Large Lung Consolidation: A Rare Presentation of Pulmonary Sarcoidosis
Abstract
1. Introduction
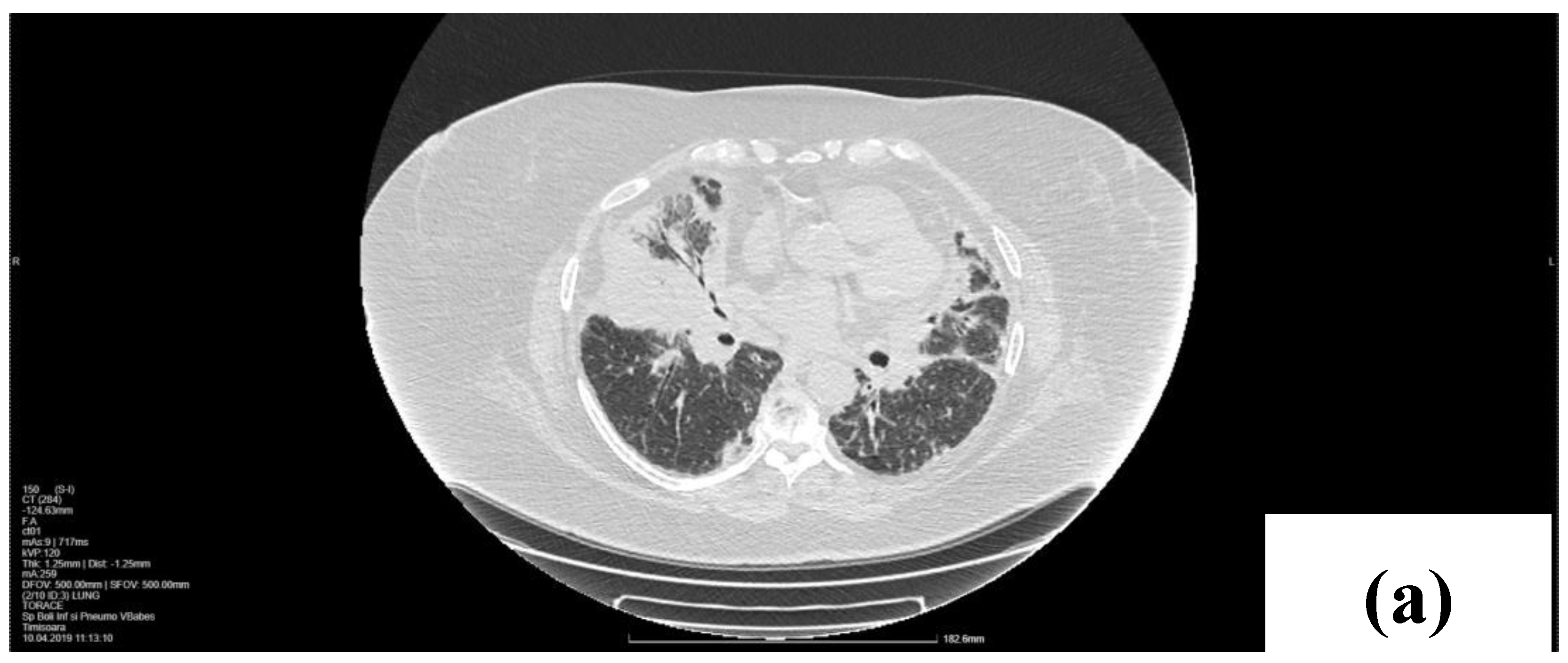
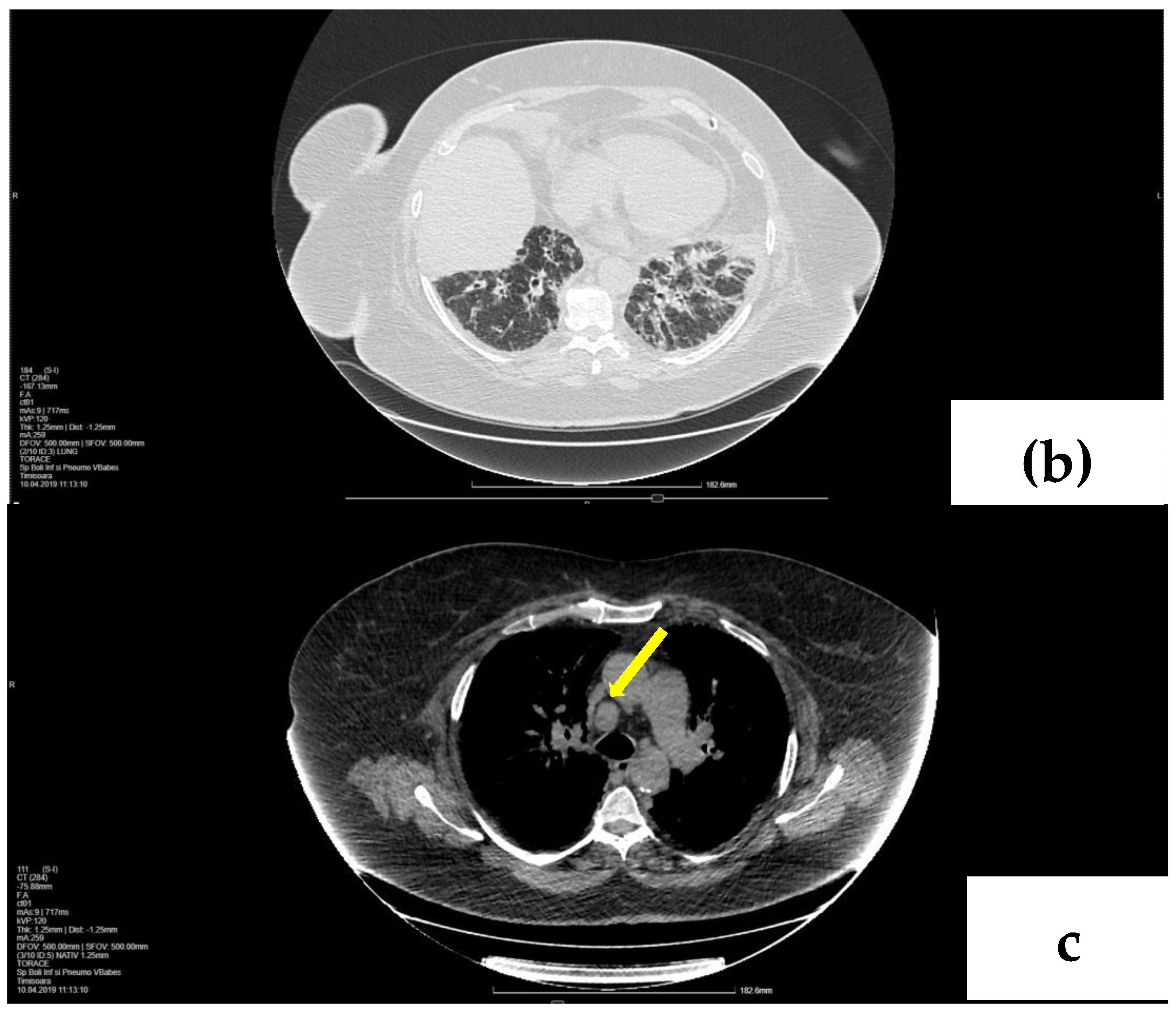
2. Case Presentation
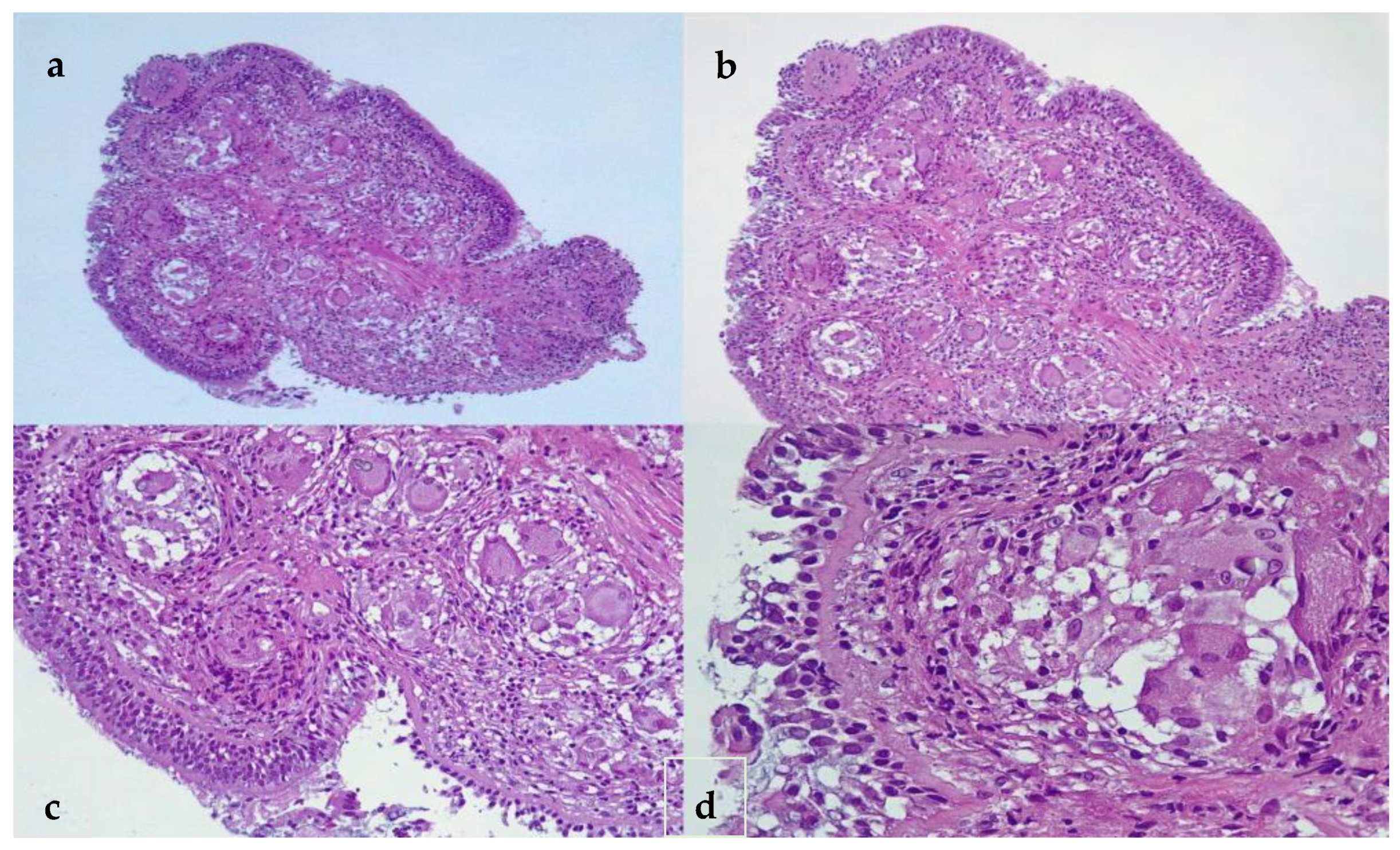
2.1. Laboratory, Radiological, and Histological Examinations
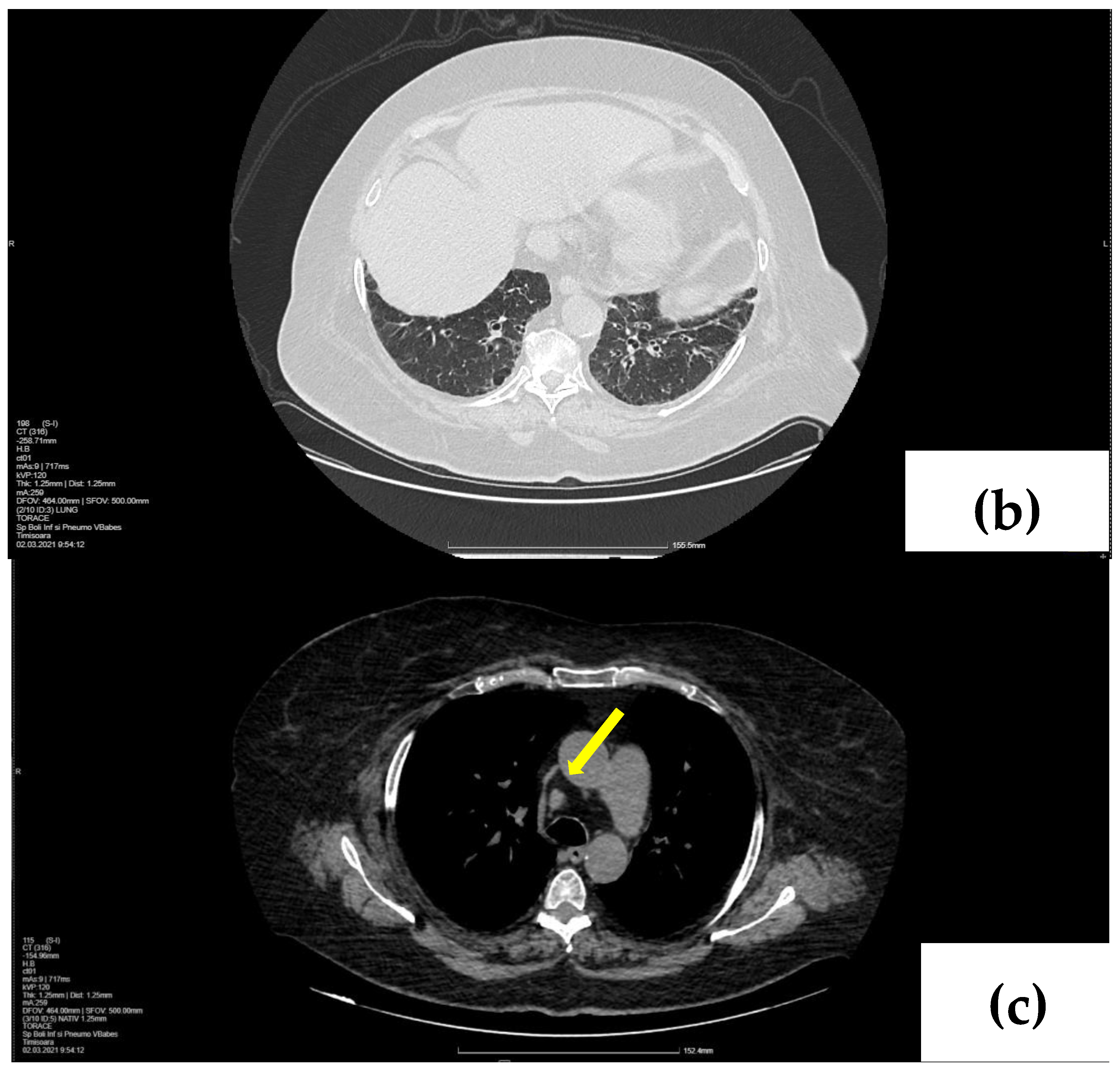
2.2. Treatment and Outcome
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martusewicz-Boros, M.M.; Piotrowski, W.J. Diagnosis of sarcoidosis-the updated ATS 2020 recommendations through the prism of everyday clinical practice. Adv. Respir. Med. 2020, 88, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Yadav, D.; Puranik, N.; Guleria, R.; Jin, J.-O. Sarcoidosis: Causes, Diagnosis, Clinical Features, and Treatments. J. Clin. Med. 2020, 9, 1081. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.P.; Culver, D.A.; Judson, M.A. A concise review of pulmonary sarcoidosis. Am. J. Respir. Crit. Care Med. 2011, 183, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, M.C.; Rybicki, B.A.; Teirstein, A.S. Sarcoidosis. N. Engl. J. Med. 2007, 357, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Criado, E.; Sánchez, M.; Ramírez, J.; Arguis, P.; De Caralt, T.M.; Perea, R.J.; Xaubet, A. Pulmonary sarcoidosis: Typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics 2010, 30, 1567–1586. [Google Scholar] [CrossRef]
- Margaritopoulos, G.A.; Proklou, A.; Lagoudaki, E.; Voloudaki, A.; Siafakas, N.M.; Antoniou, K.M. Sarcoidosis in a 65-year-old woman presenting with a lung mass and pericardial effusion: A case report. J. Med. Case Rep. 2012, 6, 259. [Google Scholar] [CrossRef]
- Crouser, E.D.; Maier, L.A.; Wilson, K.C.; Bonham, C.A.; Morgenthau, A.S.; Patterson, K.C.; Abston, E.; Bernstein, R.C.; Blankstein, R.; Chen, E.S. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 201, e26–e51. [Google Scholar] [CrossRef]
- Rosen, Y. Four decades of necrotizing sarcoid granulomatosis: What do we know now? Arch. Pathol. Lab. Med. 2015, 139, 252–262. [Google Scholar] [CrossRef]
- Keijsers, R.G.; Veltkamp, M.; Grutters, J.C. Chest Imaging. Clin. Chest Med. 2015, 36, 603–619. [Google Scholar] [CrossRef]
- Msaad, S.; Ketata, W.; Abid, N.; Abid, H.; Ayadi, L.; Mnif, J.; Boudawara, T.; Ayoub, A. Phénotype pseudotumoral de la sarcoïdose: À propos de deux cas. Rev. Mal. Respir. 2013, 30, 794–800. [Google Scholar] [CrossRef]
- Pattnaik, B.; Sryma, P.B.; Mittal, S.; Agrawal, A.; Guleria, R.; Madan, K. MicroRNAs in pulmonary sarcoidosis: A systematic review. Respir. Investig. 2020, 58, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, D.W.; Yaggi, M.; Homer, R.; Herzog, E.L.; Ryu, C. A rare presentation of pulmonary sarcoidosis as a solitary lung mass: A case report. J. Med. Case Rep. 2018, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Mulkareddy, V.; Bhalla, V.; Gurell, M. Sarcoidosis presenting as a solitary pulmonary mass. Respir. Med. Case Rep. 2020, 31, 101256. [Google Scholar] [CrossRef] [PubMed]
- d’Alessandro, M.; Conticini, E.; Bergantini, L.; Mezzasalma, F.; Cameli, P.; Baglioni, S.; Armati, M.; Abbritti, M.; Bargagli, E. PD1, CTLA4 and TIGIT Expression on T and NK Cells in Granulomatous Diseases: Sarcoidosis and ANCA-Associated Vasculitis. Int. J. Mol. Sci. 2022, 24, 256. [Google Scholar] [CrossRef]
- Haddadi, S.; Adkinson, B.C.; Holt, G.E.; Mirsaeidi, M. Sarcoidosis or cancer? That is the question. Respir. Med. Case Rep. 2021, 33, 101426. [Google Scholar] [PubMed]
- Shin, H.J.; Kim, M.S.; Kho, B.G.; Park, H.Y.; Kim, T.O.; Park, C.K.; Oh, I.J.; Kim, Y.I.; Kim, Y.C.; Choi, Y.D.; et al. Delayed diagnosis of lung cancer due to misdiagnosis as worsening of sarcoidosis: A case report. BMC Pulm. Med. 2020, 20, 71. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.J. Sarcoidosis: A reare cause of solitary pulmonary nodule. J. Lung Pulm Respir. Res. 2014, 1, 00022. [Google Scholar] [CrossRef][Green Version]
- Malaisamy, S.; Dalal, B.; Bimenyuy, C.; Soubani, A.O. The clinical and radiologic features of nodular pulmonary sarcoidosis. Lung 2009, 187, 9–15. [Google Scholar] [CrossRef]
- Taketa, T.; Nakamura, T. Pulmonary sarcoidosis presenting as a solitary nodule mimicking lung cancer. Clin. Case Rep. 2021, 9, e04208. [Google Scholar] [CrossRef]
- Tana, C.; Donatiello, I.; Coppola, M.G.; Ricci, F.; Maccarone, M.T.; Ciarambino, T.; Cipollone, F.; Giamberardino, M.A. CT findings in pulmonary and abdominal sarcoidosis. Implications for diagnosis and classification. J. Clin. Med. 2020, 9, 3028. [Google Scholar] [CrossRef]
- Tana, C.; Tchernev, G.; Chokoeva, A.A.; Wollina, U.; Lotti, T.; Fioranelli, M.; Roccia, M.G.; Maximov, G.K.; Silingardi, M. Pulmonary and abdominal sarcoidosis, the great imitators on imaging? J. Biol. Regul. Homeost Agents 2016, 30 (Suppl. S2), 45–48. [Google Scholar]
- Nunes, H.; Uzunhan, Y.; Gille, T.; Lamberto, C.; Valeyre, D.; Brillet, P.-Y. Imaging of sarcoidosis of the airways and lung parenchyma and correlation with lung function. Eur. Respir. J. 2012, 40, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Guidry, C.; Fricke, R.G.; Ram, R.; Pandey, T.; Jambhekar, K. Imaging of Sarcoidosis: A Contemporary Review. Radiol. Clin. N. Am. 2016, 54, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Arar, O.; Boni, F.; Meschi, T.; Tana, C. Pulmonary Sarcoidosis Presenting with Miliary Opacities. Curr. Med. Imaging Rev. United Arab. Emir. 2019, 15, 81–83. [Google Scholar] [CrossRef]
- Rochat, T.S.; Janssens, J.-P.; Soccal, P.M.; Adler, D. Update on the treatment of sarcoidosis. Rev. Med. Suisse. 2016, 12, 1966–1971. [Google Scholar]
- Beegle, S.H.; Barba, K.; Gobunsuy, R.; Judson, M.A. Current and emerging pharmacological treatments for sarcoidosis: A review. Drug Des. Devel Ther. 2013, 7, 325–338. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marc, M.S.; Pescaru, C.C.; Costin, E.O.; Crisan, A.F.; Maritescu, A.; Pescaru, A.; Suppini, N.; Olteanu, G.E.; Traila, D.; Oancea, C.; et al. Large Lung Consolidation: A Rare Presentation of Pulmonary Sarcoidosis. Life 2024, 14, 44. https://doi.org/10.3390/life14010044
Marc MS, Pescaru CC, Costin EO, Crisan AF, Maritescu A, Pescaru A, Suppini N, Olteanu GE, Traila D, Oancea C, et al. Large Lung Consolidation: A Rare Presentation of Pulmonary Sarcoidosis. Life. 2024; 14(1):44. https://doi.org/10.3390/life14010044
Chicago/Turabian StyleMarc, Monica Steluta, Camelia Corina Pescaru, Emanuela Oana Costin, Alexandru Florian Crisan, Adelina Maritescu, Andrei Pescaru, Noemi Suppini, Gheorghe Emilian Olteanu, Daniel Traila, Cristian Oancea, and et al. 2024. "Large Lung Consolidation: A Rare Presentation of Pulmonary Sarcoidosis" Life 14, no. 1: 44. https://doi.org/10.3390/life14010044
APA StyleMarc, M. S., Pescaru, C. C., Costin, E. O., Crisan, A. F., Maritescu, A., Pescaru, A., Suppini, N., Olteanu, G. E., Traila, D., Oancea, C., & Manolescu, D. (2024). Large Lung Consolidation: A Rare Presentation of Pulmonary Sarcoidosis. Life, 14(1), 44. https://doi.org/10.3390/life14010044








