A Continuous Extraction Protocol for the Characterisation of a Sustainably Produced Natural Indigo Pigment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Industrial Indigo Preparation
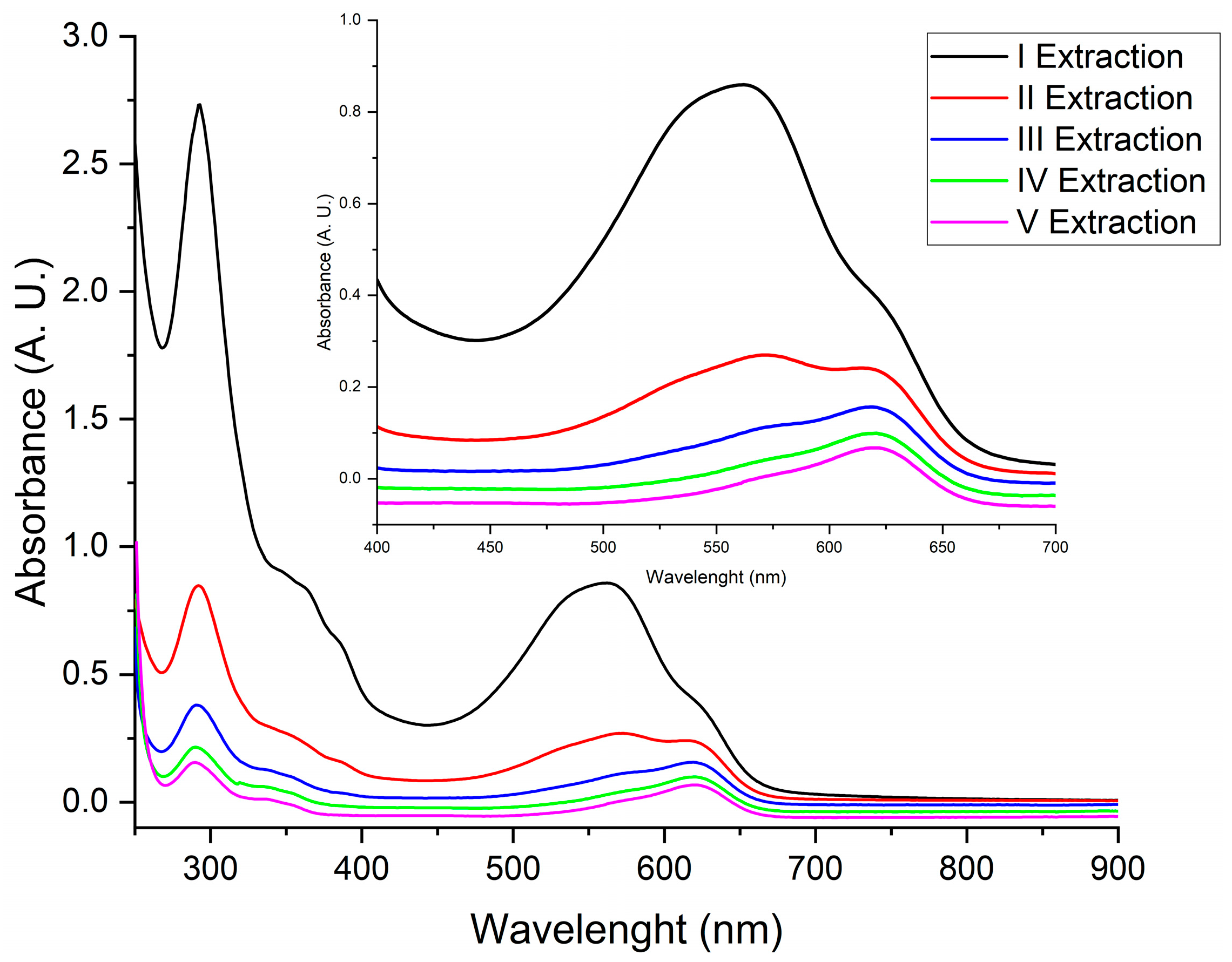
2.2. Sequential Extraction of the Organic Fraction
2.3. Continuous Extraction of the Organic Fraction
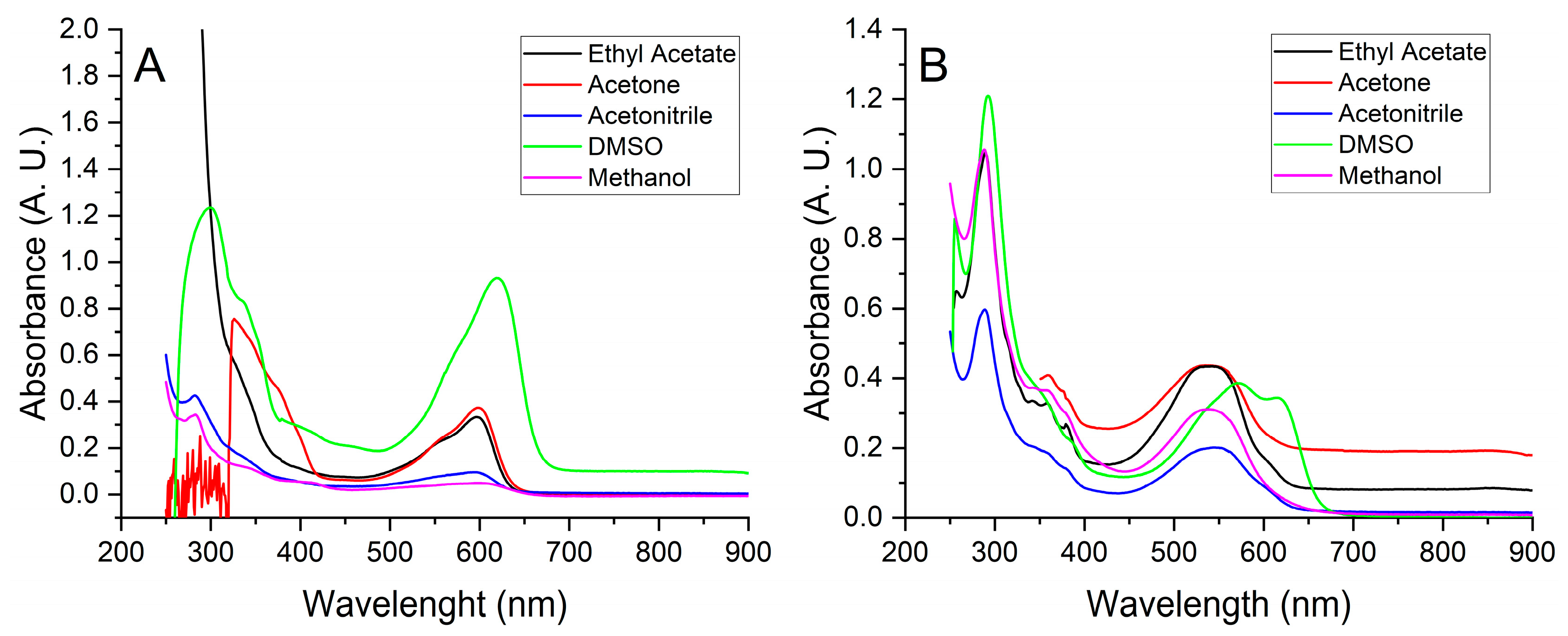
2.4. UV-VIS Spectra
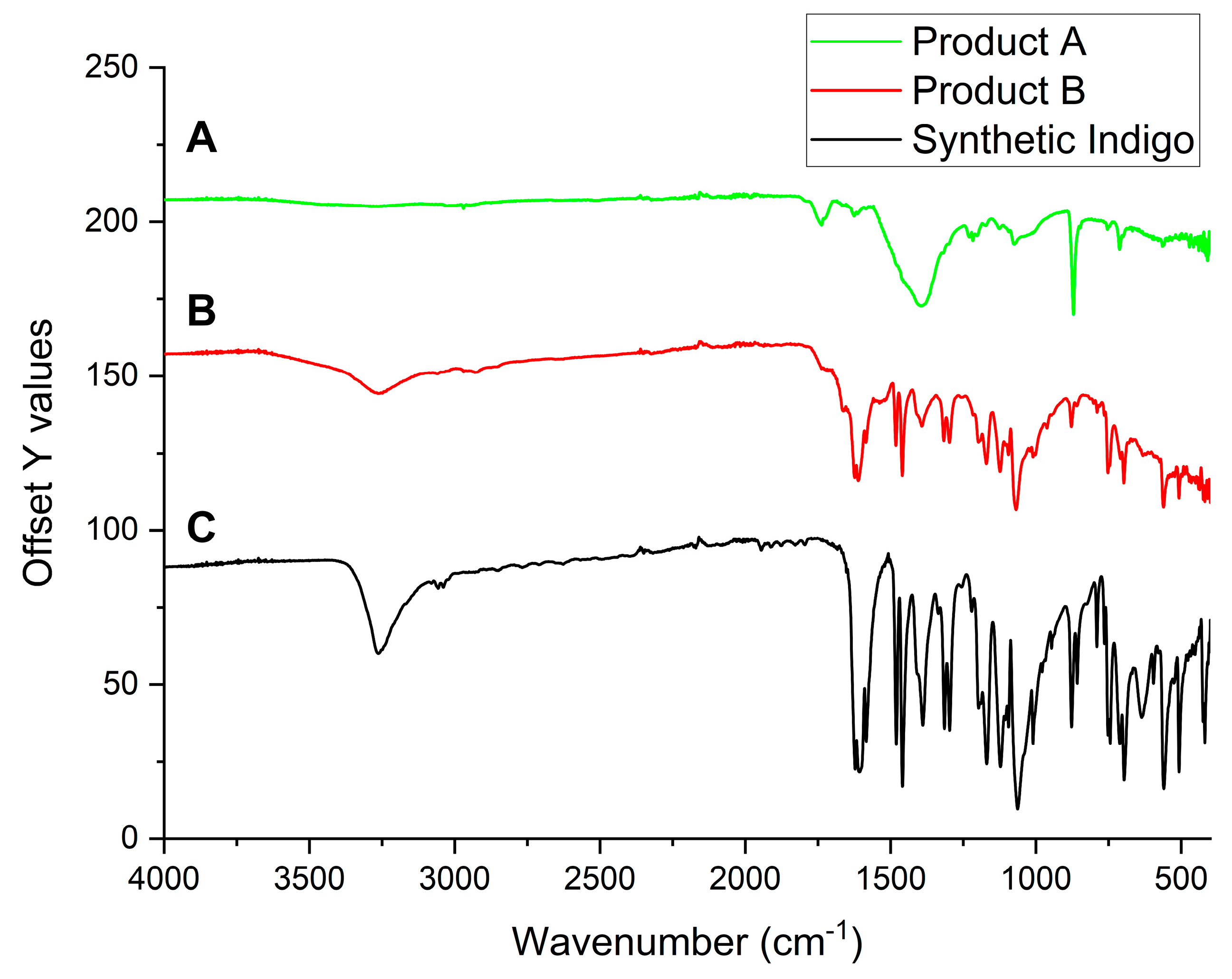
2.5. IR Spectra
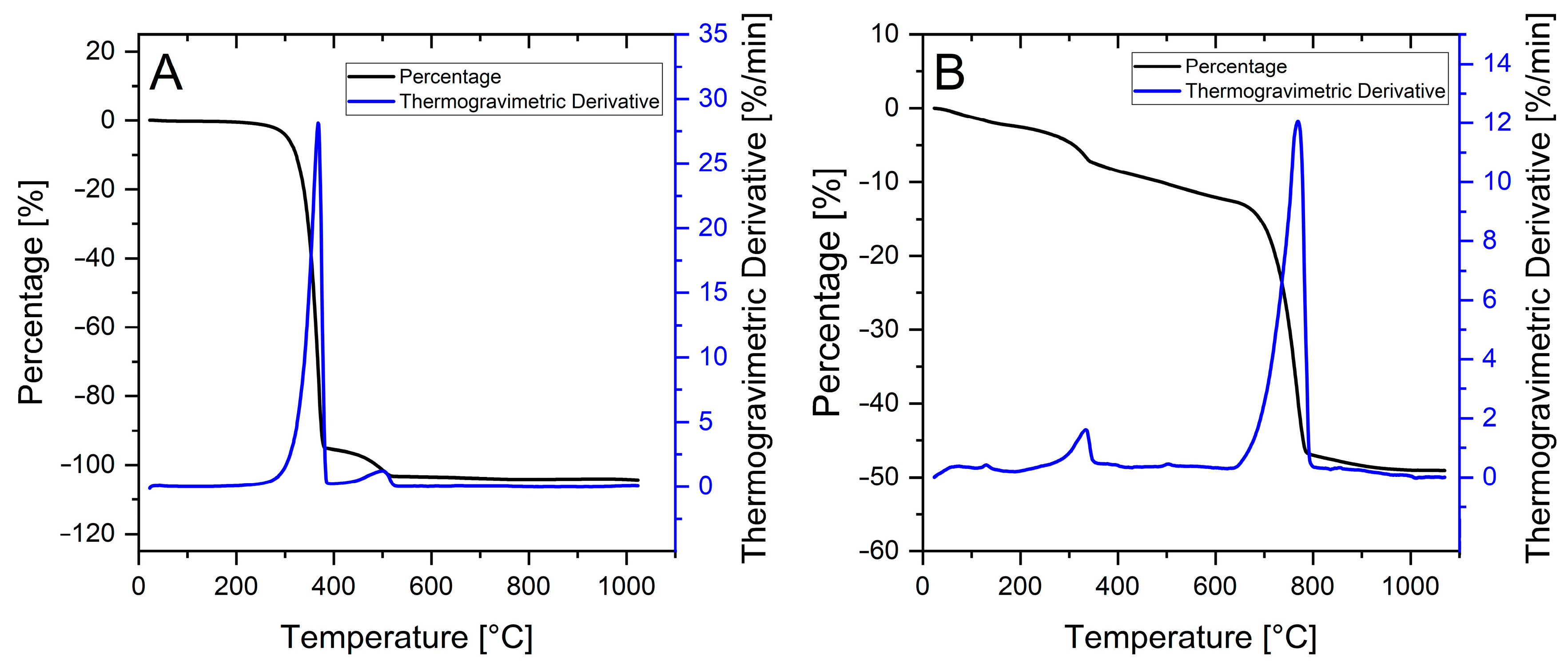
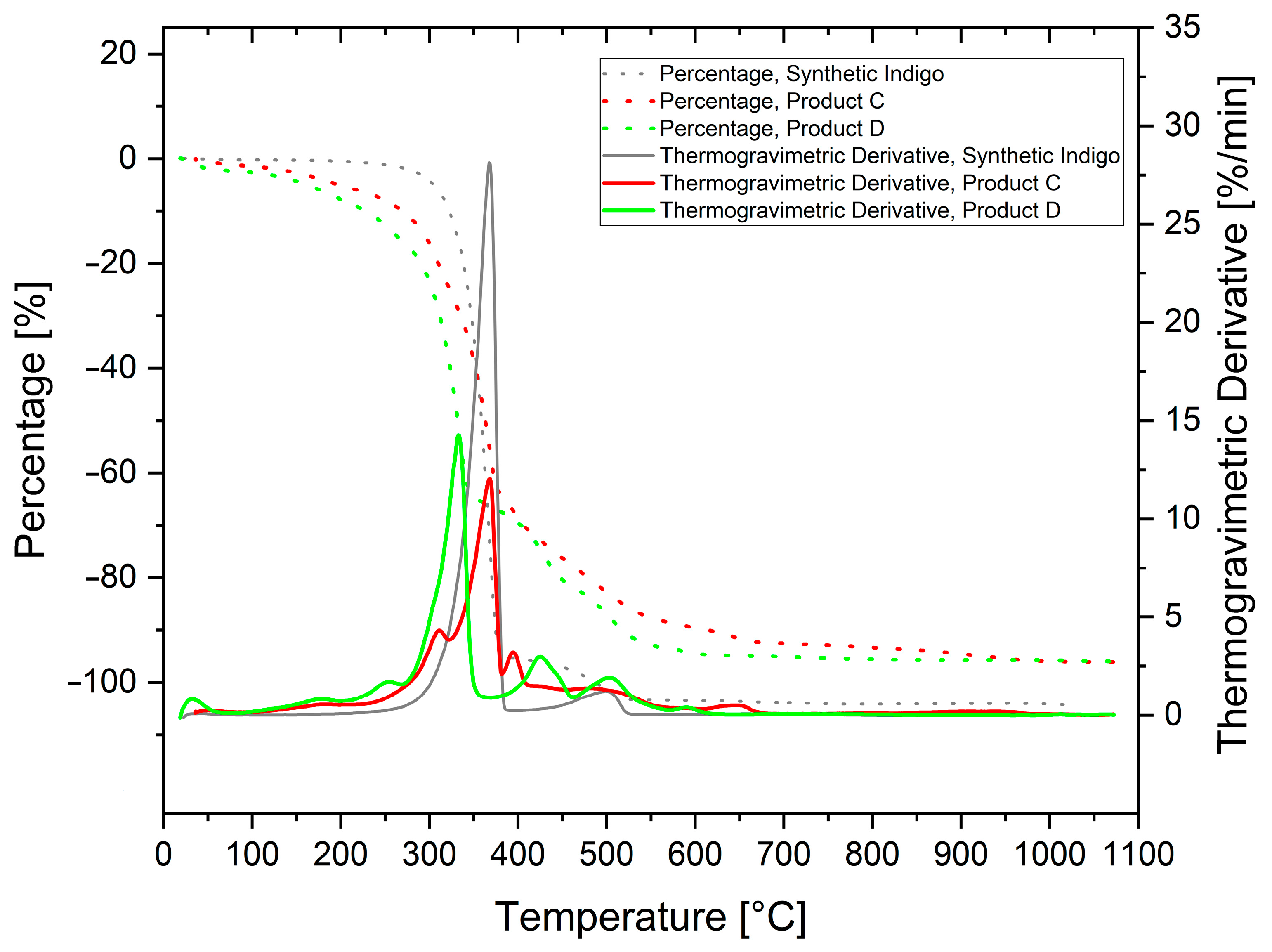
2.6. Thermogravimetric Analysis
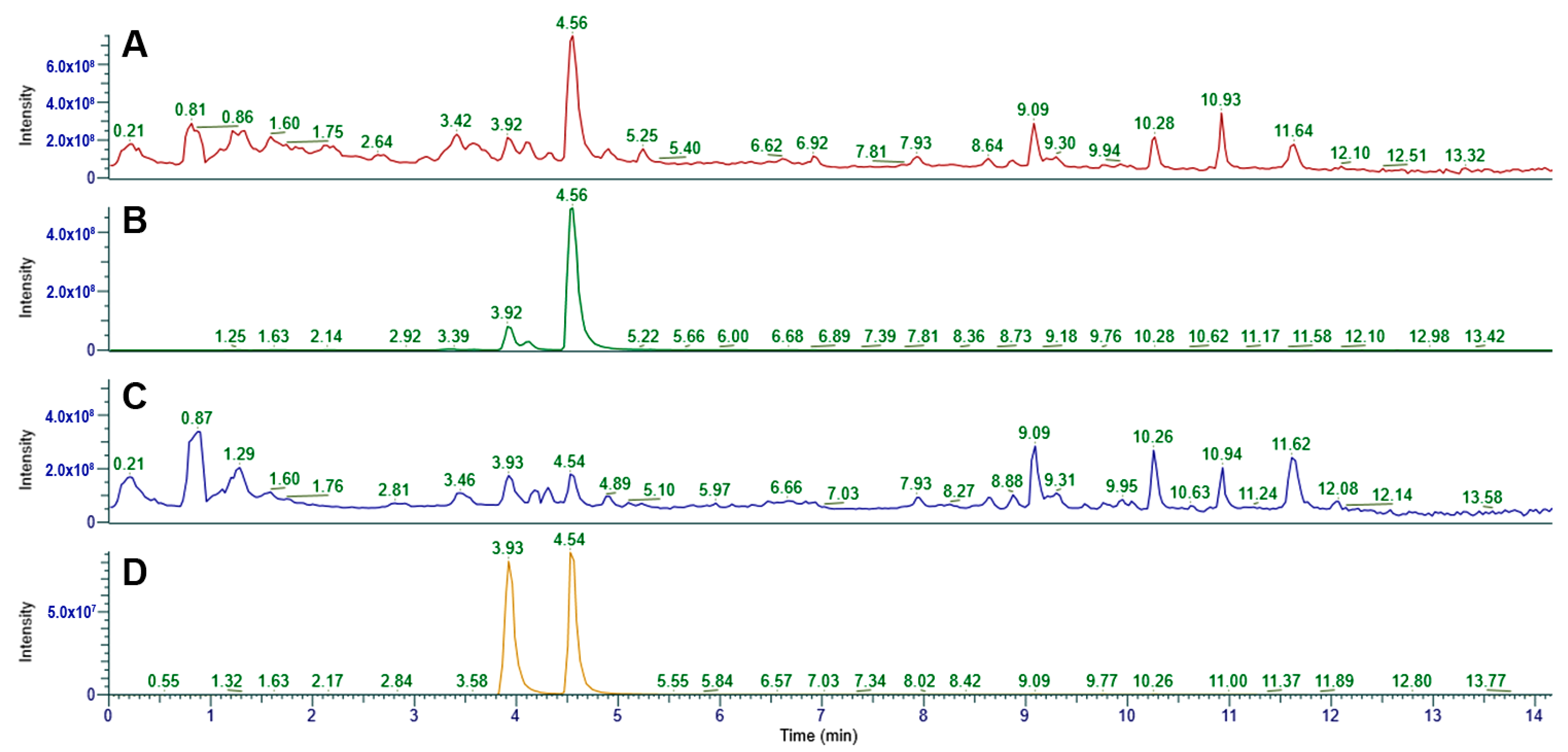
2.7. UHPLC-MS/MS Analysis
3. Results and Discussion
3.1. Sequentiual Extraction
3.2. Continuous Extraction
3.3. Trasmittance IR Spectra
3.4. Thermogravimetric Analysis
3.5. UHPLC-MS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stasiak, N.; Kukuła-Koch, W.; Głowniak, K. Modern Industrial and Pharmacological Applications of Indigo Dye and Its Derivatives—A Review. Acta Pol. Pharm. 2014, 71, 215–221. [Google Scholar] [PubMed]
- Comlekcioglu, N.; Efe, L.; Karaman, S. Extraction of Indigo from Some Isatis Species and Dyeing Standardization Using Low-Technology Methods. Braz. Arch. Biol. Technol. 2015, 58, 96–102. [Google Scholar] [CrossRef]
- Min, G.-Y.; Kim, J.-H.; Kim, T.-I.; Cho, W.-K.; Yang, J.-H.; Ma, J.-Y. Indigo Pulverata Levis (Chung-Dae, Persicaria Tinctoria) Alleviates Atopic Dermatitis-like Inflammatory Responses In Vivo and In Vitro. Int. J. Mol. Sci. 2022, 23, 553. [Google Scholar] [CrossRef]
- Ahn, C.; Zeng, X.; Obendorf, S.K. Simultaneous Analysis of the Coloring Compounds in Indigo, Phellodendron Bark, and Madder Dye Using HPLC-DAD-MS. J. Korean Soc. Cloth. Text. 2013, 37, 827–836. [Google Scholar] [CrossRef]
- Mocquard, J.; Le Lamer, A.-C.; Fabre, P.-L.; Mathieu, C.; Chastrette, C.; Vitrai, A.; Vandenbossche, V. Indigo Dyeing from Isatis Tinctoria L.: From Medieval to Modern Use. Dye. Pigment. 2022, 207, 110675. [Google Scholar] [CrossRef]
- Stoker, K.G.; Cooke, D.T.; Hill, D.J. An Improved Method for the Large-Scale Processing of Woad (Isatis Tinctoria) for Possible Commercial Production of Woad Indigo. J. Agric. Eng. Res. 1998, 71, 315–320. [Google Scholar] [CrossRef]
- Zelentskii, A.N.; Fomin, G.V.; Kutafina, N.V.; Berlin, A.A. The Mechanism of Oxidation of Indoxyl and Its Analogs to Indigo and Indigoides. Russ. Chem. Bull. 1970, 19, 1105–1107. [Google Scholar] [CrossRef]
- Sánchez-Viesca, F.; Gómez, R. On the Formation Mechanism of Indigo Blue and Indigo Red from Vegetable Source. Mod. Chem. 2021, 9, 88. [Google Scholar] [CrossRef]
- Oberthur, C.; Graf, H.; Hamburger, M. The Content of Indigo Precursors in Leaves? A Comparative Study of Selected Accessions and Post-Harvest Treatments. Phytochemistry 2004, 65, 3261–3268. [Google Scholar] [CrossRef]
- Lv, W.; Huangfu, Z.; Wang, K.; Zhang, W.; Yao, J. Efficient Degradation of Indigo Wastewater by One-Step Electrochemical Oxidation and Electro-Flocculation. Pigment. Resin Technol. 2020, 50, 32–40. [Google Scholar] [CrossRef]
- Choi, K.-Y. A Review of Recent Progress in the Synthesis of Bio-Indigoids and Their Biologically Assisted End-Use Applications. Dye. Pigment. 2020, 181, 108570. [Google Scholar] [CrossRef]
- Johansen, J.D.; Mahler, V.; Lepoittevin, J.-P.; Frosch, P.J. (Eds.) Contact Dermatitis, 6th ed.; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-36334-5. [Google Scholar]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Catucci, G.; Turella, S.; Cheropkina, H.; De Angelis, M.; Gilardi, G.; Sadeghi, S.J. Green Production of Indigo and Indirubin by an Engineered Baeyer–Villiger Monooxygenase. Biocatal. Agric. Biotechnol. 2022, 44, 102458. [Google Scholar] [CrossRef]
- Ma, L.; Sun, T.; Liu, Y.; Zhao, Y.; Liu, X.; Li, Y.; Chen, X.; Cao, L.; Kang, Q.; Guo, J.; et al. Enzymatic Synthesis of Indigo Derivatives by Tuning P450 BM3 Peroxygenases. Synth. Syst. Biotechnol. 2023, 8, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Y.; Wang, F.; Wang, J.; Shoji, O.; Xu, J. Construction of Biocatalysts Using the P450 Scaffold for the Synthesis of Indigo from Indole. Int. J. Mol. Sci. 2023, 24, 2395. [Google Scholar] [CrossRef]
- Rousseau, O.; Ebert, M.C.C.J.C.; Quaglia, D.; Fendri, A.; Parisien, A.H.; Besna, J.N.; Iyathurai, S.; Pelletier, J.N. Indigo Formation and Rapid NADPH Consumption Provide Robust Prediction of Raspberry Ketone Synthesis by Engineered Cytochrome P450 BM3. ChemCatChem 2020, 12, 837–845. [Google Scholar] [CrossRef]
- Núñez-Navarro, N.; Salazar Muñoz, J.; Castillo, F.; Ramírez-Sarmiento, C.A.; Poblete-Castro, I.; Zacconi, F.C.; Parra, L.P. Discovery of New Phenylacetone Monooxygenase Variants for the Development of Substituted Indigoids through Biocatalysis. Int. J. Mol. Sci. 2022, 23, 12544. [Google Scholar] [CrossRef]
- Linke, J.A.; Rayat, A.; Ward, J.M. Production of Indigo by Recombinant Bacteria. Bioresour. Bioprocess. 2023, 10, 20. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Wei, C.; Wang, J.; Xu, Y.; Bai, G.; Yao, Q.; Zhang, L.; Chen, Y. Anticancer Potential of Indirubins in Medicinal Chemistry: Biological Activity, Structural Modification, and Structure-Activity Relationship. Eur. J. Med. Chem. 2021, 223, 113652. [Google Scholar] [CrossRef]
- Kunikata, T.; Tatefuji, T.; Aga, H.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Indirubin Inhibits Inflammatory Reactions in Delayed-Type Hypersensitivity. Eur. J. Pharmacol. 2000, 410, 93–100. [Google Scholar] [CrossRef]
- Plitzko, I.; Mohn, T.; Sedlacek, N.; Hamburger, M. Composition of Indigo naturalis. Planta Med. 2009, 75, 860–863. [Google Scholar] [CrossRef]
- Roncaglia, F.; Forti, L.; D’Anna, S.; Maletti, L. An Expedient Catalytic Process to Obtain Solketal from Biobased Glycerol. Processes 2021, 9, 141. [Google Scholar] [CrossRef]
- Salzillo, T.; d’Agostino, S.; Rivalta, A.; Giunchi, A.; Brillante, A.; Della Valle, R.G.; Bedoya-Martínez, N.; Zojer, E.; Grepioni, F.; Venuti, E. Structural, Spectroscopic, and Computational Characterization of the Concomitant Polymorphs of the Natural Semiconductor Indigo. J. Phys. Chem. C 2018, 122, 18422–18431. [Google Scholar] [CrossRef]
- Maugard, T.; Enaud, E.; Choisy, P.; Legoy, M.D. Identification of an Indigo Precursor from Leaves of Isatis Tinctoria (Woad). Phytochemistry 2001, 58, 897–904. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chin, T.; Liang, D.T.; Chen, H.; Zheng, C. Thermogravimetric Analysis−Fourier Transform Infrared Analysis of Palm Oil Waste Pyrolysis. Energy Fuels 2004, 18, 1814–1821. [Google Scholar] [CrossRef]
- Zou, P.; Koh, H.L. Determination of Indican, Isatin, Indirubin and Indigotin inIsatis Indigotica by Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1239–1246. [Google Scholar] [CrossRef]
- Mantzouris, D.; Karapanagiotis, I.; Panayiotou, C. Comparison of Extraction Methods for the Analysis of Indigofera Tinctoria and Carthamus Tinctorius in Textiles by High Performance Liquid Chromatography. Microchem. J. 2014, 115, 78–86. [Google Scholar] [CrossRef]




| Time (min) | 15 | 60 | 180 | 240 | |
|---|---|---|---|---|---|
| Solvent | |||||
| acetone | 20% b | 42% | 100% | - | |
| methanol | 13% | 20% b | 69% | 100% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frignani, E.; D’Eusanio, V.; Grandi, M.; Pigani, L.; Roncaglia, F. A Continuous Extraction Protocol for the Characterisation of a Sustainably Produced Natural Indigo Pigment. Life 2024, 14, 59. https://doi.org/10.3390/life14010059
Frignani E, D’Eusanio V, Grandi M, Pigani L, Roncaglia F. A Continuous Extraction Protocol for the Characterisation of a Sustainably Produced Natural Indigo Pigment. Life. 2024; 14(1):59. https://doi.org/10.3390/life14010059
Chicago/Turabian StyleFrignani, Elia, Veronica D’Eusanio, Mauro Grandi, Laura Pigani, and Fabrizio Roncaglia. 2024. "A Continuous Extraction Protocol for the Characterisation of a Sustainably Produced Natural Indigo Pigment" Life 14, no. 1: 59. https://doi.org/10.3390/life14010059
APA StyleFrignani, E., D’Eusanio, V., Grandi, M., Pigani, L., & Roncaglia, F. (2024). A Continuous Extraction Protocol for the Characterisation of a Sustainably Produced Natural Indigo Pigment. Life, 14(1), 59. https://doi.org/10.3390/life14010059







