Alien Algae Species Invasions in Humic Rivers within Weakly Human Impact Basin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Area
2.2. Sample Collection, Morphological Identification, Hydrochemical and Statistical Analysis
2.3. DNA Extraction, Amplification, and Sequencing
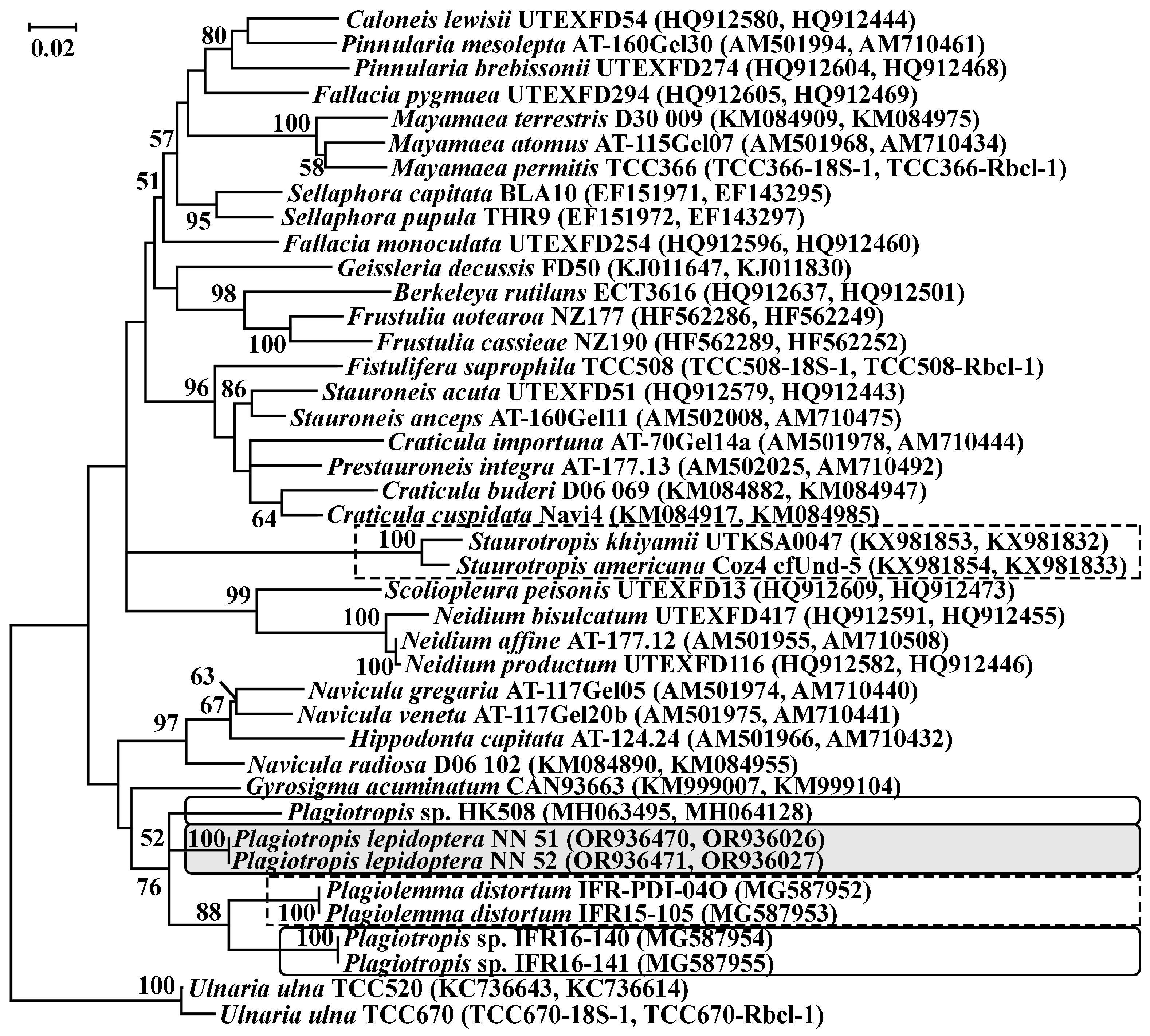
2.4. Alignment and Phylogenetic Analysis
3. Results
3.1. Environmental Indicators
3.2. Phytoplankton Species Composition
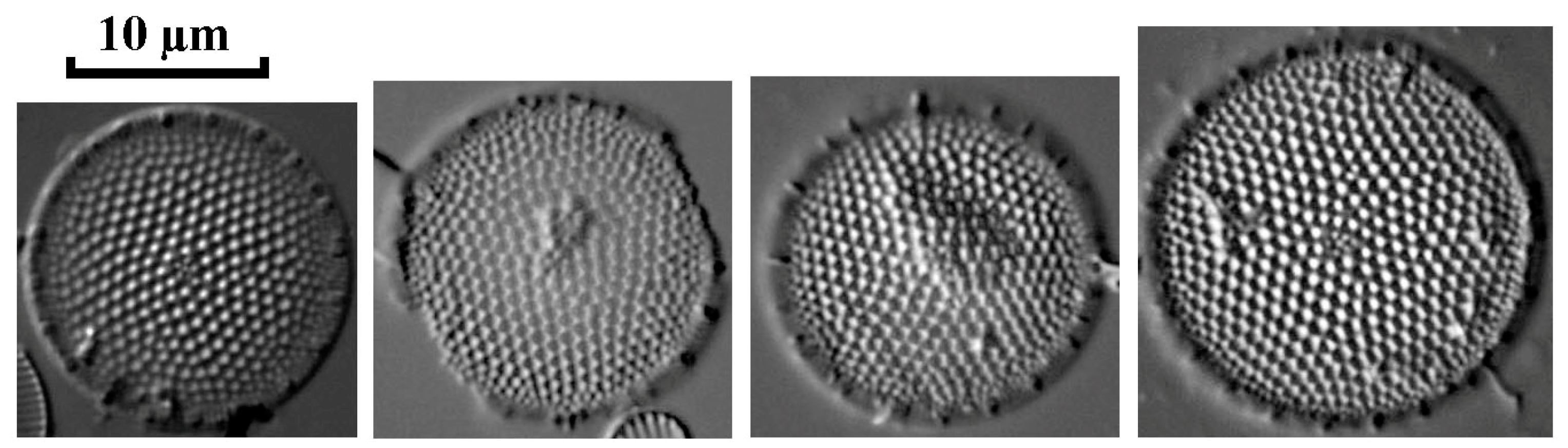
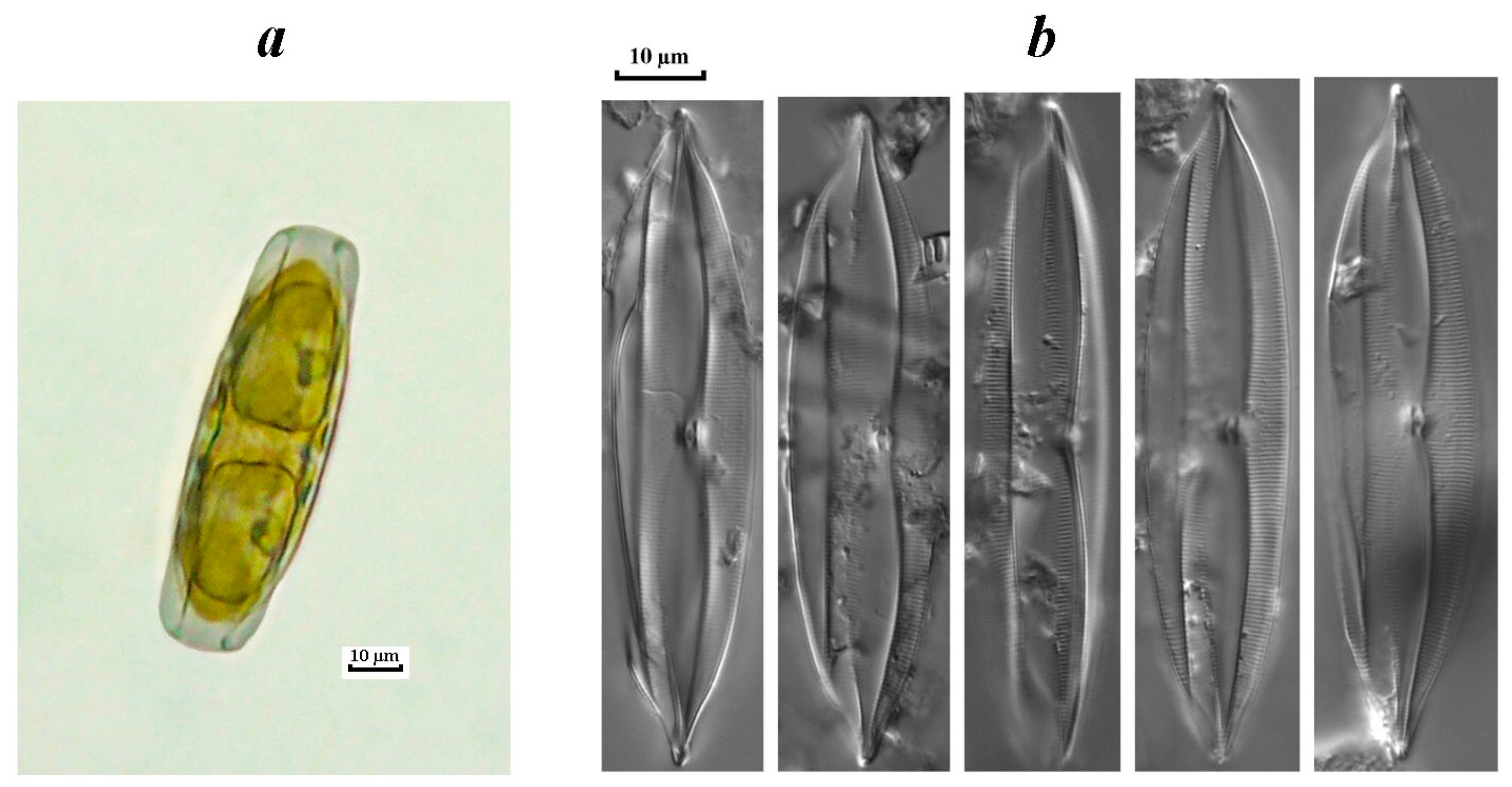
3.3. Description, Distribution, and Ecological Characteristics of Invasive Species
3.3.1. Invasive Raphydophytes
3.3.2. Invasive Dinoflagellates
3.3.3. Invasive Diatoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korneva, L.G. Invasions of alien species of planktonic microalgae into the fresh waters of Holarctic. Russ. J. Biol. Invasions 2014, 5, 65–81. [Google Scholar] [CrossRef]
- Kaštovský, J.; Hauer, T.; Mareš, J.; Krautová, M.; Bešta, T.; Komárek, J.; Desortová, B.; Heteša, J.; Hindáková, A.; Houk, V.; et al. A review of the alien and expansive species of freshwater cyanobacteria and algae in the Czech Republic. Biol. Invasions 2010, 12, 3599–3625. [Google Scholar] [CrossRef]
- Magliozzi, C.; Tsiamis, K.; Vigiak, O.; Deriu, I.; Gervasini, E.; Cardoso, A.C. Assessing invasive alien species in European catchments: Distribution and impacts. Sci. Total Environ. 2020, 732, 138677. [Google Scholar] [CrossRef] [PubMed]
- Edlund, M.B.; Taylor, C.M.; Schelske, C.L.; Stoermer, E.F. Thalassiosira baltica (Grunow) Ostenfeld (Bacillariophyta), a new exotic species in the Great Lakes. Can. J. Fish. Aquat. Sci. 2000, 57, 610–615. [Google Scholar] [CrossRef]
- Smucker, N.J.; Edlund, M.B.; Vis, M.L. The distribution, morphology, and ecology of a non-native species, Thalassiosira lacustris (Bacillariophyceae), from benthic stream habitats in North America. Nova Hedwig. 2008, 87, 201–220. [Google Scholar] [CrossRef]
- Hu, J.; Yang, Z.; Yi, Y.; Shu, Z.; Yu, P.; You, Q.; Wang, Q. Possible Origin and Distribution of an Invasive Diatom Species, Skeletonema potamos, in Yangtze River Basin (China). Water 2023, 15, 2875. [Google Scholar] [CrossRef]
- Volga and Its Life; Nauka: Leningrad, Russia, 1978; p. 348. (In Russian)
- Korneva, L.G. Recent invasion of planktonic diatom algae in the Volga River basin and their causes. Inland Water Biol. 2007, 1, 28–36. [Google Scholar]
- Alimov, A.F.; Bogutskaya, N.G. Biological Invasions in Aquatic and Terrestrial Ecosystems; Fellowship of Scientific Publications KMK (KMK Scientific Press Ltd.): Moscow, Russia; St. Petersburgh, Russia, 2004; p. 436. [Google Scholar]
- Labunskaya, E.N.; Bukharitsin, P.I. Current state of phytoplankton and water quality of the lower Volga and North Caspian. Int. J. Appl. Fundam. Res. 2010, 8, 136–138. [Google Scholar]
- Morduhai-Boltovskoi, F.D. (Ed.) Methods for Studying Biogeocenoses of Inland Water Bodies; USSR Science Academy, Nauka: Moscow, Russia, 1975; p. 240. (In Russian) [Google Scholar]
- Kulizin, P.V.; Vodeneeva, E.L.; Okhapkin, A.G. Phytoplankton of some rivers in the south of broadleaved forests subzone of the Middle Volga basin in a long-term perspective. Samarsk. Nauchn. Vestn. 2021, 10, 45–53. [Google Scholar] [CrossRef]
- Vodeneeva, E.L.; Kulizin, P.V.; Okhapkin, A.G. Algae of the Kerzhensky Nature Reserve. Annotated List of Species; Flora and Fauna of Nature Reserves: Moscow, Russia, 2022; Volume 146, p. 145. (In Russian) [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2021; Available online: http://www.algaebase.org (accessed on 14 September 2023).
- Zimmermann, J.; Jahn, R.; Gemeinholzer, B. Barcoding diatoms: Evaluation of the V4 subregion on the 18S rRNA gene, including new primers and protocols. Org. Divers. Evol. 2011, 11, 173–192. [Google Scholar] [CrossRef]
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Belevich, T.A.; Ilyash, L.V.; Milyutina, I.A.; Logacheva, M.D.; Goryunov, D.V.; Troitsky, A.V. Metagenomic analyses of White Sea picoalgae: First data. Biochemistry 2015, 80, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Alverson, A.J.; Jansen, R.K.; Theriot, E.C. Bridging the Rubicon: Phylogenetic analysis reveals repeated colonizations of marine and fresh waters by thalassiosiroid diatoms. Mol. Phylogenetics Evol. 2007, 45, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across ComputingPlatforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Rimet, F.; Gusev, E.; Kahlert, M.; Kelly, M.G.; Kulikovskiy, M.; Maltsev, Y.; Mann, D.G.; Pfannkuchen, M.; Trobajo, R.; Vasselon, V.; et al. Diat. barcode, an open-access curated barcode library for diatoms. Sci. Rep. 2019, 9, 15116. [Google Scholar] [CrossRef] [PubMed]
- Nézan, E.; Bilien, G.; Boulben, S.; Mertens, K.N.; Chomérat, N. Description and phylogenetic position of Plagiolemma distortum sp. nov., a new raphid diatom (Bacillariophyceae) from French coastal waters. Diatom Res. 2018, 33, 13–24. [Google Scholar] [CrossRef]
- Pruesse, E.; Peplies, J.; Glöckner, F.O. SINA: Accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 2012, 28, 1823–1829. [Google Scholar] [CrossRef]
- Katoh, K.; Toh, H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 2010, 26, 1899–1900. [Google Scholar] [CrossRef]
- Hagman, C.H.C.; Rohrlack, T.; Riise, G. The success of Gonyostomum semen (Raphidophyceae) in a boreal lake is due to environmental changes rather than a recent invasion. Limnologica 2020, 84, 125818. [Google Scholar] [CrossRef]
- Vetrova, Z.I.; Okhapkin, A.G. Representatives of Raphidophyta in reservoirs of the Soviet Union. Bot. Zhurnal 1990, 5, 631–636. [Google Scholar]
- Korneva, L.G. Ecological aspects of mass development of Gonyostomum semen (Ehr.) Dies. (Raphidophyta). Int. J. Algae 2001, 3, 40–54. [Google Scholar] [CrossRef]
- Krahmalny, A.F. A new species of the genus Peridiniopsis Lemm. (Peridiniales, Dynophyta). Int. J. Algae 2002, 4, 51–58. [Google Scholar] [CrossRef]
- Krahmalny, A.F. Dinophyta of Ukraine (Illustrated Book for Identification); Tsarenko, P.M., Ed.; Alterpress: Kyiv, Ukraine, 2011; p. 444. (In Russian) [Google Scholar]
- Genkal, S.I.; Kuzmin, G.V. On taxonomy and biology of little-known fresh-water species Sceletonema Grev. (Bacillariophyta). Hydrobiol. J. 1980, 16, 25–30. [Google Scholar]
- Genkal, S.I.; Korneva, L.G. New findings of diatoms (Centrophyceae) from the Volga reservoirs (Russia). Algology 2001, 11, 457–461. (In Russian) [Google Scholar]
- Brondizio, E.S.; Settele, J.; Díaz, S.; Ngo, H.T. (Eds.) IPBES (2019): Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019; p. 1148. [Google Scholar] [CrossRef]
- Sandvik, H.; Taugbøl, A.; Bærum, K.M.; Hesthagen, T.; Jensen, T.C.; Johnsen, S.I.; Sandlund, O.T.; Schartau, A.K. Alien species and the Water Framework Directive: Recommendations for assessing ecological status in fresh waters in Norway. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 1101–1233. [Google Scholar] [CrossRef]
- Sidelev, S.; Koksharova, O.; Babanazarova, O.; Fastner, J.; Chernova, E.; Gusev, E. Phylogeographic, toxicological and ecological evidence for the global distribution of Raphidiopsis raciborskii and its northernmost presence in Lake Nero, Central Western Russia. Harmful Algae 2020, 98, 101889. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.A.; Crooks, J.A. Functional Consequences of Invasive Species in Coastal and Estuarine Systems. In Treatise on Estuarine and Coastal Science, 1st ed.; Wolanski, E., McLusky, D., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 17–51. [Google Scholar] [CrossRef]
- Halpern, B.S.; Frazier, M.; Potapenko, J.; Casey, K.S.; Koenig, K.; Longo, K.; Lowndes, J.S.; Rockwood, R.C.; Selig, E.R.; Selkoe, K.A.; et al. Spatial and temporal changes in cumulative human impacts on the world’s ocean. Nat. Commun. 2015, 6, 7615. [Google Scholar] [CrossRef]
- Corrales, X.; Katsanevakis, S.; Coll, M.; Heymans, J.J.; Piroddi, C.; Ofir, E.; Gal, G. Advances and challenges in modelling the impacts of invasive alien species on aquatic cosystems. Biol. Invasions 2020, 22, 907–934. [Google Scholar] [CrossRef]
- Jenkins, M. Prospects for biodiversity. Science 2003, 302, 1175–1177. [Google Scholar] [CrossRef]
- Okhapkin, A.G. Phytoplankton of the Cheboksary Reservoir; Institute of Ecology of the Volga Basin RAS: Togliatti, Russia, 1994; p. 275. (In Russian) [Google Scholar]
- Korneva, L.G. Spatiotemporal distribution of diatoms invading the water bodies of the Volga basin. In Proceedings of the Russian-American Symposium on Invasive Species “Invasions of Alien Species in the Holarctic”, Borok, Russia, 27–31 August 2001; pp. 76–84. (In Russian). [Google Scholar]
- Korneva, L.G. Phytoplankton of Volga River Basin Reservoirs; Kostroma Printing House: Kostroma, Russia, 2015; p. 284. (In Russian) [Google Scholar]
- Korneva, L.G.; Solovyova, V.V.; Makarova, O.S. Diversity and dynamics of planktonic algocenoses in reservoirs of the Upper and Middle Volga (Rybinsk, Gorky, Cheboksary) under conditions of eutrophication and climate change. Proc. Inst. Inland Water Biol. Russ. Acad. Sci. 2016, 76, 35–45. (In Russian) [Google Scholar]
- Padisak, J.; Reynolds, C.S. Selection of phytoplankton association in Lake Balaton, Hungary, in response to eutrophication and restoration measures, with special reference to the cyanoprokaryotes. Hydrobiologia 1998, 384, 41–53. [Google Scholar] [CrossRef]
- Korneva, L.G. Formation of Phytoplankton in Reservoirs of the Volga Basin under the Influence of Natural and Anthropogenic Factors. Ph.D. Thesis, Institute of Limnology of the Russian Academy of Sciences, Saint-Petersburgh, Russia, 20 November 2009; p. 47. (In Russian). [Google Scholar]
- Babanazarova, O.V.; Alexandrina, E.M.; Rakhmangulov, R.A. The outbreak of the subtropical potentially toxic species Cylindrospermopsis raciborskii in the hypertrophic Lake Nero (Russia). In Proceedings of the V International Scientific Conference “Lake Ecosystems: Biological Processes, Anthropogenic Transformation, Water Quality”, Naroch, Belarus, 12–17 September 2011; p. 48. (In Russian). [Google Scholar]
- Rengefors, K.; Weyhenmeyer, G.A.; Bloch, I. Temperature as a driver for the expansion of the microalga Gonyostomum semen in Swedish lakes. Harmful Algae 2012, 18, 65–73. [Google Scholar] [CrossRef]
- Hagman, C.H.C.; Ballot, A.; Hjermann, D.Ø.; Skjelbred, B.; Brettum, P.; Ptacnik, R. The occurrence and spread of Gonyostomum semen (Ehr.) Diesing (Raphidophyceae) in Norwegian lakes. Hydrobiologia 2015, 744, 1–14. [Google Scholar] [CrossRef]
- Grigorszky, I.; Vasas, F.; Borics, G.; Klee, R.; Schmidt, A.; Borbély, G. Peridiniopsis kevei sp. nov., a new freshwater dinoflagellate species (Peridiniaceae, Dinophyta) from Hungary. Acta Bot. Hung. 2001, 43, 163–174. [Google Scholar] [CrossRef]
- Sakharova, E.G.; Korneva, L.G. Influence of Temperature and Water Level on the Phytoplankton in the Estuarine Zone of the Rybinsk Reservoir Tributary. Inland Water Biol. 2019, 12, 25–32. [Google Scholar] [CrossRef]
- Horiguchi, T. Origin and Evolution of Dinoflagellates with a Diatom Endosymbiont. In Proceedings of the International Symposium on “Dawn of a New Natural History–International of Geoscience and Biodiversity Studies”, Sapporo, Japan, 5–6 March 2004; pp. 53–59. [Google Scholar]
- Okhapkin, A.G.; Genkal, S.I.; Scharagina, E.M.; Vodeneeva, E.L. Structure and dynamics of phytoplankton in the Oka river mouth at the beginning of the 21th century. Inland Water Biol. 2014, 7, 57–365. [Google Scholar] [CrossRef]
- Genkal, S.I.; Okhapkin, A.G. Centric diatoms (Centrophyceae) of the lower reaches of the Oka river (Russian Federation). Hydrobiol. J. 2013, 49, 44–49. [Google Scholar] [CrossRef]
- Okhapkin, A.G.; Genkal, S.I.; Vodeneeva, E.L.; Sharagina, E.M.; Bondarev, O.O. To ecology and morphology of Thalassiosira incerta Makarova (Bacillariophyta). Inland Water Biol. 2016, 9, 126–134. [Google Scholar] [CrossRef]
- Lebret, K.; Tesson, S.V.; Kritzberg, E.S.; Tomas, C.; Rengefors, K. Phylogeography of the freshwater raphidophyte Gonyostomum semen confirms a recent expansion in northern Europe by a single haplotype. J. Phycol. 2015, 51, 768–781. [Google Scholar] [CrossRef]
- Balashova, N.B.; Kiselev, G.A.; Stepanova, V.A.; Tobias, A.V. Benthic diatoms of the southern coast of the Gulf of Finland (Lebyazhiy Nature Reserve). Vestn. SpbSU Bull. St. Petersburg State Univ. 2016, 4, 9–25. [Google Scholar] [CrossRef]
- Lilitskaya, G.G.; Tsarenko, P.M. Bacillariophyta of Lake Donuzlav (Crimea, Ukraine). Int. J. Algae 2013, 15, 135–152. [Google Scholar] [CrossRef]
- Karpinsky, M.G. On peculiarities of introduction of marine species into the Caspian Sea. Russ. J. Biol. Invasions 2010, 1, 7–10. [Google Scholar] [CrossRef]
- Karayeva, N.I. Diatoms of Benthos of the Caspian Sea; El’m: Baku, Azerbaijan, 1972; p. 258. (In Russian) [Google Scholar]
- Proshkina-Lavrenko, A.I. Benthic Diatoms of the Black Sea; Publishing House of the Academy of Sciences of the USSR: Moscow, Russia; Leningrad, Russia, 1963; p. 243. [Google Scholar]
- Czarnecki, D.B.; Blinn, D.W. Observations on Southwestern diatoms. 1. Plagiotropis arizonica n.sp. (Bacillariophyta, Entomoneidaceae), a large mesohalobous diatom. Trans. Am. Microsc. Soc. 1978, 97, 393–396. [Google Scholar] [CrossRef]

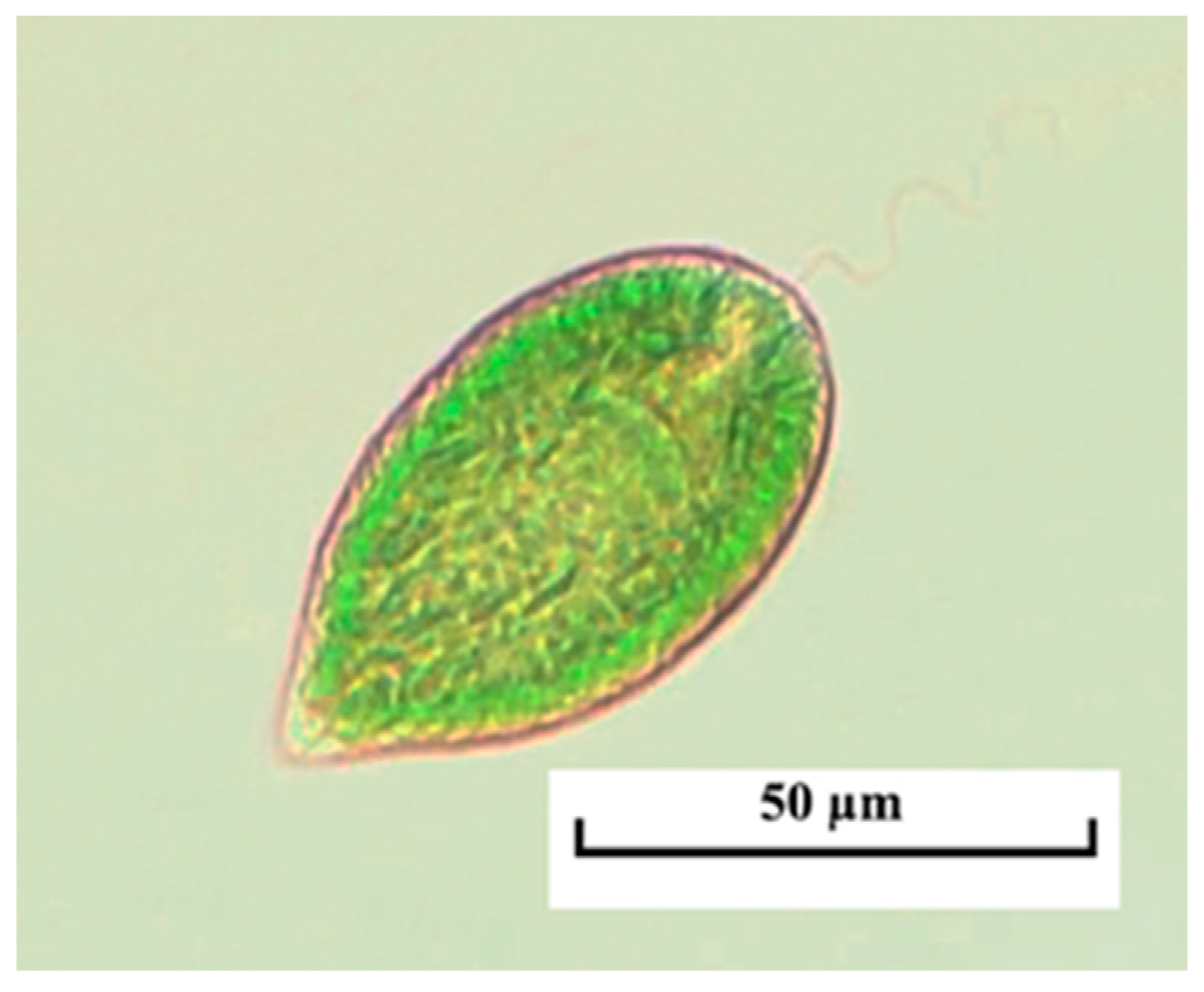
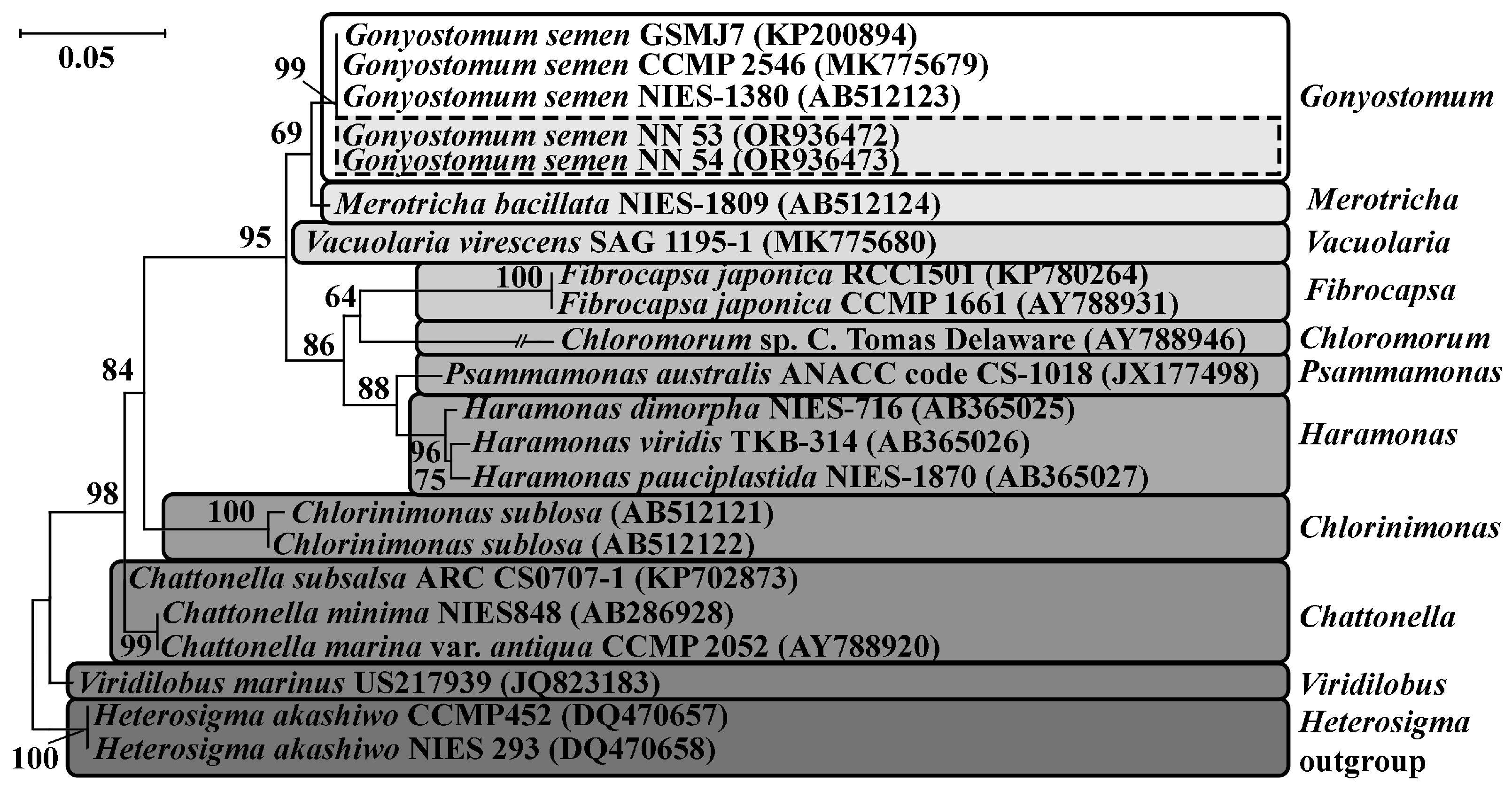


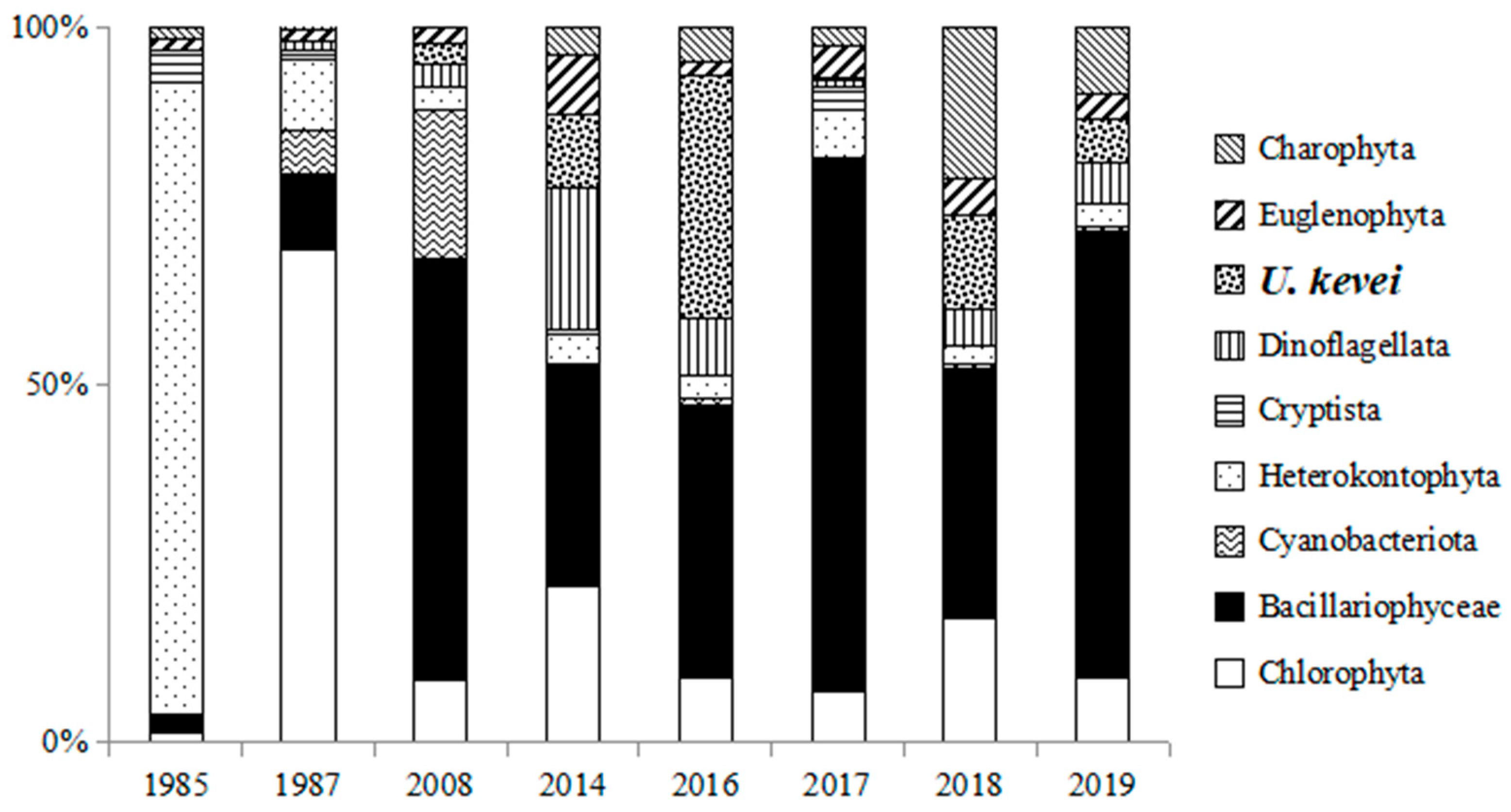
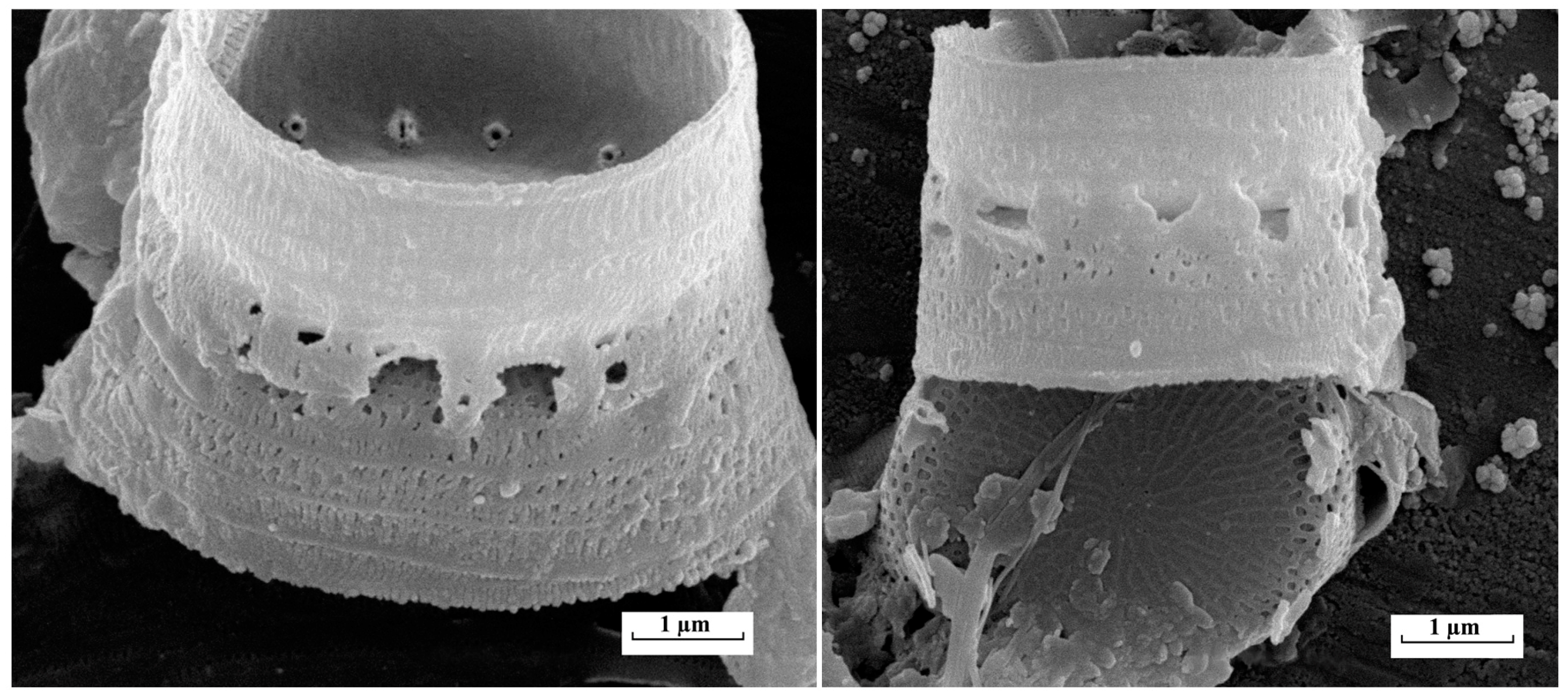

| Station | River | GPS Coordinates | Collection Years |
|---|---|---|---|
| 1 | Vetluga | 57.39 N, 45.10 E | 2010 |
| 2 | Vetluga | 57.12 N, 45.18 E | 1987 |
| 3 | Vetluga | 56.85 N, 45.43 E | 2014, 2016, 2018 |
| 4 | Vetluga | 56.32 N, 46.36 E | 1972–1979 |
| 5 | Kerzhenets | 56.64 N, 44.67 E | 1987 |
| 6 | Kerzhenets | 56.58 N, 44.65 E | 1985 |
| 7 | Kerzhenets | 56.49 N, 44.79 E | 2008, 2014, 2016, 2018, 2019 |
| 8 | Kerzhenets | 56.08 N, 44.97 E | 1972–1979, 1981–1990 |
| 9 | Vishnya | 56.50 N, 44.81 E | 2002, 2016 |
| Parameters | Vetluga | Kerzhenets | Vishnya |
|---|---|---|---|
| Length, km | 889 | 290 | 27 |
| Catchment area, km2 | 39,400 | 6140 | 250 |
| Chromaticity, Pt-Co | 92.8 | 96.5 | 207.13 |
| pH | 7.9 | 7.09 | 6.54 |
| Mineralization, mg/L | 145 | 100.7 | 75.3 |
| HCO3−, mg/L | 88.6 | 61.2 | 42.1 |
| SO42−, mg/L | 8.02 | 7.4 | 4.0 |
| Cl−, mg/L | 5.6 | 4.8 | 1.0 |
| Ca2+ + Mg2+, mg/L | 27 | 21.1 | 17.5 |
| Fetotal., mg/L | 0.22 | 0.38 | 0.45 |
| Ptotal, mg/L | 35 | 55 | 63 |
| Nmin., mgN/L | 0.45 | 0.5 | 0.82 |
| O2, mg/L | 12.5 | 9.6 | 6.63 |
| COD, mg O2/L | 32.1 | 32.7 | 42.6 |
| BOD5, mg O2/L | 0.55 | 0.77 | 1.36 |
| Water Body, the Year of Sampling | N, 103 cells/L | N/N Total, % | B, g/m3 | B/B Total, % |
|---|---|---|---|---|
| Vetluga, 2010 | 0.02 | 0.31 | 0.44 | 32.8 |
| Vetluga, 2014 | 0.02 | 2 | 0.31 | 12.4 |
| Vetluga, 2016 | 0.021 ± 0.01 | 1 | 0.31 ± 0.11 | 29.7 |
| Kerzhenets, 2008 | 0.07 ± 0.06 | 1.1 | 0.39 ± 0.3 | 15.1 |
| Kerzhenets, 2014 | 0.048 ± 0.01 | 1.1 | 0.46 ± 0.11 | 26.2 |
| Kerzhenets, 2016 | 0.04 ± 0.01 | 1.1 | 0.56 ± 0.15 | 39.7 |
| Kerzhenets, 2017 | 0.01 | 0.5 | 0.057 | 2.1 |
| Kerzhenets, 2018 | 0.02 ± 0.01 | 0.46 | 0.30 ± 0.07 | 24.8 |
| Kerzhenets, 2019 | 0.02 ± 0.01 | 0.7 | 0.22 ± 0.06 | 12.4 |
| Vishnya, 2016 | 0.001 | 0.5 | 0.01 | 3.5 |
| Parameters | Water Level | T, °C | pH | Conductivity | Chromaticity |
|---|---|---|---|---|---|
| U. kevei biomass | −0.79 | 0.59 | 0.75 | 0.61 | 0.64 |
| U. kevei share in total biomass | −0.83 | 0.52 | 0.75 | 0.59 | 0.61 |
| Water Object, the Year of Investigation | N, 106 cells/L | N/N Total, % | B, g/m3 | B/B Total, % |
|---|---|---|---|---|
| Vetluga, 2014 | 0.01 ± 0.01 | 0.2 | 0.22 ± 0.10 | 14.6 |
| Vetluga, 2016 | 0.02 ± 0.01 | 0.4 | 0.46 ± 0.19 | 44.1 |
| Kerzhenets, 2019 | 0.004 | 0.3 | 0.069 | 6.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulizin, P.; Vodeneeva, E.; Martynenko, N.; Sharagina, E.; Okhapkin, A. Alien Algae Species Invasions in Humic Rivers within Weakly Human Impact Basin. Life 2024, 14, 61. https://doi.org/10.3390/life14010061
Kulizin P, Vodeneeva E, Martynenko N, Sharagina E, Okhapkin A. Alien Algae Species Invasions in Humic Rivers within Weakly Human Impact Basin. Life. 2024; 14(1):61. https://doi.org/10.3390/life14010061
Chicago/Turabian StyleKulizin, Pavel, Ekaterina Vodeneeva, Nikita Martynenko, Ekaterina Sharagina, and Alexander Okhapkin. 2024. "Alien Algae Species Invasions in Humic Rivers within Weakly Human Impact Basin" Life 14, no. 1: 61. https://doi.org/10.3390/life14010061
APA StyleKulizin, P., Vodeneeva, E., Martynenko, N., Sharagina, E., & Okhapkin, A. (2024). Alien Algae Species Invasions in Humic Rivers within Weakly Human Impact Basin. Life, 14(1), 61. https://doi.org/10.3390/life14010061






