Diversity of Endophytes of Actinidia arguta in Different Seasons
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Genomic DNA Extraction, PCR Amplification, and Sequencing
2.3. Environmental Factor Determination
2.4. Statistical Analysis
3. Results
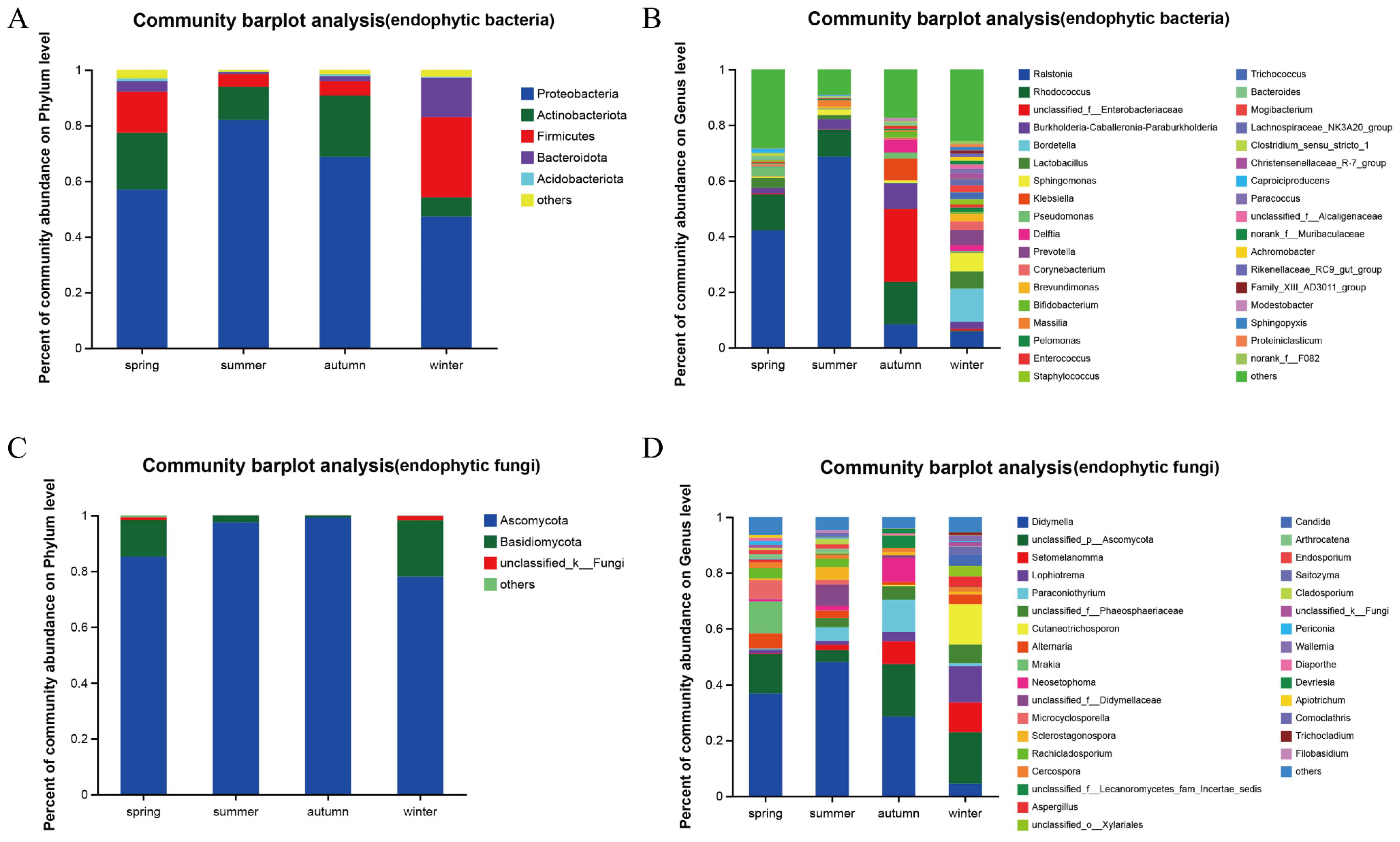
3.1. Diversity Analysis of Endophytic Bacteria in Actinidia arguta
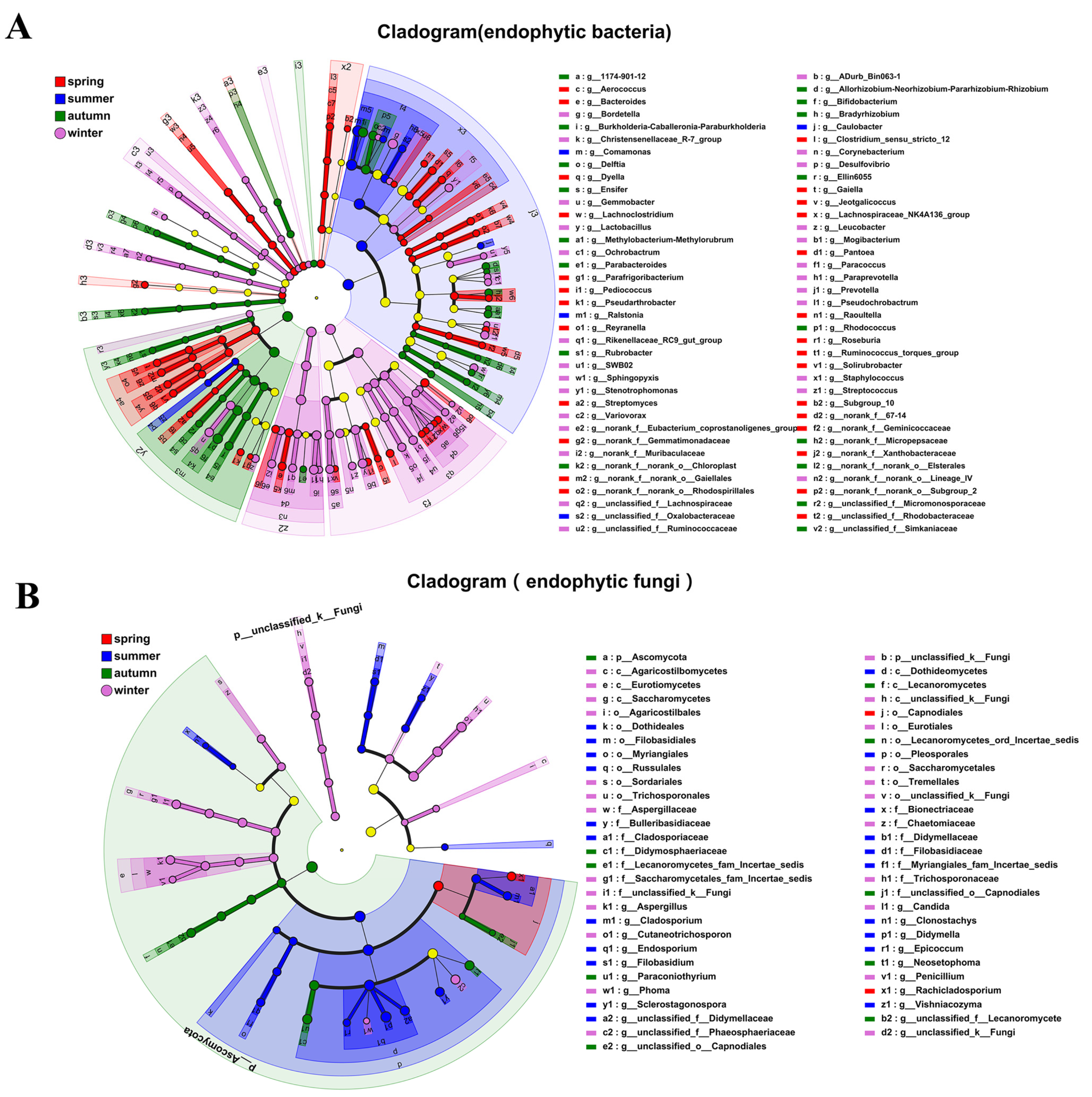
3.2. Community Composition and Diversity Analysis of Actinidia arguta Endophytes
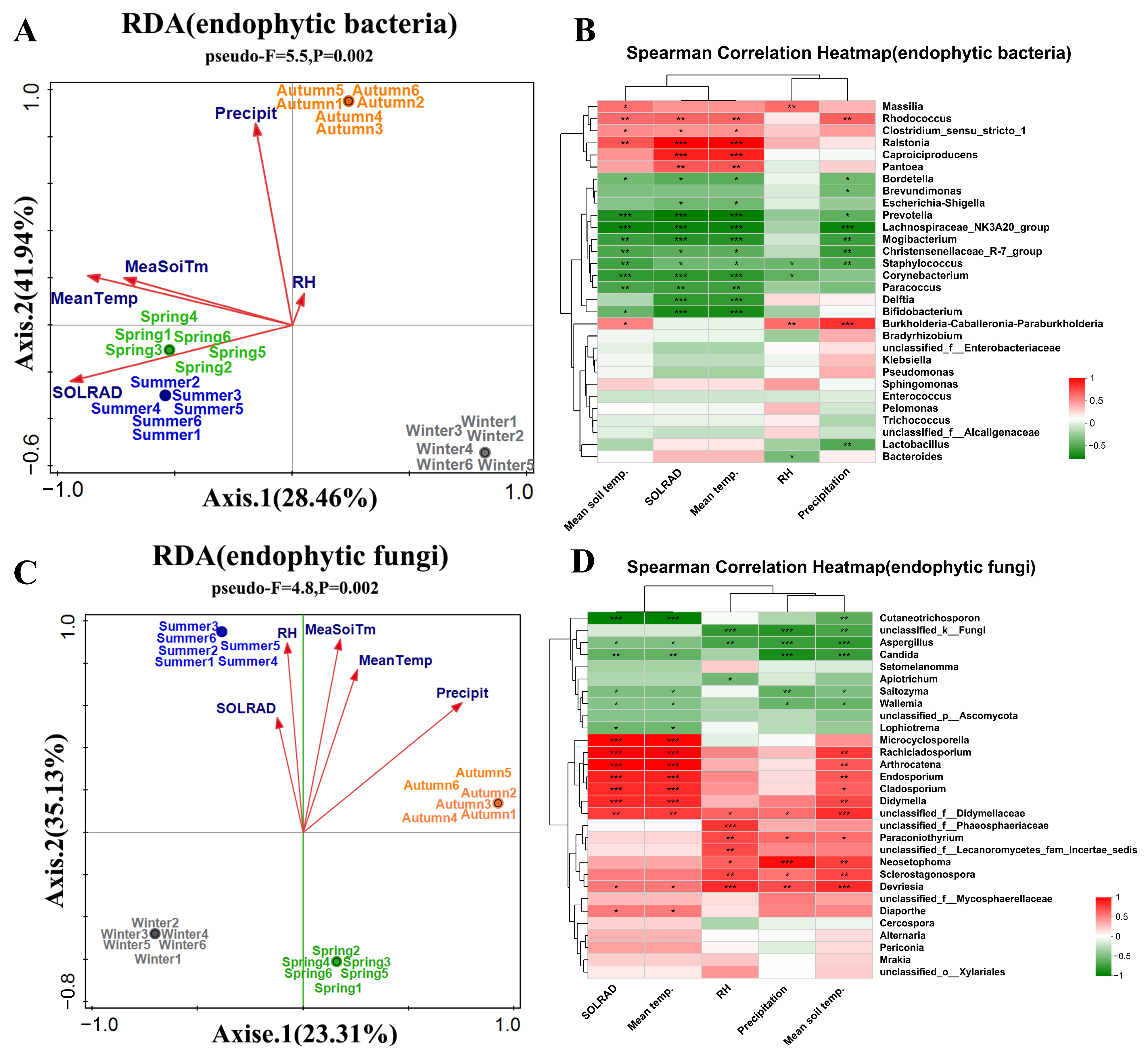
3.3. Effects of Environmental Factors on the Community Structure of Endophytic Bacteria in Actinidia arguta
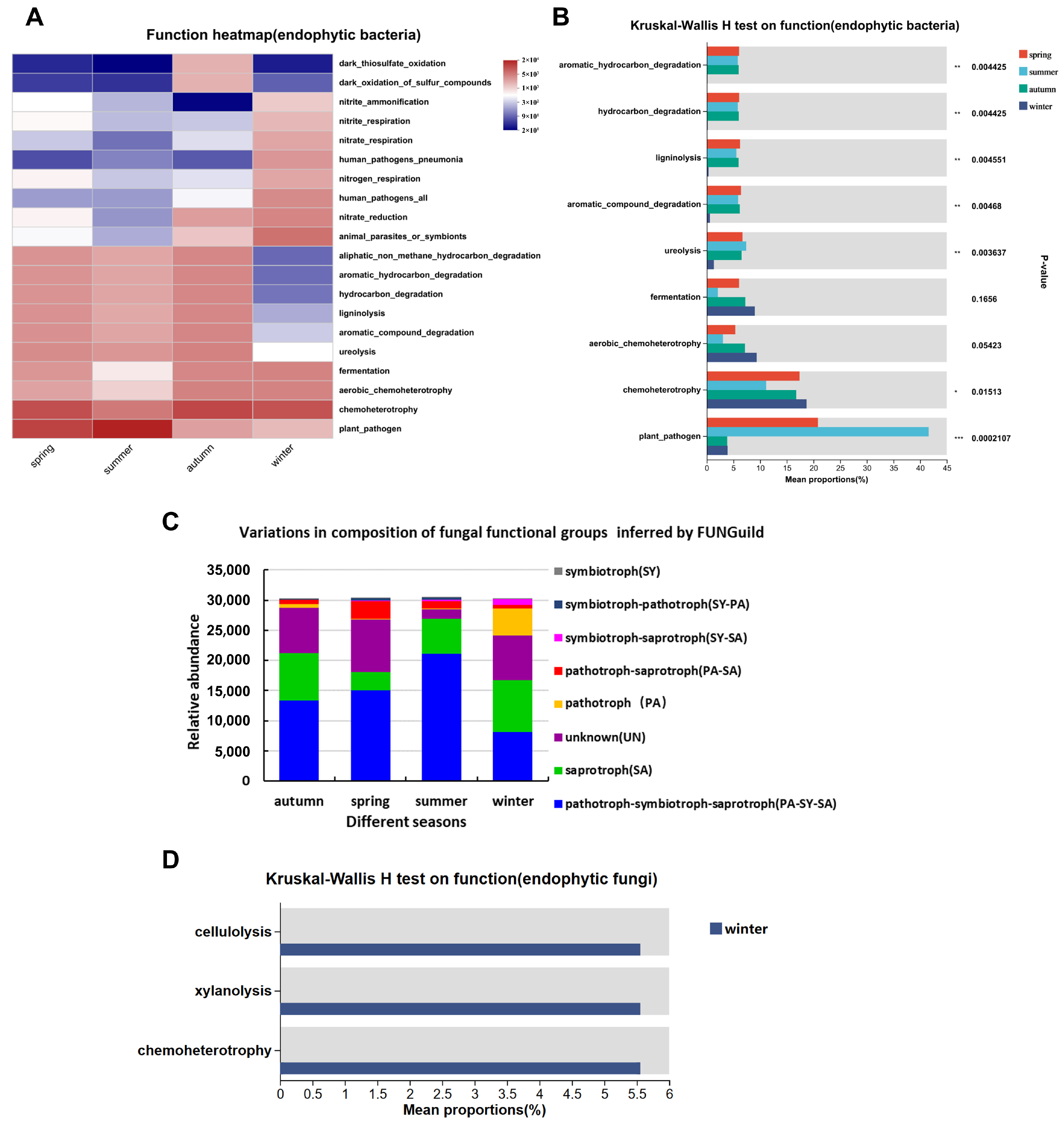
3.4. Analysis of Functional Composition and Inter-Group Differences of Endophytic Bacteria in Actinidia arguta
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrini, O. Fungal endophytes of tree leaves. In Microbial Ecology of Leaves; Springer: New York, NY, USA, 1991; pp. 179–197. [Google Scholar]
- Gange, A.C.; Eschen, R.; Wearn, J.A.; Thawer, A.; Sutton, B.C. Differential effects of foliar endophytic fungi on insect herbivores attacking a herbaceous plant. Oecologia 2012, 168, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.L.; Balsemão-Pires, E.; Saraiva, R.M.; Ferreira, P.C.; Hemerly, A.S. Nitrogen signalling in plant interactions with associative and endophytic diazotrophic bacteria. J. Exp. Bot. 2014, 65, 5631–5642. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda Mdel, C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic bacteria: A new source of bioactive compounds. 3 Biotech 2017, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Hu, T.; Luo, H.; Alam, N.U.; Xin, J.; Li, H.; Lin, Y.; Huang, J.; Huang, K.; Meng, Y.; et al. A carotenoid- and poly-β-hydroxybutyrate-free mutant strain of sphingomonas elodea atcc 31461 for the commercial production of gellan. mSphere 2019, 4, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Barik, M.; Das, C.P.; Verma, A.K.; Sahoo, S.; Sahoo, N.K. Metabolic profiling of phenol biodegradation by an indigenous rhodococcus pyridinivorans strain pdb9t n-1 isolated from paper pulp wastewater. Int. Biodeterior. Biodegrad. 2021, 158, 105168. [Google Scholar] [CrossRef]
- Oukala, N.; Aissat, K.; Pastor, V. Bacterial endophytes: The hidden actor in plant immune responses against biotic stress. Plants 2021, 10, 1012. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Li, J.; Zhang, W.; Pan, Y.; Liu, X. Investigating the Role of Endophytic Fungi in Gentiana scabra bge. by Cross-Growth Period Inoculation. Indian J. Microbiol. 2018, 58, 319–325. [Google Scholar] [CrossRef]
- Adhikari, P.; Pandey, A. Bioprospecting plant growth promoting endophytic bacteria isolated from Himalayan yew (Taxus wallichiana Zucc.). Microbiol. Res. 2020, 239, 126536. [Google Scholar] [CrossRef]
- Li, D.; Bodjrenou, D.M.; Zhang, S.; Wang, B.; Pan, H.; Yeh, K.W.; Lai, Z.; Cheng, C. The Endophytic Fungus Piriformospora indica Reprograms Banana to Cold Resistance. Int. J. Mol. Sci. 2021, 22, 4973. [Google Scholar] [CrossRef]
- Jaiswal, S.; Ojha, A.; Mishra, S.K. Assessment of Plant Growth-Promoting Parameters of Endophytes Isolated from Calotropis procera and Their Performance Under Irrigated and Non-irrigated Conditions. Curr. Microbiol. 2023, 81, 49. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, W.; Xu, W.; Ji, C.; Zhou, Y.; Sun, J.; Tian, Y.; Li, Y.; Zhao, F.; Tian, Y. Antimicrobial and cytotoxic activity of endophytic fungi from lagopsis supina. J. Microbiol. Biotechnol. 2023, 33, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chen, Z.; Wei, X.; Chen, S.; Nan, Z. A toxic grass achnatherum inebrians serves as a diversity refuge for the soil fungal community in rangelands of northern china. Plant Soil 2020, 448, 425–438. [Google Scholar] [CrossRef]
- Xu, G.; Liu, Y.; Cao, P.; Liu, X. Diversity of endophytic bacteria of Oxytropis glacialis in different ecological environments on the Tibetan Plateau. Plateau Sci. Res. 2020, 4, 20–29. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, J.; Sun, X.; Tang, Q.Y.; Zhang, Z.D. Seasonal Dynamics and Persistency of Endophyte Communities in Kalidium schrenkianum Shifts Under Radiation Stress. Front. Microbiol. 2021, 12, 778327. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wan, D.; Tan, L.; Liu, H. Dynamic changes of endophytic bacteria in the bark and leaves of medicinal plant Eucommia ulmoides in different seasons. Microbiol. Res. 2023, 280, 127567. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Liu, X.; Yang, J.; Wang, Y.; Yao, K.; Huo, Q.; Fu, Y.; Wei, Y.; Guo, B. The temporal and spatial endophytic fungal community of Huperzia serrata: Diversity and relevance to huperzine A production by the host. BMC Microbiol. 2022, 22, 281. [Google Scholar] [CrossRef]
- Ou, T.; Xu, W.F.; Wang, F.; Strobel, G.; Zhou, Z.Y.; Xiang, Z.H.; Liu, J.; Xie, J. A Microbiome Study Reveals Seasonal Variation in Endophytic Bacteria Among different Mulberry Cultivars. Comput. Struct. Biotechnol. J. 2019, 17, 1091–1100. [Google Scholar] [CrossRef]
- Niu, Q.; Shen, J.; Liu, Y.; Nie, C.; Scripcenco, N.V.; Liu, D. Research progress on main active constituents and pharmacological activities of Actinidia arguta. Sci. Technol. Food Ind. 2019, 40, 333–338+344. (In Chinese) [Google Scholar] [CrossRef]
- Gong, D.S.; Sharma, K.; Kang, K.W.; Kim, D.W.; Oak, M.H. Endothelium-dependent relaxation effects of actinidia arguta extracts in coronary artery: Involvement of enos/akt pathway. J. Nanosci. Nanotechnol. 2020, 20, 5381–5384. [Google Scholar] [CrossRef]
- Sun, S.; Qi, X.; Wang, R.; Lin, M.; Fang, J. Evaluation of freezing tolerance inactinidiagermplasm based on relative electrolyte leakage. Hortic. Environ. Biotechnol. 2020, 61, 755–765. [Google Scholar] [CrossRef]
- Wen, X.; Qin, H.; Ai, J.; Wang, Y.; Han, X.; Li, C. Establishment and evaluation of resistance identification method for Pseudomonass yringae pv. actinidiae disease in Actinidia arguta germplasm resources. Plant Prot. 2021, 47, 193–199. (In Chinese) [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Q.; Ju, M.; Yan, S.; Zhang, Q.; Gu, P. The endophytic fungi diversity, community structure, and ecological function prediction of sophora alopecuroides in ningxia, china. Microorganisms 2022, 10, 2099. [Google Scholar] [CrossRef] [PubMed]
- Wijayawardene, N.N.; Bahram, M.; Sánchez-Castro, I.; Dai, D.Q.; Ariyawansa, K.; Jayalal, U.; Suwannarach, N. Current insight into culture-dependent and culture-independent methods in discovering ascomycetous taxa. J. Fungi 2021, 7, 703. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Dong, W.; Yan, D.H. Organs, cultivars, soil, and fruit properties affect structure of endophytic mycobiota of pinggu peach trees. Microorganisms 2019, 7, 322. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y. Diversity analysis of cultured endophytic bacteria in Codonopsis pilosula and effects on promoting host growth. LanZhou Univ. Technol. 2019. (In Chinese) [Google Scholar] [CrossRef]
- Liu, C.; Zhao, D.; Ma, W.; Guo, Y.; Wang, A.; Wang, Q.; Lee, D.J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Wang, H.F.; Zhang, Y.G.; Chen, J.Y.; Guo, J.W.; Li, L.; Hozzein, W.N.; Zhang, Y.M.; Wadaan, M.A.M.; Li, W.J. Frigoribacterium endophyticum sp. Nov., an endophytic actinobacterium isolated from the root of anabasis elatior (c. A. Mey.) schischk. Int. J. Syst. Evol. Microbiol. 2015, 65, 1207–1212. [Google Scholar] [CrossRef]
- Liu, H.; Qin, Y.; Fu, Y. A Low Temperature Tolerant Bacterium Pseudochrobactrum A4 and Its Application in Crop Disease Control. Patent CN106544289B, 27 August 2019. (In Chinese). [Google Scholar]
- Finkel, O.M.; Salas-González, I. A single bacterial genus maintains root growth in a complex microbiome. Nature 2020, 587, 103–108. [Google Scholar] [CrossRef]
- Viaene, T.; Langendries, S.; Beirinckx, S.; Maes, M.; Goormachtig, S. Streptomyces as a plant’s best friend? FEMS Microbiol. Ecol. 2016, 92, fiw119. [Google Scholar] [CrossRef]
- Cabrera, G.; Pérez, R.; Gómez, J.M.; Abalos, A.; Cantero, D. Toxic effects of dissolved heavy metals on Desulfovibrio vulgaris and Desulfovibrio sp. Strains. J. Hazard. Mater. 2006, 135, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Chen, Y.; Wang, D.; Shi, T.; Wu, X.; Ma, X.; Li, X.; Hua, R.; Tang, X.; Li, Q.X. Rapid biodegradation of organophosphorus pesticides by Stenotrophomonas sp. G1. J. Hazard. Mater. 2015, 297, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; Andolfi, A.; Nicoletti, R. The genus Cladosporium: A rich source of diverse and bioactive natural compounds. Molecules 2021, 26, 3959. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shao, S.; Liu, C.; Song, Z.; Liu, S.; Wu, S. The genus Paraconiothyrium: Species concepts, biological functions, and secondary metabolites. Crit. Rev. Microbiol. 2021, 47, 781–810. [Google Scholar] [CrossRef] [PubMed]
- Vailleau, F.; Genin, S. Ralstonia solanacearum: An arsenal of virulence strategies and prospects for resistance. Annu. Rev. Phytopathol. 2023, 61, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Truchon, A.N.; Dalsing, B.L.; Khokhani, D.; MacIntyre, A.; McDonald, B.R.; Ailloud, F.; Klassen, J. Plant-pathogenic Ralstonia phylotypes evolved divergent respiratory strategies and behaviors to thrive in xylem. Mbio 2023, 14, e0318822. [Google Scholar] [CrossRef]
- De Oliveira, M.; Atalla, A.A.; Farias Frihling, B.E.; Cavalheri, P.S.; Migliolo, L.; Magalhaes Filho, F.J.C. Ibuprofen and caffeine removal in vertical flow and free-floating macrophyte constructed wetlands with heliconia rostrata and Eichornia crassipes. Chem. Eng. J. 2019, 373, 458–467. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, R.; Zhang, Y.; Wu, Q.; Xie, B.; Yang, J.; Chen, J.; Sun, Z. Research progress of Rhodococcus and its biodegradation. Food Sci. 2016, 37, 254–258. (In Chinese) [Google Scholar]
- Bell, K.S.; Philp, J.C.; Aw, D.W.; Christofi, N. The genus Rhodococcus. J. Appl. Microbiol. 1998, 85, 195–210. [Google Scholar] [CrossRef]
- Nazari, M.T.; Simon, V.; Machado, B.S.; Crestani, L.; Marchezi, G.; Concolato, G.; Ferrari, V.; Colla, L.M.; Piccin, J.S. Rhodococcus: A promising genus of actinomycetes for the bioremediation of organic and inorganic contaminants. J. Environ. Manag. 2022, 323, 116220. [Google Scholar] [CrossRef] [PubMed]
- Tapia, D.; Sanchez-Villamil, J.I.; Torres, A.G. Emerging role of biologics for the treatment of melioidosis and glanders. Expert Opin. Biol. Ther. 2019, 19, 1319–1332. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, P.; Sun, J. Isolation, identification, phylogeny and growth promoting characteristics of endophytic diazotrophs from tuber and root crops. Sci. Agric. Sin. 2017, 50, 104–122. (In Chinese) [Google Scholar]
- Kim, B.; Westerhuis, J.A.; Smilde, A.K.; Floková, K.; Suleiman, A.K.A.; Kuramae, E.E. Effect of strigolactones on recruitment of the rice root-associated microbiome. FEMS Microbiol. Ecol. 2022, 98, fiac010. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wang, L.; Deng, L.; Mei, X.; Liu, Y.; Huang, H.; Du, F.; Zhu, S.; Yang, M. Enrichment of Burkholderia in the rhizosphere by autotoxic ginsenosides to alleviate negative plant-soil feedback. Microbiol. Spectr. 2021, 9, e0140021. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Ou, Y.; Shen, Z.; Wang, B.; Li, R.; Shen, Q. Stable microbial community and specific beneficial taxa associated. J. Microbiol. Biotechnol. 2019, 29, 1624–1628. [Google Scholar] [CrossRef]
- Gu, Y.; Banerjee, S.; Dini-Andreote, F.; Xu, Y.; Shen, Q.; Jousset, A.; Wei, Z. Small changes in rhizosphere microbiome composition predict disease outcomes earlier than pathogen density variations. ISME J. 2022, 16, 2448–2456. [Google Scholar] [CrossRef]
- Hingurao, K.; Nerurkar, A. A novel methylotrophic bacterial consortium for treatment of industrial effluents. Appl. Biochem. Biotechnol. 2018, 185, 691–704. [Google Scholar] [CrossRef]
- Tyler, C.A.; Kopit, L.; Doyle, C.; Yu, A.O.; Hugenholtz, J.; Marco, M.L. Polyol production during heterofermentative growth of the plant isolate Lactobacillus florum 2f. J. Appl. Microbiol. 2016, 120, 1336–1345. [Google Scholar] [CrossRef]
- Silva, E.; Marques, A.R.; Fialho, A.M.; Granja, A.T.; Sá-Correia, I. Proteins encoded by Sphingomonas elodea atcc 31461 rmla and ugpG genes, involved in gellan gum biosynthesis, exhibit both dtdp- and udp-glucose pyrophosphorylase activities. Appl Environ. Microbiol. 2005, 71, 4703–4712. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Z.; Wu, M.; Shi, Z.; Zhu, F.; Li, G.; Ma, T. Improved production of carotenoid-free welan gum in a genetic-engineered Alcaligenes sp. Atcc31555. Biotechnol. Lett. 2016, 38, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Kumar, V.; Usmani, Z.; Rani, R.; Chandra, A.; Gupta, V.K. Implications of plant growth promoting Klebsiella sp. Cpsb4 and Enterobacter sp. Cpsb49 in luxuriant growth of tomato plants under chromium stress. Chemosphere 2020, 240, 124944. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Shityakov, S.; Das, P.P.; Ghosh, C. Enhanced biodegradation of mixed pahs by mutated naphthalene 1,2-dioxygenase encoded by Pseudomonas putida strain kd6 isolated from petroleum refinery waste. 3 Biotech 2017, 7, 365. [Google Scholar] [CrossRef] [PubMed]
- Yarte, M.E.; Gismondi, M.I.; Llorente, B.E.; Larraburu, E.E. Isolation of endophytic bacteria from the medicinal, forestal and ornamental tree Handroanthus impetiginosus. Environ. Technol. 2022, 43, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Mello, I.S.; Targanski, S.; Pietro-Souza, W.; Frutuoso Stachack, F.F.; Terezo, A.J.; Soares, M.A. Endophytic bacteria stimulate mercury phytoremediation by modulating its bioaccumulation and volatilization. Ecotoxicol. Environ. Saf. 2020, 202, 110818. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, Z.; Ma, G.H.; Du, B.H.; Shen, B.; Ding, Y.Q.; Xu, K. Diversity and potential application of endophytic bacteria in ginger. Genet. Mol. Res. 2014, 13, 4918–4931. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yao, J.; Ding, S.; Wu, D.; Zhang, P.; Tie, B.; Lou, S. Effects of adding Delftia sp. B9 on the morphological distribution of soil Cd and the uptake and accumulation of Cd in rice. J. Agric. Resour. Environ. 2023, 40, 1339–1348. (In Chinese) [Google Scholar] [CrossRef]
- Agafonova, N.V.; Doronina, N.V.; Kaparullina, E.N.; Fedorov, D.N.; Gafarov, A.B.; Sazonova, O.I.; Sokolov, S.L.; Trotsenko, Y.A. A novel delftia plant symbiont capable of autotrophic methylotrophy. Mikrobiologiia 2017, 86, 88–98. (In Russian) [Google Scholar] [CrossRef]
- Avgustin, G.; Wallace, R.J.; Flint, H.J. Phenotypic diversity among ruminal isolates of Prevotella ruminicola: Proposal of Prevotella brevis sp. Nov., Prevotella bryantii sp. Nov., and Prevotella albensis sp. Nov. And redefinition of Prevotella ruminicola. Int. J. Syst. Bacteriol. 1997, 47, 284–288. [Google Scholar] [CrossRef][Green Version]
- Zorec, M.; Vodovnik, M.; Marinšek-Logar, R. Potential of selected rumen bacteria for cellulose and hemicellulose degradation. Food Technol. Biotechnol. 2014, 52, 210–221. [Google Scholar]
- Arafat, Y.; Ud Din, I.; Tayyab, M.; Jiang, Y.; Chen, T.; Cai, Z.; Zhao, H.; Lin, X.; Lin, W.; Lin, S. Soil sickness in aged tea plantation is associated with a shift in microbial communities as a result of plant polyphenol accumulation in the tea gardens. Front. Plant Sci. 2020, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A.; Akasaka, H.; Satoh, A.; Suzuki, D.; Ueki, K. Prevotella paludivivens sp. Nov., a novel strictly anaerobic, gram-negative, hemicellulose-decomposing bacterium isolated from plant residue and rice roots in irrigated rice-field soil. Int. J. Syst. Evol. Microbiol. 2007, 57, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Bodhankar, S.; Grover, M.; Hemanth, S.; Reddy, G.; Rasul, S.; Yadav, S.K.; Desai, S.; Mallappa, M.; Mandapaka, M.; Srinivasarao, C. Maize seed endophytic bacteria: Dominance of antagonistic, lytic enzyme-producing Bacillus spp. 3 Biotech 2017, 7, 232. [Google Scholar] [CrossRef] [PubMed]
- Michalko, J.; Medo, J.; Ferus, P.; Konôpková, J.; Košútová, D.; Hoťka, P.; Barta, M. Changes of endophytic bacterial community in mature leaves of Prunus laurocerasus L. During the seasonal transition from winter dormancy to vegetative growth. Plants 2022, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Merzaeva, O.V.; Shirokikh, I.G. Production of auxins by the endophytic bacteria of winter rye. Appl. Biochem. Microbiol. 2010, 46, 51–57. (In Russian) [Google Scholar] [CrossRef]
- Liaqat, F.; Eltem, R. Identification and characterization of endophytic bacteria isolated from in vitro cultures of peach and pear rootstocks. 3 Biotech 2016, 6, 120. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Tian, Z.; Feng, L.; Xu, L.; Wang, H. Diversity analysis of the rhizospheric and endophytic bacterial communities of Senecio vulgaris l. (asteraceae) in an invasive range. PeerJ 2019, 6, e6162. [Google Scholar] [CrossRef]
- Feng, G.D.; Yang, S.Z.; Li, H.P.; Zhu, H.H. Massilia putida sp. Nov., a dimethyl disulfide-producing bacterium isolated from wolfram mine tailing. Int. J. Syst. Evol. Microbiol. 2016, 66, 50–55. [Google Scholar] [CrossRef]
- Kumari, S.; Regar, R.K.; Manickam, N. Improved polycyclic aromatic hydrocarbon degradation in a crude oil by individual and a consortium of bacteria. Bioresour. Technol. 2018, 254, 174–179. [Google Scholar] [CrossRef]
- Du, Y.; Yu, X.; Wang, G. Massilia tieshanensis sp. Nov., isolated from mining soil. Int. J. Syst. Evol. Microbiol. 2012, 62, 2356–2362. [Google Scholar] [CrossRef]
- Zheng, B.X.; Bi, Q.F.; Hao, X.L.; Zhou, G.W.; Yang, X.R. Massilia phosphatilytica sp. Nov., a phosphate solubilizing bacteria isolated from a long-term fertilized soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, M.A.; Fazeli, M.; Yazdi, M.T.; Adrangi, S.; Mohseni, F.A. Optimization of cultural conditions for production of chitinase by a soil isolate of Massilia timonae. Biotechnology 2009, 8, 93–99. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Peng, W.; Zhai, C.; Yi, L. Identification, genome sequence and production of polysaccharide hydrolases of Massilii eurypsychrophila 709. In Abstract Books of Eleventh China Symposium on Enzyme Engineering; Enzyme Engineering Committee of China Society of Microbiology: Wuhan, China, 2017. (In Chinese) [Google Scholar]
- Dodou, H.V.; de Morais Batista, A.H.; Sales, G.W.P.; de Medeiros, S.C.; Rodrigues, M.L.; Nogueira, P.C.N.; Silveira, E.R.; Nogueira, N.A.P. Violacein antimicrobial activity on Staphylococcus epidermidis and synergistic effect on commercially available antibiotics. J. Appl. Microbiol. 2017, 123, 853–860. [Google Scholar] [CrossRef]
- Han, X.; Satoh, Y.; Kuriki, Y.; Seino, T.; Fujita, S.; Suda, T.; Kobayashi, T.; Tajima, K. Polyhydroxyalkanoate production by a novel bacterium Massilia sp. Umi-21 isolated from seaweed, and molecular cloning of its polyhydroxyalkanoate synthase gene. J. Biosci. Bioeng. 2014, 118, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Raj, G.; Shadab, M.; Deka, S.; Das, M.; Baruah, J.; Bharali, R.; Talukdar, N.C. Seed interior microbiome of rice genotypes indigenous to three agroecosystems of indo-burma biodiversity hotspot. BMC Genom. 2019, 20, 924. [Google Scholar] [CrossRef] [PubMed]
- Keinath, A.P. Survival of Didymella bryoniae in buried watermelon vines in south carolina. Plant Dis. 2002, 86, 32–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuo, J.; Chang, C.F.; Chi, W.C. Isolation of endophytic fungi with antimicrobial activity from medicinal plant Zanthoxylum simulans hance. Folia Microbiol. 2021, 66, 385–397. [Google Scholar] [CrossRef]
- Turbat, A.; Rakk, D.; Vigneshwari, A.; Kocsubé, S.; Thu, H.; Szepesi, Á.; Bakacsy, L.D.; Škrbić, B.; Jigjiddorj, E.A.; Vágvölgyi, C.; et al. Characterization of the plant growth-promoting activities of endophytic fungi isolated from Sophora flavescens. Microorganisms 2020, 8, 683. [Google Scholar] [CrossRef]
- Ma, N.; Yin, D.; Liu, Y.; Gao, Z.; Cao, Y.; Chen, T.; Huang, Z.; Jia, Q.; Wang, D. Succession of endophytic fungi and rhizosphere soil fungi and their correlation with secondary metabolites in Fagopyrum dibotrys. Front. Microbiol. 2023, 14, 1220431. [Google Scholar] [CrossRef]
- Kosawang, C.; Amby, D.B.; Bussaban, B.; McKinney, L.V.; Xu, J.; Kjær, E.D.; Collinge, D.B.; Nielsen, L.R. Fungal communities associated with species of Fraxinus tolerant to ash dieback, and their potential for biological control. Fungal Biol. 2018, 122, 110–120. [Google Scholar] [CrossRef]
- Gakuubi, M.M.; Ching, K.C.; Munusamy, M.; Wibowo, M.; Liang, Z.X.; Kanagasundaram, Y.; Ng, S.B. Enhancing the discovery of bioactive secondary metabolites from fungal endophytes using chemical elicitation and variation of fermentation media. Front. Microbiol. 2022, 13, 898976. [Google Scholar] [CrossRef]
- Azevedo, R.P.; Alves, N.M.; Costa, I.A.; Domingues, M.I.S.; Bandória, N.A.; de Figueiredo, U.J.; de Medeiros, F.H.V.; Silva, B.M.; Cardoso, P.G. Endophytic fungi assures tropical forage grass growth by water stress tolerances. Curr. Microbiol. 2021, 78, 4060–4071. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.I.; Tomida, J.; Hirai, T.; Kawamura, Y.; Inoue, M. Paraconiothins a-j: Sesquiterpenoids from the endophytic fungus Paraconiothyrium brasiliense ecn258. J. Nat. Prod. 2019, 82, 3347–3356. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Cui, H.; Yao, H.; Yuan, T. Five sesquiterpenes from Paraconiothyrium sp. And their anti-inflammatory activity. Chem. Biodivers. 2023, 20, e202300477. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Naveen, K.V.; Han, K.S.; Zhang, X.; Jeong, M.S.; Wang, M.H. Combination of Paraconiothyrium brasiliense fabricated titanium dioxide nanoparticle and antibiotics enhanced antibacterial and antibiofilm properties: A toxicity evaluation. Environ. Res. 2022, 212, 113237. [Google Scholar] [CrossRef] [PubMed]
- Shaigani, P.; Fuchs, T.; Graban, P.; Prem, S.; Haack, M.; Masri, M.; Mehlmer, N.; Brueck, T. Mastering targeted genome engineering of gc-rich oleaginous yeast for tailored plant oil alternatives for the food and chemical sector. Microb. Cell Factories 2023, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.L.; Ren, H.; Xi, J.M.; Fang, J.; Zhang, J.Z.; Wu, Q.X. Diverse anti-inflammation and anti-cancer polyketides isolated from the endophytic fungi Alternaria sp. Mg1. Fitoterapia 2021, 153, 105000. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Zhang, Q.; Wang, R.; Yan, S.; Li, Z.; Li, P.; Gu, P. Correlation in endophytic fungi community diversity and bioactive compounds of Sophora alopecuroides. Front. Microbiol. 2022, 13, 955647. [Google Scholar] [CrossRef]
- Hatamzadeh, S.; Rahnama, K.; White, J.F.; Oghaz, N.A.; Nasrollahnejad, S.; Hemmati, K. Investigation of some endophytic fungi from five medicinal plants with growth promoting ability on maize (Zea mays l.). J. Appl. Microbiol. 2023, 134, lxac015. [Google Scholar] [CrossRef]
- Tsuji, M. Cold-stress responses in the antarctic basidiomycetous yeast Mrakia blollopis. R. Soc. Open Sci. 2016, 3, 160106. [Google Scholar] [CrossRef] [PubMed]
- Yuivar, Y.; Alcaino, J.; Cifuentes, V.; Baeza, M. Characterization of gelatinase produced by antarctic Mrakia sp. J. Basic Microbiol. 2019, 59, 846–852. [Google Scholar] [CrossRef]
- Cha, T.; Kim, H.H.; Keum, J.; Kwak, M.J.; Park, J.Y.; Hoh, J.K.; Kim, C.R.; Jeon, B.H.; Park, H.K. Gut microbiome profiling of neonates using nanopore minion and illumina miseq sequencing. Front. Microbiol. 2023, 14, 1148466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Guan, P.; Hao, C.; Yang, J.; Xie, Z.; Wu, D. Changes in assembly processes of soil microbial communities in forest-to-cropland conversion in changbai mountains, northeastern china. Sci. Total Environ. 2022, 818, 151738. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, B. Current progression: Application of high-throughput sequencing technique in space microbiology. Biomed. Res. Int. 2020, 2020, 4094191. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Do, H.; Cho, G.; Jeong, R.D.; Kwak, Y.S. Comparison of microbial community of rhizosphere and endosphere in kiwifruit. Plant Pathol. J. 2019, 35, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.; Kim, M.J.; Kwon, Y.; Kwak, Y.S. Comparison of endophytic microbial community in kiwifruit plant cultivars. Plant Pathol. J. 2018, 34, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Blanco, A.; Sicardi, M.; Frioni, L. Plant genotype and nitrogen fertilization effects on abundance and diversity of diazotrophic bacteria associated with maize (Zea mays L.). Biol. Fertil. Soils 2015, 51, 391–402. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef]
- Ding, T.; Melcher, U. Influences of plant species, season and location on leaf endophytic bacterial communities of non-cultivated plants. PLoS ONE 2016, 11, e0150895. [Google Scholar] [CrossRef]
- Ali, M.; Ali, Q.; Sohail, M.A.; Ashraf, M.F.; Saleem, M.H.; Hussain, S.; Zhou, L. Diversity and Taxonomic Distribution of Endophytic Bacterial Community in the Rice Plant and Its Prospective. Int. J. Mol. Sci. 2021, 22, 10165. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, Y.; Shi, W.; Zhao, J.; Hou, Q.; Zhang, H.; Jia, L.; Sun, K. Analysis of endophyte diversity of Rheum palmatum among different tissues and ages. Arch. Microbiol. 2022, 205, 14. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Takagi, K.; Ito, K.; Kataoka, R. Changes in endophytic bacterial communities during different growth stages of cucumber (Cucumis sativus L.). World J. Microbiol. Biotechnol. 2019, 35, 104. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef] [PubMed]
- Maela, M.P.; Serepa-Dlamini, M.H. Data on metagenomic profiles of bacterial endophyte communities associated with Dicoma anomala. Data Brief 2022, 42, 108112. [Google Scholar] [CrossRef] [PubMed]
- Lumibao, C.Y.; Borer, E.T.; Condon, B.; Kinkel, L.; May, G.; Seabloom, E.W. Site-specific responses of foliar fungal microbiomes to nutrient addition and herbivory at different spatial scales. Ecol. Evol. 2019, 9, 12231–12244. [Google Scholar] [CrossRef] [PubMed]
- Al Ashhab, A.; Meshner, S.; Alexander-Shani, R.; Dimerets, H.; Brandwein, M.; Bar-Lavan, Y.; Winters, G. Temporal and spatial changes in phyllosphere microbiome of acacia trees growing in arid environments. Front. Microbiol. 2021, 12, 656269. [Google Scholar] [CrossRef]
- Li, J.; Luo, Z.; Zhang, C.; Qu, X.; Chen, M.; Song, T.; Yuan, J. Seasonal Variation in the Rhizosphere and Non-Rhizosphere Microbial Community Structures and Functions of Camellia yuhsienensis Hu. Microorganisms 2020, 8, 1385. [Google Scholar] [CrossRef]
- Kowal, J.; Arrigoni, E.; Serra, J.; Bidartondo, M. Prevalence and phenology of fine root endophyte colonization across populations of Lycopodiella inundata. Mycorrhiza 2020, 30, 577–587. [Google Scholar] [CrossRef]
- de Souza, L.C.; Procópio, L. The adaptations of the microbial communities of the savanna soil over a period of wildfire, after the first rains, and during the rainy season. Environ. Sci. Pollut. Res. Int. 2022, 29, 14070–14082. [Google Scholar] [CrossRef]
- Turchetti, B.; De Francesco, G.; Mugnai, G.; Sileoni, V.; Alfeo, V.; Buzzini, P.; Yurkov, A.; Marconi, O. Species and temperature-dependent fermentative aptitudes of Mrakia genus for innovative brewing. Food Res. Int. 2023, 170, 113004. [Google Scholar] [CrossRef]
- HongYang, W.; ChuanZhi, K.; Sheng, W.; DaiQuan, J.; Zheng, P.; Yang, X.; YongXi, D.; Yan, Z.; DaHui, L.; LanPing, G. Research strategies for endophytes in medicinal plants based on high-throughput sequencing and traditional culture and isolation methods. Zhongguo Zhong Yao Za Zhi 2021, 46, 1910–1919. (In Chinese) [Google Scholar] [CrossRef]
- Kastanis, G.J.; Santana-Quintero, L.V.; Sanchez-Leon, M.; Lomonaco, S.; Brown, E.W.; Allard, M.W. In-depth comparative analysis of illumina(®) miseq run metrics: Development of a wet-lab quality assessment tool. Mol. Ecol. Resour. 2019, 19, 377–387. [Google Scholar] [CrossRef]


| Sampling Season | Sampling Date (Y.M.D) | RH (%) | Precipit (mm) | SOLRAD (W/m2) | Mean Temp (°C) | MeaSoiTm (°C) |
|---|---|---|---|---|---|---|
| Winter | 15 January 2021 | 73.66 | 13.60 | 80.05 | −16.09 | −4.54 |
| Spring | 15 April 2021 | 56.32 | 21.30 | 183.60 | 6.50 | 4.45 |
| Summer | 15 July 2021 | 78.43 | 24.20 | 245.01 | 23.38 | 24.50 |
| Autumn | 15 October 2021 | 66.69 | 22.70 | 127.78 | 6.32 | 9.92 |
| Sampling Season | RH (%) | Precipit (mm) | SOLRAD (W/m2) | Mean Temp (°C) | MeaSoiTm (°C) |
|---|---|---|---|---|---|
| Spring | 63.75 | 59.20 | 177.24 | 7.01 | 5.77 |
| Summer | 80.77 | 179.00 | 213.81 | 21.82 | 22.61 |
| Autumn | 75.99 | 299.10 | 109.38 | 6.39 | 10.53 |
| Winter | 72.24 | 19.90 | 88.99 | −13.27 | −2.92 |
| Endophyte Type | Index Type | Spring Stem (n = 6) | Summer Stem (n = 6) | Autumn Stem (n = 6) | Winter Stem (n = 6) | p Value |
|---|---|---|---|---|---|---|
| Endophytic Bacteria | Coverage | 0.9990 ± 0.0002 | 0.9990 ± 0.0002 | 0.9966 ± 0.0021 | 0.9984 ± 0.0006 | 0.0009 *** |
| Chao | 347.73 ± 155.68 | 232.73 ± 28.25 | 454.46 ± 197.44 | 467.86 ± 180.96 | 0.005 ** | |
| Shannon | 3.01 ± 0.71 | 1.7211 ± 0.30 | 2.81 ± 0.50 | 3.86 ± 1.23 | 0.008 ** | |
| Endophytic Fungi | Coverage | 0.9999 ± 0.0000 | 0.9999 ± 0.0000 | 1.0000 ± 0.0000 | 0.9999 ± 0.0000 | 0.001 *** |
| Chao | 31.75 ± 6.31 | 68.74 ± 13.07 | 32.58 ± 4.68 | 56.94 ± 24.15 | 0.0009 *** | |
| Shannon | 1.65 ± 0.40 | 1.95 ± 0.46 | 1.61 ± 0.5212 | 1.99 ± 0.40 | 0.436 |
| Endophyte Type | Index Type | p Value (Autumn–Spring) | p Value (Autumn–Summer) | p Value (Winter–Autumn) | p Value (Spring–Summer) | p Value (Spring–Winter) | p Value (Summer–Winter) |
|---|---|---|---|---|---|---|---|
| Endophytic Bacteria | Coverage | <0.01 | <0.01 | <0.1 | ≥0.1 | ≥0.1 | ≥0.1 |
| Chao | <0.05 | <0.01 | ≥0.1 | ≥0.1 | ≥0.1 | <0.1 | |
| Shannon | ≥0.1 | ≥0.1 | ≥0.1 | <0.1 | ≥0.1 | <0.01 | |
| Endophytic Fungi | Coverage | ≥0.1 | <0.01 | ≥0.1 | <0.01 | ≥0.1 | ≥0.1 |
| Chao | ≥0.1 | <0.001 | <0.1 | <0.001 | ≥0.1 | <0.1 | |
| Shannon | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 | ≥0.1 |
| Endophytic Bacterial Genus | Phylum | Spring (%) | Summer (%) | Autumn (%) | Winter (%) | Functions |
|---|---|---|---|---|---|---|
| Ralstonia | Proteobacteria | 42.11 | 66.68 | 8.35 | 5.71 | Some species within this genus are potential pathogens [38]; some possess unique protein synthesis pathways to adapt to specific lifestyles [39]; in some plants, an anomalous increase in Ralstonia may alter the overall ecosystem of the microbial community [40]. |
| Rhodococcus | Actinobacteria | 12.76 | 9.61 | 15.18 | 0.34 | Biodegradation [41]; biosurfactants and bioflocculants production [42]; indicators in hydrocarbon deposits for crude oil exploration; enhancing the aroma of noni juice [40]; adaptation to harsh environments and tolerance to toxic substances [43]; capabilities in biormediation and biotransformation [7]. |
| Unclassified_f_Enterobacteriaceae | Proteobacteria | 0.45 | 0.23 | 26.16 | 0.62 | Genera related to plant defense [44]. |
| Burkholderia-Caballeronia-Paraburkholderia | Proteobacteria | 1.99 | 3.58 | 9.27 | 2.64 | Certain species within the genus are potential plant pathogens [45]; some produce siderophores [46]; solubilize phosphates [47]; part of the ginsenoside-enriching microbial community [48]. |
| Bordetella | Proteobacteria | 11.89 | Suppressing pathogens and enhancing plant disease resistance [49]; a genus characterized by high protein expression [50]; potential for degrading fertilizers and pesticides [51]. | |||
| Sphingomonas | Proteobacteria | 0.48 | 1.95 | 0.82 | 6.72 | Involved in carbon, nitrogen, and sugar metabolism [52]; capable of synthesizing secondary metabolites such as welan gum and carotenoids to enhance plant stress resistance [53]; producing beneficial phytohormones [54]. |
| Klebsiella | Proteobacteria | 0.21 | 7.72 | Promoting plant growth; biocontrol; degrading tebuconazole-class pesticides [9]; tolerance to the heavy metal Cd [55]; resilience to salinity and high temperatures [11]. | ||
| Pseudomonas | Proteobacteria | 3.59 | 0.34 | 2.15 | 0.66 | Degradation of polycyclic aromatic hydrocarbons like phenanthrene and chloroacetamide herbicides [56]; phosphate solubilization and production of IAA for growth promotion [57]; remediation of mercury contamination [58]; disease resistance [59]. |
| Delftia | Proteobacteria | 4.61 | 1.98 | Remediation of Cd contamination [60]; promotion of plant growth [61]. | ||
| Prevotella | Bacteroidetes | 5.51 | Possessing the capability to degrade proteins and peptides [62]; xylanolytic activity [63]; promoting growth [64]; hemicellulose decomposition [65]. | |||
| Corynebacterium | Acidobacteria | 0.55 | 0.52 | 3.02 | Antagonizing pathogenic fungi [66]; tolerance to salinity and osmotic stress [67]; growth promotion [68]. | |
| Brevundimonas | Proteobacteria | 0.54 | 0.31 | 2.67 | Production of indoleacetic acid (IAA), nitrogen fixation, phosphate solubilization, and siderophore production for growth promotion [69]; heavy metal tolerance [70]. | |
| Massilia | Proteobacteria | 2.38 | 0.41 | Soil fumigants [71]; degradation of polycyclic aromatic hydrocarbons like phenanthrene and chloroacetamide herbicides [72]; heavy metal resistance [73]; phosphate solubilization [74]; chitinase production [75]; production of cold-active enzymes such as mannanase, amylase, cellulase, and other polysaccharide hydrolases [76]; production of secondary metabolites like violacein [77] and polyhydroxyalkanoates [78]. | ||
| Bifidobacterium | Actinobacteria | 2.08 | 0.66 | Probiotics commonly found in animal guts are also present on plants, including Actinidia arguta, with their functions yet to be determined [79]. | ||
| Total proportion | 65.22 | 84.55 | 76.56 | 37.36 |
| Endophytic Fungi Genera | Phylum | Spring (%) | Summer (%) | Autumn (%) | Winter (%) | Function |
|---|---|---|---|---|---|---|
| Didymella | Ascomycota | 36.76 | 47.96 | 28.55 | 4.41 | Can survive in the soil for more than six months without a host [80]; possesses antibacterial activity [81] and promotes growth [82]. |
| Unclassified_p_Ascomycota | Ascomycota | 14.06 | 4.31 | 18.85 | 18.39 | Affects the production of plant secondary metabolites such as saponins and flavonoids [83]. |
| Setomelanomma | Ascomycota | 0.26 | 1.81 | 8.04 | 10.80 | Possesses biocontrol properties [84]. |
| Lophiotrema | Ascomycota | 1.35 | 1.41 | 3.32 | 13.07 | Exhibits strong antibacterial effects [85]. |
| Paraconiothyrium | Ascomycota | 4.88 | 11.48 | 0.93 | Has a wide range of hosts [37]; enhances plant drought resistance [86]; produces sesquiterpenoid compounds [87]; possesses biocontrol properties [88]; synthesizes titanium dioxide nanoparticles to reduce antibiotic use and lower the risk of antimicrobial resistance [89]. | |
| Unclassified_f__Phaeosphaeriaceae | Ascomycota | 3.43 | 4.96 | 6.66 | Unknown. | |
| Cutaneotrichosporon | Basidiomycota | 14.42 | Produces oleaginous yeasts [90]. | |||
| Alternaria | Ascomycota | 5.39 | 1.79 | 1.21 | 3.55 | Produces antimicrobial multicopper compounds [91]; generates nitrogen metabolites, steroids, terpenes, pyrones, quinones, and phenolic compounds [92]; promotes growth [93]; produces substances with antimicrobial activity [13]. |
| Mrakia | Basidiomycota | 11.43 | Some species are cold-tolerant yeasts that ferment various sugars at low temperatures in response to the cold [94]; some produce gelatinase [95]. | |||
| Neosetophoma | Ascomycota | 0.75 | 1.79 | 8.52 | Unknown. | |
| Total proportion | 70.00 | 67.38 | 84.93 | 72.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Lu, W.; Li, Y.; Zhai, B.; Zhang, B.; Qin, H.; Xu, P.; Yang, Y.; Fan, S.; Wang, Y.; et al. Diversity of Endophytes of Actinidia arguta in Different Seasons. Life 2024, 14, 149. https://doi.org/10.3390/life14010149
Liu Y, Lu W, Li Y, Zhai B, Zhang B, Qin H, Xu P, Yang Y, Fan S, Wang Y, et al. Diversity of Endophytes of Actinidia arguta in Different Seasons. Life. 2024; 14(1):149. https://doi.org/10.3390/life14010149
Chicago/Turabian StyleLiu, Yingxue, Wenpeng Lu, Yang Li, Boyu Zhai, Baoxiang Zhang, Hongyan Qin, Peilei Xu, Yiming Yang, Shutian Fan, Yue Wang, and et al. 2024. "Diversity of Endophytes of Actinidia arguta in Different Seasons" Life 14, no. 1: 149. https://doi.org/10.3390/life14010149
APA StyleLiu, Y., Lu, W., Li, Y., Zhai, B., Zhang, B., Qin, H., Xu, P., Yang, Y., Fan, S., Wang, Y., Li, C., Zhao, J., & Ai, J. (2024). Diversity of Endophytes of Actinidia arguta in Different Seasons. Life, 14(1), 149. https://doi.org/10.3390/life14010149






