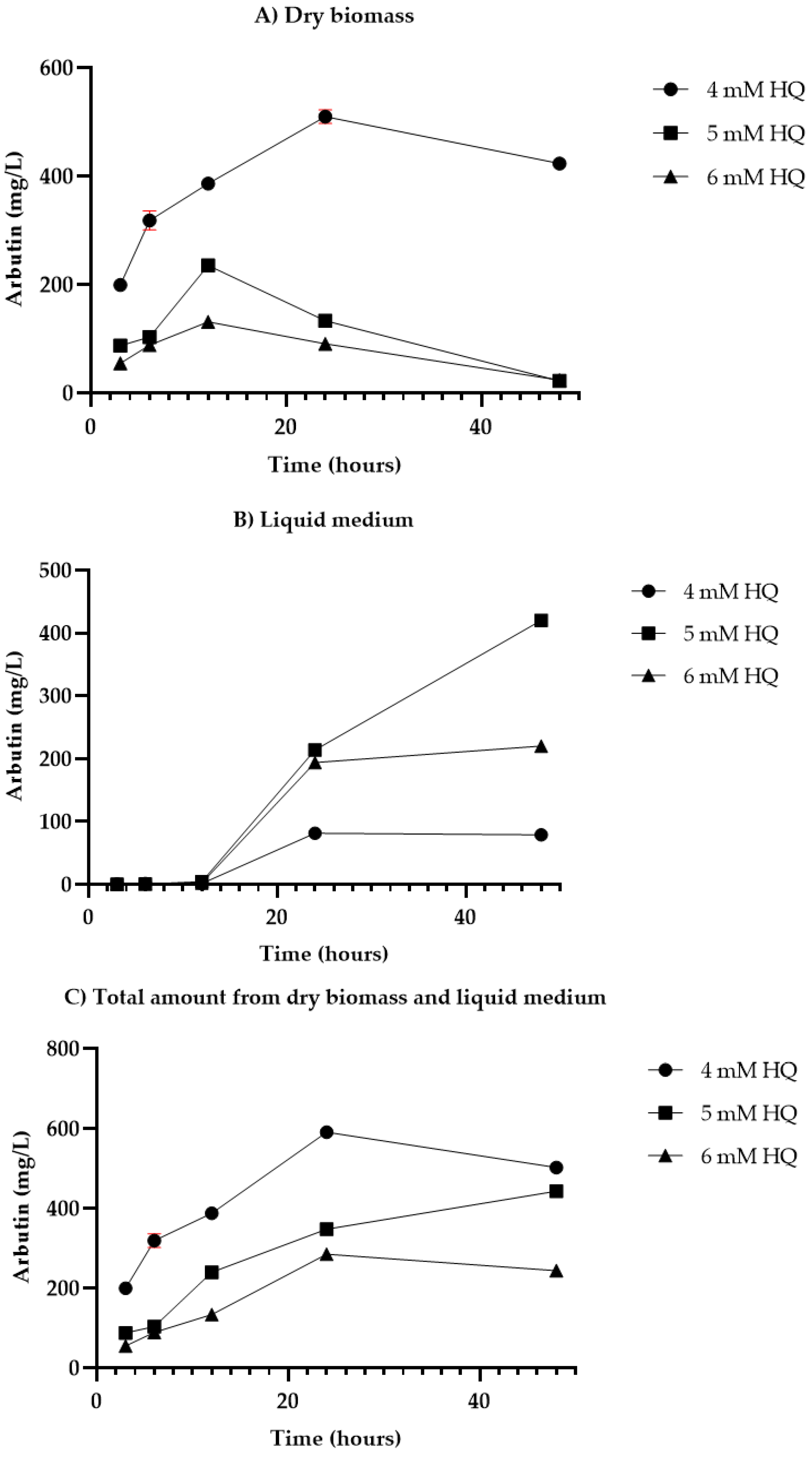
3.2. Feeding Protocols Outcomes
3.3. Biotransformation Experiments Results
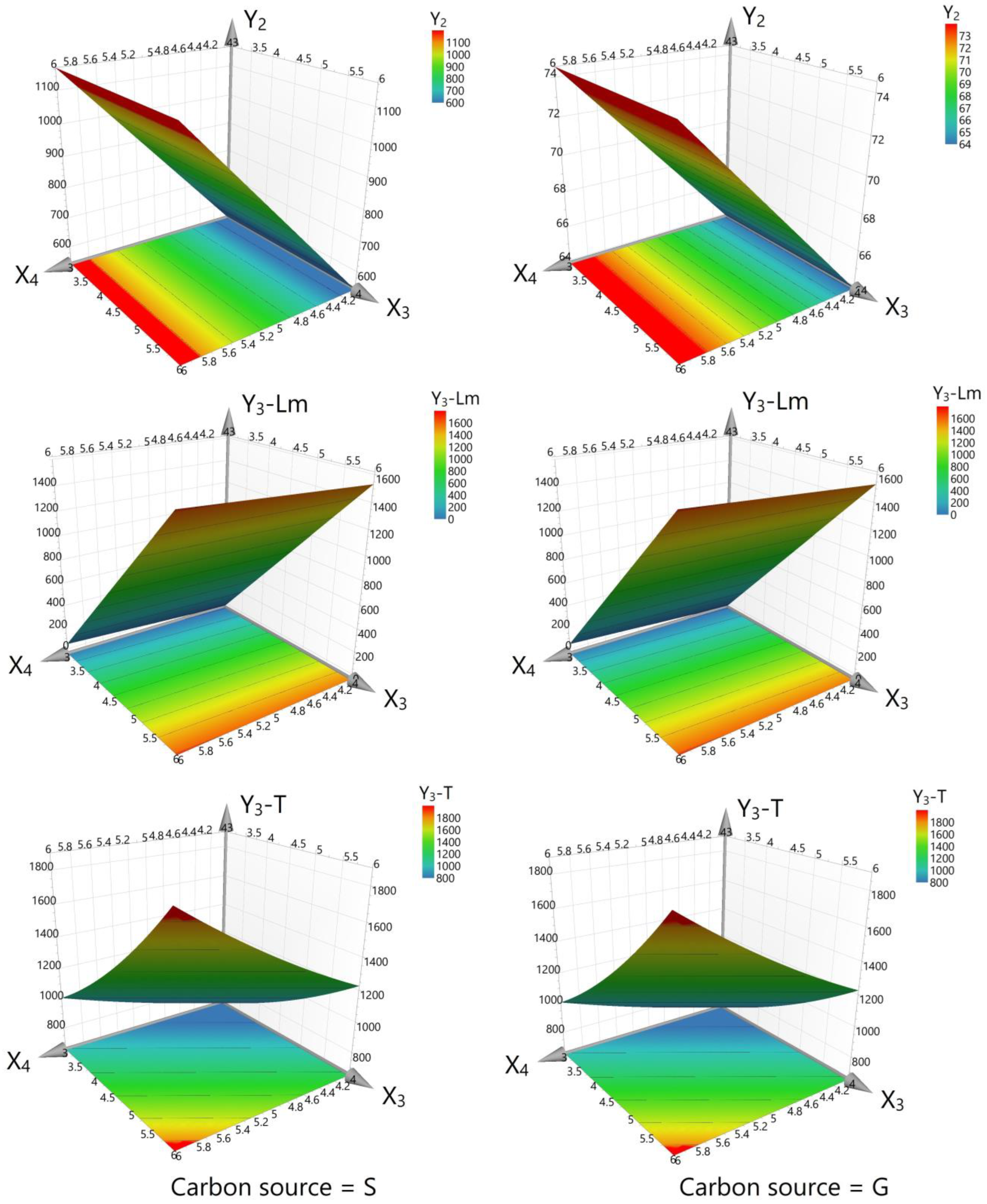
3.4. Outcomes of Fitting the Data with the Models
4. Discussion
4.1. Growth Profile
4.2. Biotransformation Experiments
4.3. Interpretation of the Experimental Design Results after Fitting the Data with the Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boo, Y.C. Arbutin as a Skin Depigmenting Agent with Antimelanogenic and Antioxidant Properties. Antioxidants 2021, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Asensio, E.; Vitales, D.; Pérez, I.; Peralba, L.; Viruel, J.; Montaner, C.; Vallès, J.; Garnatje, T.; Sales, E. Phenolic Compounds Content and Genetic Diversity at Population Level across the Natural Distribution Range of Bearberry (Arctostaphylos uva-ursi, Ericaceae) in the Iberian Peninsula. Plants 2020, 9, 1250. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Nakamura, K.; Ma, L.; Li, J.-Z.; Kayahara, H. Analyses of Arbutin and Chlorogenic Acid, the Major Phenolic Constituents in Oriental Pear. J. Agric. Food Chem. 2005, 53, 3882–3887. [Google Scholar] [CrossRef] [PubMed]
- Migas, P.; Krauze-Baranowska, M. The Significance of Arbutin and Its Derivatives in Therapy and Cosmetics. Phytochem. Lett. 2015, 13, 35–40. [Google Scholar] [CrossRef]
- Sârbu, I.; Ştefan, N.; Oprea, A. Plante Vasculare din Romania: Determinator Ilustrat de Teren; Victor, B., Ed.; Victor: Bucureşti, Romania, 2013; ISBN 978-606-8149-08-0. [Google Scholar]
- FARMACOPEEA Romana, 10th ed.; Editura Medicală: Bucharest, Romania, 2010; ISBN 978-973-39-0662-9.
- European Directorate for the Quality of Medicines. European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2022. [Google Scholar]
- Hefner, T. Arbutin Synthase, a Novel Member of the NRD1β Glycosyltransferase Family, Is a Unique Multifunctional Enzyme Converting Various Natural Products and Xenobiotics. Bioorg. Med. Chem. 2002, 10, 1731–1741. [Google Scholar] [CrossRef]
- Hefner, T.; Stöckigt, J. Probing Suggested Catalytic Domains of Glycosyltransferases by Site-directed Mutagenesis. Eur. J. Biochem. 2003, 270, 533–538. [Google Scholar] [CrossRef]
- Yokoyama, M.; Inomata, S. Catharanthus roseus (Periwinkle): In Vitro Culture, and High-Level Production of Arbutin by Biotransformation. In Medicinal and Aromatic Plants X; Bajaj, Y.P.S., Ed.; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 1998; Volume 41, pp. 67–80. ISBN 978-3-642-63748-3. [Google Scholar]
- Sugimoto, K.; Nishimura, T.; Nomura, K.; Sugimoto, K.; Kuriki, T. Inhibitory Effects of α-Arbutin on Melanin Synthesis in Cultured Human Melanoma Cells and a Three-Dimensional Human Skin Model. Biol. Pharm. Bull. 2004, 27, 510–514. [Google Scholar] [CrossRef]
- Seyfizadeh, N.; Tazehkand, M.Q.; Palideh, A.; Maroufi, N.F.; Hassanzadeh, D.; Rahmati-Yamchi, M.; Elahimanesh, F.; Borzoueisileh, S. Is Arbutin an Effective Antioxidant for the Discount of Oxidative and Nitrosative Stress in Hep-G2 Cells Exposed to Tert-Butyl Hydroperoxide? Bratisl. Med. J. 2019, 120, 569–575. [Google Scholar] [CrossRef]
- Jurica, K.; Gobin, I.; Kremer, D.; Čepo, D.V.; Grubešić, R.J.; Karačonji, I.B.; Kosalec, I. Arbutin and Its Metabolite Hydroquinone as the Main Factors in the Antimicrobial Effect of Strawberry Tree (Arbutus unedo L.) Leaves. J. Herb. Med. 2017, 8, 17–23. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, K.-W. Anti-Inflammatory Effects of Arbutin in Lipopolysaccharide-Stimulated BV2 Microglial Cells. Inflamm. Res. 2012, 61, 817–825. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, H.; Huang, Z.; Dong, P.; Chen, X. Anticancer Effect of Arbutin on Diethylnitrosamine-Induced Liver Carcinoma in Rats via the GRP and GADD Pathway. J. Environ. Pathol. Toxicol. Oncol. 2022, 41, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Kumar, M.; Sharma, J.; Yuan, Z. Arbutin Effectively Ameliorates the Symptoms of Parkinson’s Disease: The Role of Adenosine Receptors and Cyclic Adenosine Monophosphate. Neural. Regen. Res. 2021, 16, 2030. [Google Scholar] [CrossRef]
- Saeedi, M.; Khezri, K.; Seyed Zakaryaei, A.; Mohammadamini, H. A Comprehensive Review of the Therapeutic Potential of A-arbutin. Phytother. Res. 2021, 35, 4136–4154. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-H.; Liang, Q.; Zhang, Y.-J.; Zhao, P. Naturally Occurring Arbutin Derivatives and Their Bioactivities. Chem. Biodivers. 2015, 12, 54–81. [Google Scholar] [CrossRef] [PubMed]
- Tůmová, L.; Dolečková, I.; Hendrychová, H.; Kašparová, M. Arbutin Content and Tyrosinase Activity of Bergenia Extracts. Nat. Prod. Commun. 2017, 12, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, C.; Ichitani, M.; Kunimoto, K.-K.; Asada, C.; Nakamura, Y. Extraction of Arbutin and Its Comparative Content in Branches, Leaves, Stems, and Fruits of Japanese Pear Pyrus pyrifolia cv. Kousui. Biosci. Biotechnol. Biochem. 2014, 78, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-Y.; Park, K.Y.; Lee, K.H.; Lee, H.J.; Lee, S.-H.; Cho, J.A.; Kim, W.-S.; Shin, S.-C.; Park, K.-H.; Moon, J.-H. Recovery of Arbutin in High Purity from Fruit Peels of Pear (Pyrus pyrifolia Nakai). Food Sci. Biotechnol. 2011, 20, 801–807. [Google Scholar] [CrossRef]
- Kwiecień, I.; Szopa, A.; Madej, K.; Ekiert, H. Arbutin Production via Biotransformation of Hydroquinone in In Vitro Cultures of Aronia melanocarpa (Michx.) Elliott. Acta Biochim. Pol. 2013, 60, 865–870. [Google Scholar] [CrossRef]
- Xu, K.-X.; Xue, M.-G.; Li, Z.; Ye, B.-C.; Zhang, B. Recent Progress on Feasible Strategies for Arbutin Production. Front. Bioeng. Biotechnol. 2022, 10, 914280. [Google Scholar] [CrossRef]
- Lutterbach, R.; Stöckigt, J. High-Yield Formation of Arbutin from Hydroquinone by Cell-Suspension Cultures of Rauwolfia serpentina. Helv. Chim. Acta 1992, 75, 2009–2011. [Google Scholar] [CrossRef]
- Inomata, S.; Yokoyama, M.; Seto, S.; Yanagi, M. High-Level Production of Arbutin from Hydroquinone in Suspension Cultures of Catharanthus roseus Plant Cells. Appl. Microbiol. Biotechnol. 1991, 36, 315–319. [Google Scholar] [CrossRef]
- Casas, D.A.; Pitta-Alvarez, S.I.; Giulietti, A.M. Biotransformation of Hydroquinone by Hairy Roots of Brugmansia Candida and Effect of Sugars and Free-Radical Scavengers. Appl. Biochem. Biotechnol. 1998, 69, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Kittipongpatana, N.; Maneerat, P.; Pattanakitkosol, P.; Kittipongpatana, O. Effect of Some Factors on the Growth of Capsicum annuum L. Cell Suspension Culture and Biotransformation of Hydroquinone to Arbutin. CMU J. Nat. Sci. 2007, 6, 207–218. [Google Scholar]
- Whayne, T.F. Clinical Use of Digitalis: A State of the Art Review. Am. J. Cardiovasc. Drugs 2018, 18, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Pop, C.E.; Butiuc-Keul, A.; Munteanu-Deliu, C.; Deliu, C.; Halmagyi, A. Aspects Concerning Hydroquinone Effect on Cell Cultures of Arctostaphyllos uva-ursi, Catharantus Roseus and Digitalis Lanata. Contrib. Bot. 2003, 38, 93–104. [Google Scholar]
- Pop, C.E.; Deliu, C.; Tămaș, M. Biotransformarea Hidrochinonei În Arbutozidă În Culturi Celulare In Vitro de Digitalis Lanata. Clujul Med. 2005, 78, 182–187. [Google Scholar]
- Pop, C.E.; Deliu, C.; Tămaș, M.; Coste, A. Use of Digitalis Purpurea Cell Cultures for the Biotransformation of Hydroquinone into Arbutin. In Proceedings of the 4th Conference on Medicinal and Aromatic Plants of South-East European Countries, Iasi, Romania, 28–31 May 2006; pp. 220–225. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Evans, D.E.; Coleman, J.O.D.; Kearns, A. Plant Cell Culture, 1st ed.; Taylor & Francis: Milton Park, UK, 2020; ISBN 978-1-00-307694-0. [Google Scholar]
- Pop, C.E.; Vlase, L.; Tămaș, M. Natural Resources Containing Arbutin. Determination of Arbutin in the Leaves of Bergenia crassifolia L. Fritsch. Acclimated in Romania. Not. Bot. Horti Agrobot. 2009, 37, 129–132. [Google Scholar]
- Vlase, A.-M.; Toiu, A.; Tomuță, I.; Vlase, L.; Muntean, D.; Casian, T.; Fizeșan, I.; Nadăș, G.C.; Novac, C.Ș.; Tămaș, M.; et al. Epilobium Species: From Optimization of the Extraction Process to Evaluation of Biological Properties. Antioxidants 2022, 12, 91. [Google Scholar] [CrossRef]
- Solcan, M.-B.; Fizeșan, I.; Vlase, L.; Vlase, A.-M.; Rusu, M.E.; Mateș, L.; Petru, A.-E.; Creștin, I.-V.; Tomuțǎ, I.; Popa, D.-S. Phytochemical Profile and Biological Activities of Extracts Obtained from Young Shoots of Blackcurrant (Ribes nigrum L.), European Blueberry (Vaccinium myrtillus L.), and Mountain Cranberry (Vaccinium vitis-idaea L.). Horticulturae 2023, 9, 1163. [Google Scholar] [CrossRef]
- Carvalho, E.B.; Curtis, W.R. The Effect of Inoculum Size on the Growth of Cell and Root Cultures of Hyoscyamus muticus: Implications for Reactor Inoculation. Biotechnol. Bioprocess Eng. 1999, 4, 287–293. [Google Scholar] [CrossRef]
- Gorret, N.; Bin Rosli, S.K.; Oppenheim, S.F.; Willis, L.B.; Lessard, P.A.; Rha, C.; Sinskey, A.J. Bioreactor Culture of Oil Palm (Elaeis guineensis) and Effects of Nitrogen Source, Inoculum Size, and Conditioned Medium on Biomass Production. J. Biotechnol. 2004, 108, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, J.; He, G. Effects of Inoculum Size and Age on Biomass Growth and Paclitaxel Production of Elicitor-Treated Taxus Yunnanensis Cell Cultures. Appl. Microbiol. Biotechnol. 2002, 60, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.K.; Lim, P.S.; Choo, M.L.; Boey, P.L. Establishment of Cyperus aromaticus Cell Suspension Cultures for the Production of Juvenile Hormone III. Vitr. Cell. Dev. Biol. Plant 2010, 46, 8–12. [Google Scholar] [CrossRef]
- Lo, K.Y.; Nadali, B.J.; Chan, L.-K. Investigation on the Effect of Subculture Frequency and Inoculum Size on the Artemisinin Content in a Cell Suspension Culture of Artemisia annua L. Aust. J. Crop Sci. 2012, 6, 801–807. [Google Scholar]
- Che Saad, N.; Mazlan, F.I.; Abd Karim, K. Factors Affecting the Establishment and Growth of Pogostemon Cablin Cell Suspension Cultures. Int. J. Adv. Res. Sci. Eng. Technol. 2016, 3, 2790–2796. [Google Scholar]
- Sang-Yoon, L.; Kim, D.-I. Effects of Pluronic F-68 on Cell Growth of Digitalis Lanata in Aqueous Two-Phase Systems. J. Microbiol. Biotechnol. 2004, 14, 1129–1133. [Google Scholar]
- Tomilova, S.V.; Kochkin, D.V.; Tyurina, T.M.; Glagoleva, E.S.; Labunskaya, E.A.; Galishev, B.A.; Nosov, A.M. Specificity of Growth and Synthesis of Secondary Metabolites in Cultures In Vitro Digitalis Lanata Ehrh. Russ. J. Plant. Physiol. 2022, 69, 25. [Google Scholar] [CrossRef]
- Dantas, L.A.; Faria, P.S.A.; Dário, B.M.M.; Arantes, A.L.M.; Silva, F.G.; Avila, R.G.; Pereira, P.S.; Neto, A.R. The Impact of Carbon Source on Cell Growth and the Production of Bioactive Compounds in Cell Suspensions of Hancornia speciosa Gomes. Sci. Rep. 2021, 11, 24315. [Google Scholar] [CrossRef]
- See, K.; Bhatt, A.; Keng, C. Effect of Sucrose and Methyl Jasmonate on Biomass and Anthocyanin Production in Cell Suspension Culture of Melastoma malabathricum (Melastomaceae). Rev. Biol. Trop. 2011, 59, 597–606. [Google Scholar]
- Solís-Ramos, L.; Carballo, L.; Valdez-Melara, M. Establishment of Cell Suspension Cultures of Two Costa Rican Jatropha Species Euphorbiaceae. Rev. De Biol. Trop. 2013, 61, 1095–1107. [Google Scholar] [CrossRef][Green Version]
- Modarres, M.; Esmaeilzadeh Bahabadi, S.; Taghavizadeh Yazdi, M.E. Enhanced Production of Phenolic Acids in Cell Suspension Culture of Salvia leriifolia Benth. Using Growth Regulators and Sucrose. Cytotechnology 2018, 70, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Inomata, S.; Seto, S.; Yanagi, M. Effects of Sugars on the Glucosylation of Exogenous Hydroquinone by Catharanthus roseus Cells in Suspension Culture. Plant Cell Physiol. 1990, 31, 551–555. [Google Scholar] [CrossRef]
- Yokoyama, M.; Inomata, S.; Yanagi, M.; Wachi, Y. Change of Maximal Cellular Productivity of Arbutin by Biotransformation Depending on the Culture Stage of Catharanthus roseus Cells. Plant Tissue Cult. Lett. 1996, 13, 285–290. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshioka, T.; Tabata, M.; Fujita, Y. Potential of Datura innoxia Cell Suspension Cultures for Glucosylating Hydroquinone. Plant Cell Rep. 1987, 6, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Tofighi, Z.; Amini, M.; Shirzadi, M.; Mirhabibi, H.; Ghazi Saeedi, N.; Yassa, N. Vigna Radiata as a New Source for Biotransformation of Hydroquinone to Arbutin. Pharm. Sci. 2016, 22, 126–131. [Google Scholar] [CrossRef][Green Version]
- Kumar, P.P.; Joy, R.W.; Thorpe, T.A. Ethylene and Carbon Dioxide Accumulation, and Growth of Cell Suspension Cultures of Picea glauca (White Spruce). J. Plant Physiol. 1990, 135, 592–596. [Google Scholar] [CrossRef]
- Akalezi, C.O.; Liu, S.; Li, Q.S.; Yu, J.T.; Zhong, J.J. Combined Effects of Initial Sucrose Concentration and Inoculum Size on Cell Growth and Ginseng Saponin Production by Suspension Cultures of Panax Ginseng. Process Biochem. 1999, 34, 639–642. [Google Scholar] [CrossRef]
- Singh, M.; Chaturvedi, R. Evaluation of Nutrient Uptake and Physical Parameters on Cell Biomass Growth and Production of Spilanthol in Suspension Cultures of Spilanthes acmella Murr. Bioprocess Biosyst. Eng. 2012, 35, 943–951. [Google Scholar] [CrossRef]
- Jayaraman, S.; Daud, N.H.; Halis, R.; Mohamed, R. Effects of Plant Growth Regulators, Carbon Sources and pH Values on Callus Induction in Aquilaria malaccensis Leaf Explants and Characteristics of the Resultant Calli. J. For. Res. 2014, 25, 535–540. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H.; Ahmad, N.; Ali, S.S.; Ali, S.; Ali, G.S. Sucrose-Enhanced Biosynthesis of Medicinally Important Antioxidant Secondary Metabolites in Cell Suspension Cultures of Artemisia absinthium L. Bioprocess Biosyst. Eng. 2016, 39, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Kittipongpatana, N.; Chaiwan, A.; Pusod, U.; Kittipongpatana, O.S. High-Performance Liquid Chromatographic Method for Separation and Quantitative Analysis of Arbutin in Plant Tissue Cultures. CMU J. Nat. Sci. 2007, 6, 65–74. [Google Scholar]
- Duskova, J.; Dusek, J.; Jahodar, L.; Poustka, F. Arbutin, Salicin: The Possibilities of Their Biotechnological Production. Ceska Slov. Farm. 2005, 54, 78–81. [Google Scholar] [PubMed]
- Yan, C.-Y.; Zhang, Z.; Yu, R.-M.; Kong, L.-Y. Studies on Biotransformation of Arbutin by 4-Hydroxy Phenol in Hairy Root of Polygonum Multiflorum. Zhongguo Zhong Yao Za Zhi 2007, 32, 192–195. [Google Scholar]


| Variables | Levels | |||
|---|---|---|---|---|
| Independent Variables (Factors) | ||||
| Feeding protocol (X1) | 1—single administration | 2—sequential administration | ||
| Carbon source (X2) | Sucrose (S) | Glucose (G) | ||
| Carbon source level (X3) | 3% | 6% | 3% | 6% |
| Hidroquinone concentration (X4) | 4 mM | 5 mM | 6 mM | |
| Dependent variables (responses) | ||||
| Cell growth (Y1) | ||||
| Dry biomass (dry weight) (Y2) (g/L) | ||||
| Arbutin content (Y3) (mg/L) | Dry biomass (Y3-Dm) | |||
| Liquid medium (Y3-Lm) | ||||
| Total amount (Y3-T) | ||||
| Time (hours) | Dry Biomass (g/L) | |||
|---|---|---|---|---|
| Control * | 4 mM HQ ** | 5 mM HQ | 6 mM HQ | |
| 3 | 13.00 ± 1.00 | 12.00 ± 0.26 | 11.12 ± 0.15 | 10.84 ± 0.45 # |
| 6 | 12.00 ± 0.36 | 11.38 ± 0.41 | 10.76 ± 0.12 | 10.56 ± 0.15 |
| 12 | 11.40 ± 0.38 | 11.06 ± 0.01 | 10.28 ± 0.32 | 10.25 ± 0.12 |
| 24 | 10.40 ± 0.17 | 9.81 ± 0.16 | 9.74 ± 0.21 | 9.39 ± 0.19 # |
| 48 | 9.60 ± 0.20 | 7.99 ± 0.19 # | 7.87 ± 0.26 # | 7.23 ± 0.11 # |
| Carbon Source | Dry Biomass (g/L) | |||
|---|---|---|---|---|
| Hidroquinone Concentration | Control (No Treatment) | |||
| 4 mM | 5 mM | 6 mM | ||
| 3% sucrose | 8.00 ± 0.60 # | 7.50 ± 0.10 # | 7.00 ± 0.78 # | 9.50 ± 0.30 |
| 6% sucrose | 9.00 ± 0.66 | 8.60 ± 0.20 | 8.00 ± 0.56 | |
| 3% glucose | 7.80 ± 0.54 # | 7.30 ± 0.36 # | 7.00 ± 0.30 # | |
| 6% glucose | 8.20 ± 0.30 # | 7.80 ± 0.18 # | 7.10 ± 0.20 # | |
| Arbutin amount (mg/L) | ||||||
| 4 mM HQ * | 5 mM HQ | 6 mM HQ | ||||
| Sucrose | 3% | 6% | 3% | 6% | 3% | 6% |
| Dry biomass | 498.40 ± 2.26 | 611.50 ± 2.46 | 761.60 ± 11.13 | 878.06 ± 3.63 | 952.45 ± 4.62 | 1004.20 ± 3.51 |
| Liquid medium | 0.23 ± 0.05 | 1049.56 ± 3.03 | 0.86 ± 0.06 | 1786.99 ± 9.31 | 2.77 ± 0.13 | 1356.33 ± 14.74 |
| Total | 498.63 ± 2.30 | 1661.06 ± 5.46 | 762.46 ± 11.07 | 2665.05 ± 5.73 | 955.22 ± 4.67 | 2360.53 ± 11.74 |
| Conversion rate | 45.827% | 96.46% | 56.66% | 97.82% | 58.53% | 96.8% |
| Arbutin amount (mg/L) | ||||||
| 4 mM HQ | 5 mM HQ | 6 mM HQ | ||||
| Glucose | 3% | 6% | 3% | 6% | 3% | 6% |
| Dry biomass | 23.10 ± 1.15 | 110.24 ± 3.39 | 42.00 ± 2.00 | 84.95 ± 2.05 | 77.00 ± 2.65 | 77.70 ±1.73 |
| Liquid medium | 0.30 ± 0.03 | 1125.80 ± 2.11 | 0.34 ± 0.06 | 2964.25 ± 4.68 | 0.00 ± 0.00 | 1537.10 ± 14.08 |
| Total | 23.40 ± 1.18 | 1236.04 ± 5.37 | 42.34 ± 2.04 | 3049.20 ± 4.68 | 77.00 ± 2.65 | 1650.80 ± 12.90 |
| Conversion rate | 9.46% | 94.8% | 17.12% | 98.89% | 31.13% | 97.6% |
| Parameter | Cell Growth | Arbutin Content | |||||
|---|---|---|---|---|---|---|---|
| Dry Biomass | Liquid Medium | Total Amount | |||||
| FP 1 * | FP 2 ** | FP 1 | FP 2 | FP 1 | FP 2 | ||
| R2 | 0.794 | 0.689 | 0.964 | 0.876 | 0.725 | 0.787 | 0.591 |
| Q2 | 0.670 | 0.408 | 0.880 | 0.548 | 0.665 | 0.656 | 0.423 |
| p (ANOVA) | 0.004 # | 0.0039 # | <0.001 # | <0.001 # | 0.0029 # | <0.001 # | 0.0177 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, C.E.; Coste, A.; Vlase, A.-M.; Deliu, C.; Tămaș, M.; Casian, T.; Vlase, L. Selection of a Digitalis purpurea Cell Line with Improved Bioconversion Capacity of Hydroquinone into Arbutin. Life 2024, 14, 84. https://doi.org/10.3390/life14010084
Pop CE, Coste A, Vlase A-M, Deliu C, Tămaș M, Casian T, Vlase L. Selection of a Digitalis purpurea Cell Line with Improved Bioconversion Capacity of Hydroquinone into Arbutin. Life. 2024; 14(1):84. https://doi.org/10.3390/life14010084
Chicago/Turabian StylePop, Carmen Elena, Ana Coste, Ana-Maria Vlase, Constantin Deliu, Mircea Tămaș, Tibor Casian, and Laurian Vlase. 2024. "Selection of a Digitalis purpurea Cell Line with Improved Bioconversion Capacity of Hydroquinone into Arbutin" Life 14, no. 1: 84. https://doi.org/10.3390/life14010084
APA StylePop, C. E., Coste, A., Vlase, A.-M., Deliu, C., Tămaș, M., Casian, T., & Vlase, L. (2024). Selection of a Digitalis purpurea Cell Line with Improved Bioconversion Capacity of Hydroquinone into Arbutin. Life, 14(1), 84. https://doi.org/10.3390/life14010084









