Changes in the Microbiome of Sugarcane (Saccharum spp. Hybrids.) Rhizosphere in Response to Manganese Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Sample Collection
2.3. Determination of Soil pH, Water-Soluble Mn, Exchangeable Mn, and Enzyme Activities
2.4. DNA Extraction and Sequencing of Partial 16S rRNA Genes
3. Results
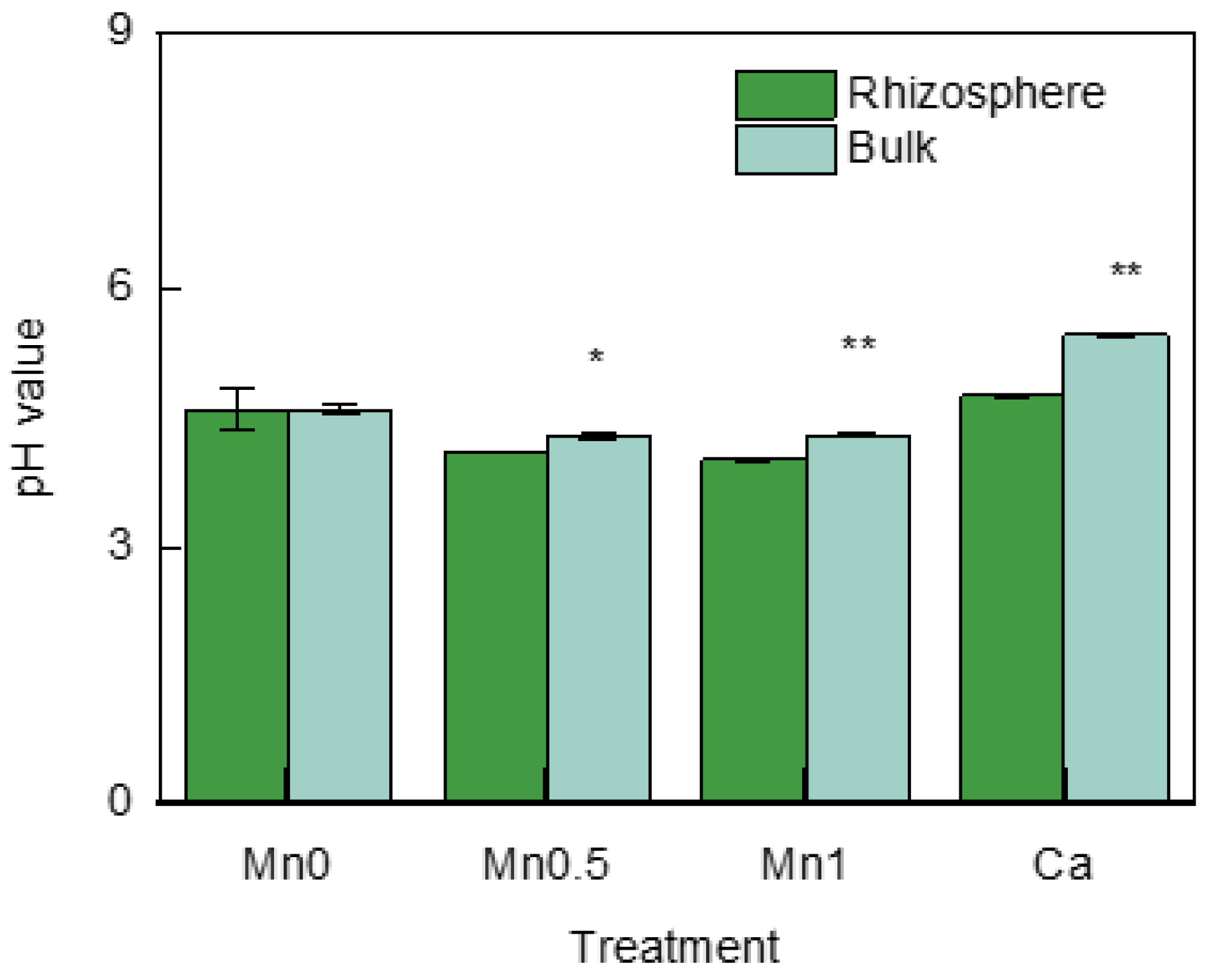
3.1. pH Values in the Rhizosphere and Bulk Soil
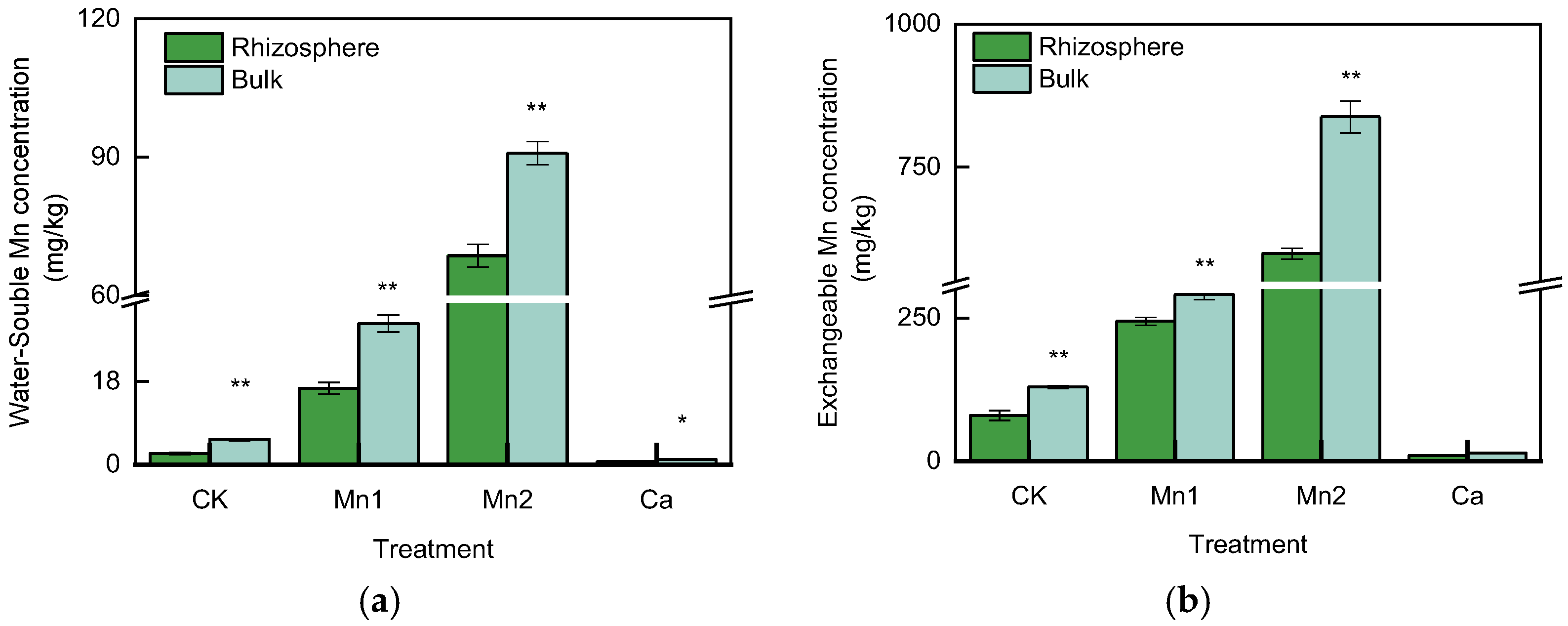
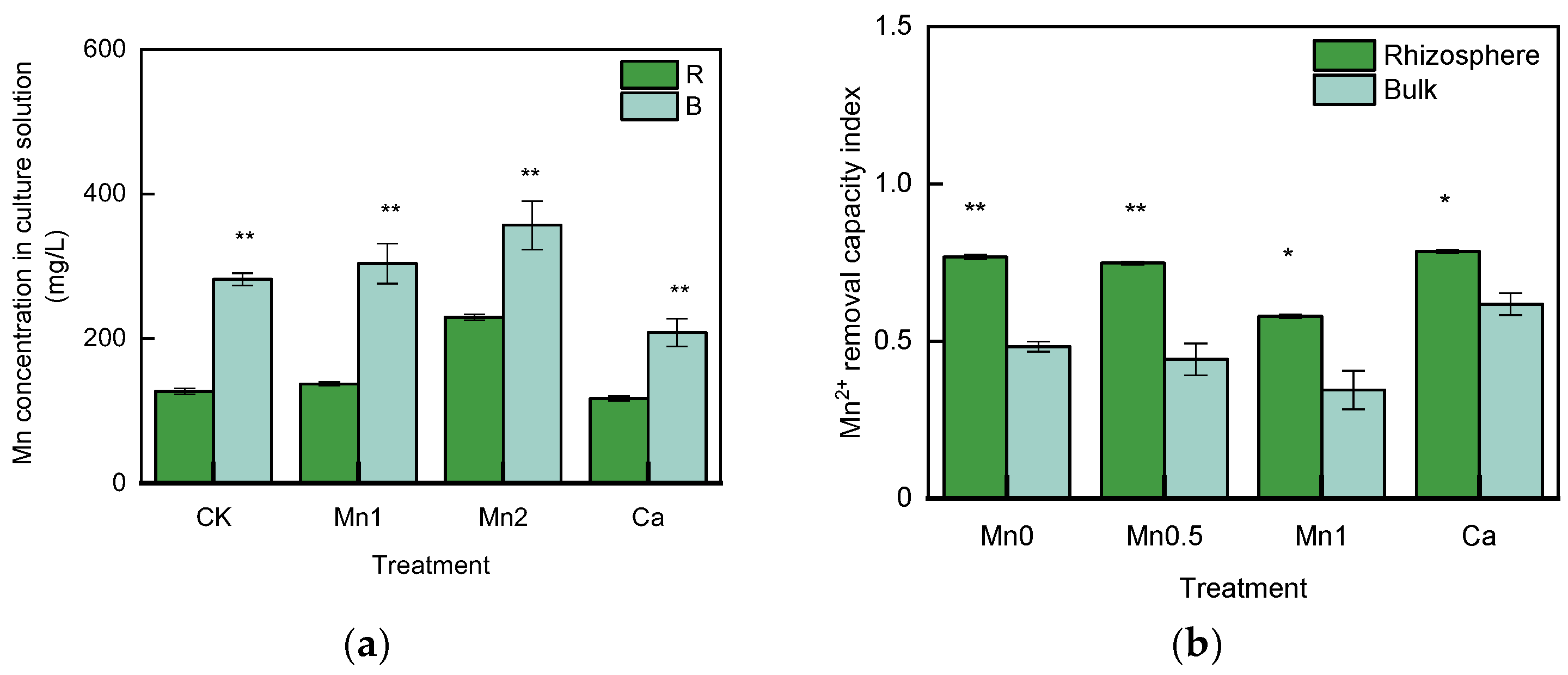
3.2. WS–Mn and EX–Mn Concentration in the Rhizosphere and Bulk Soil
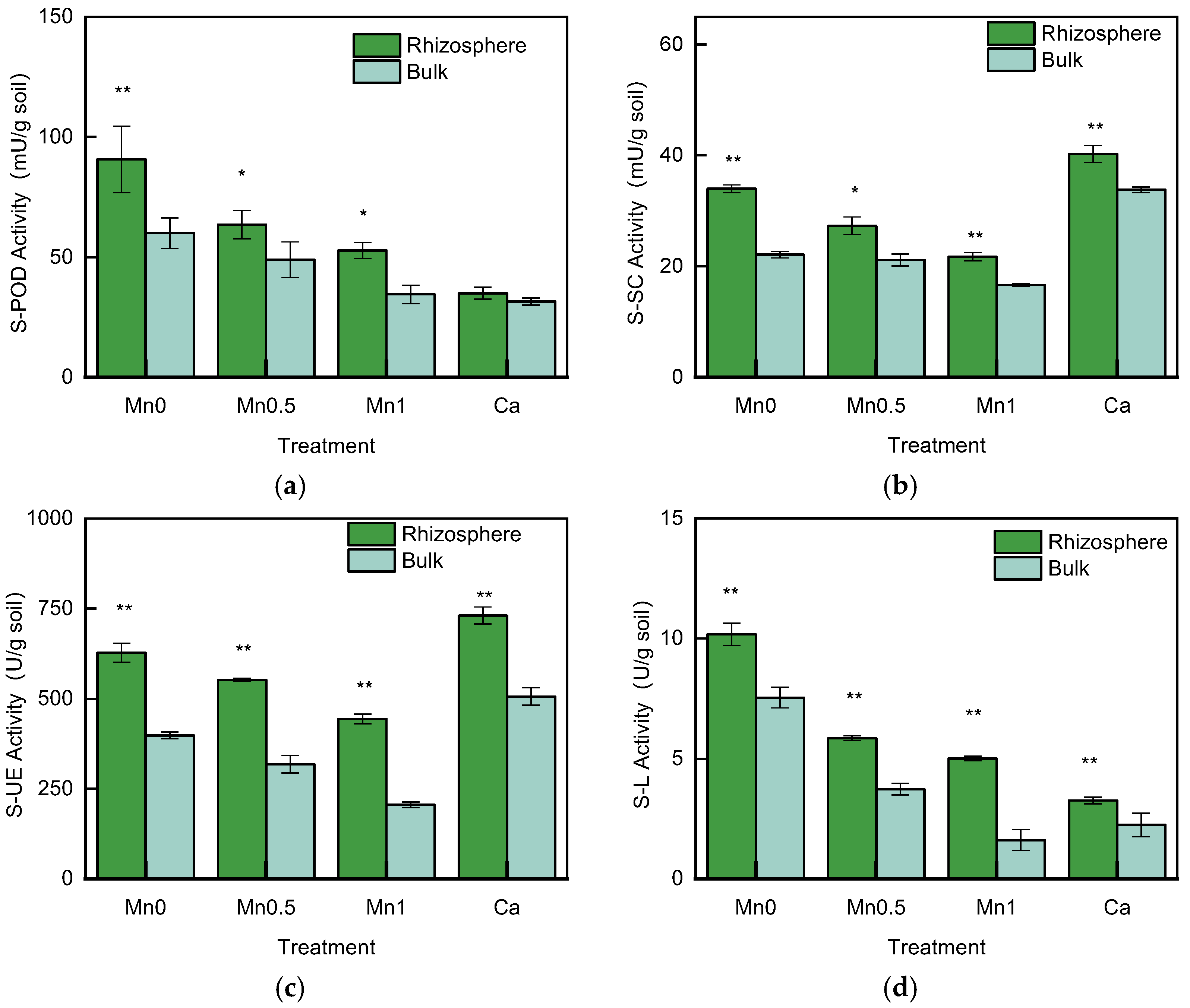
3.3. Soil Enzyme Activities in the Rhizosphere and Bulk Soil
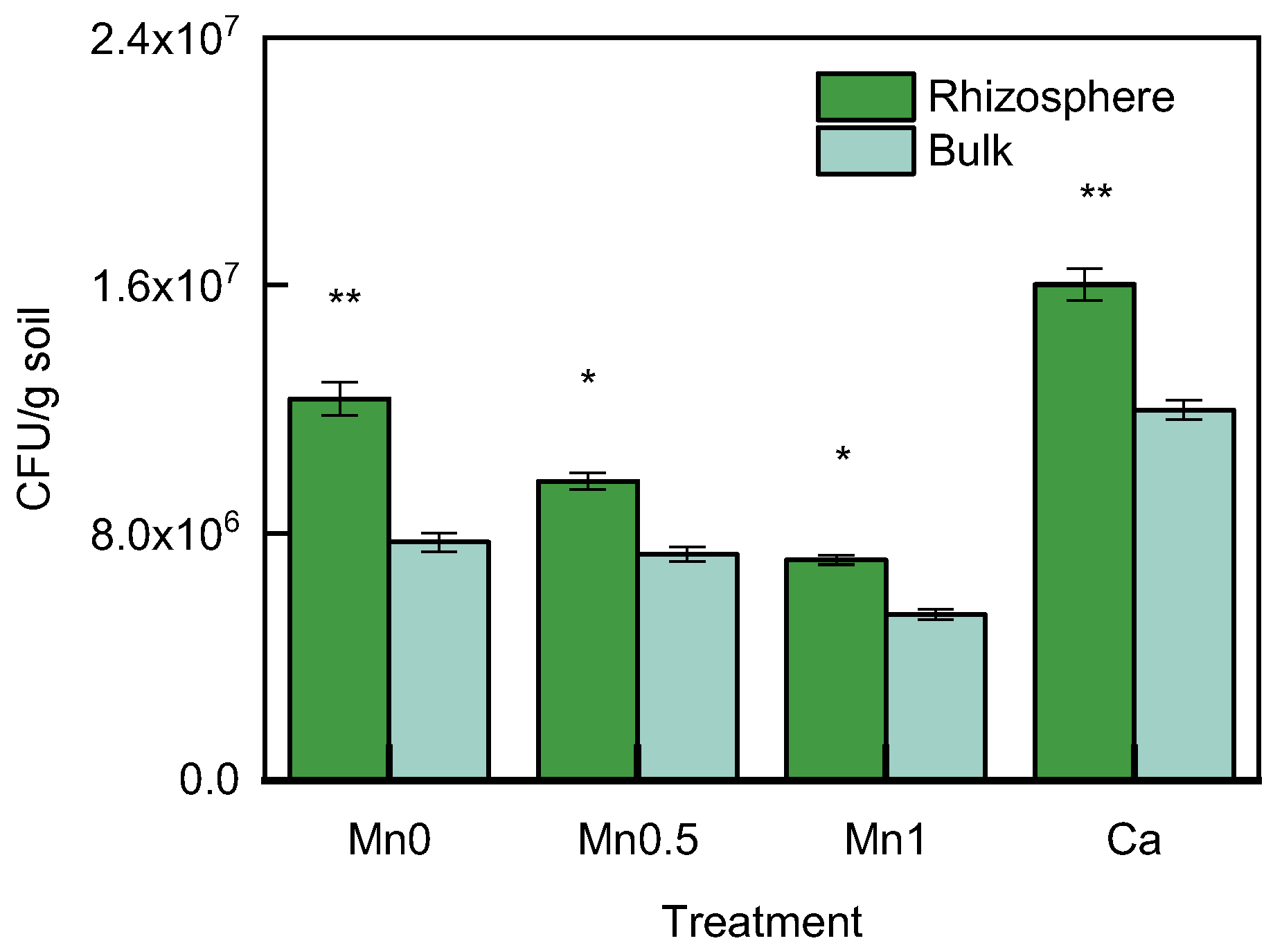
3.4. Comparison of the Number of Cultivable Bacteria in the Rhizosphere and Bulk Soil
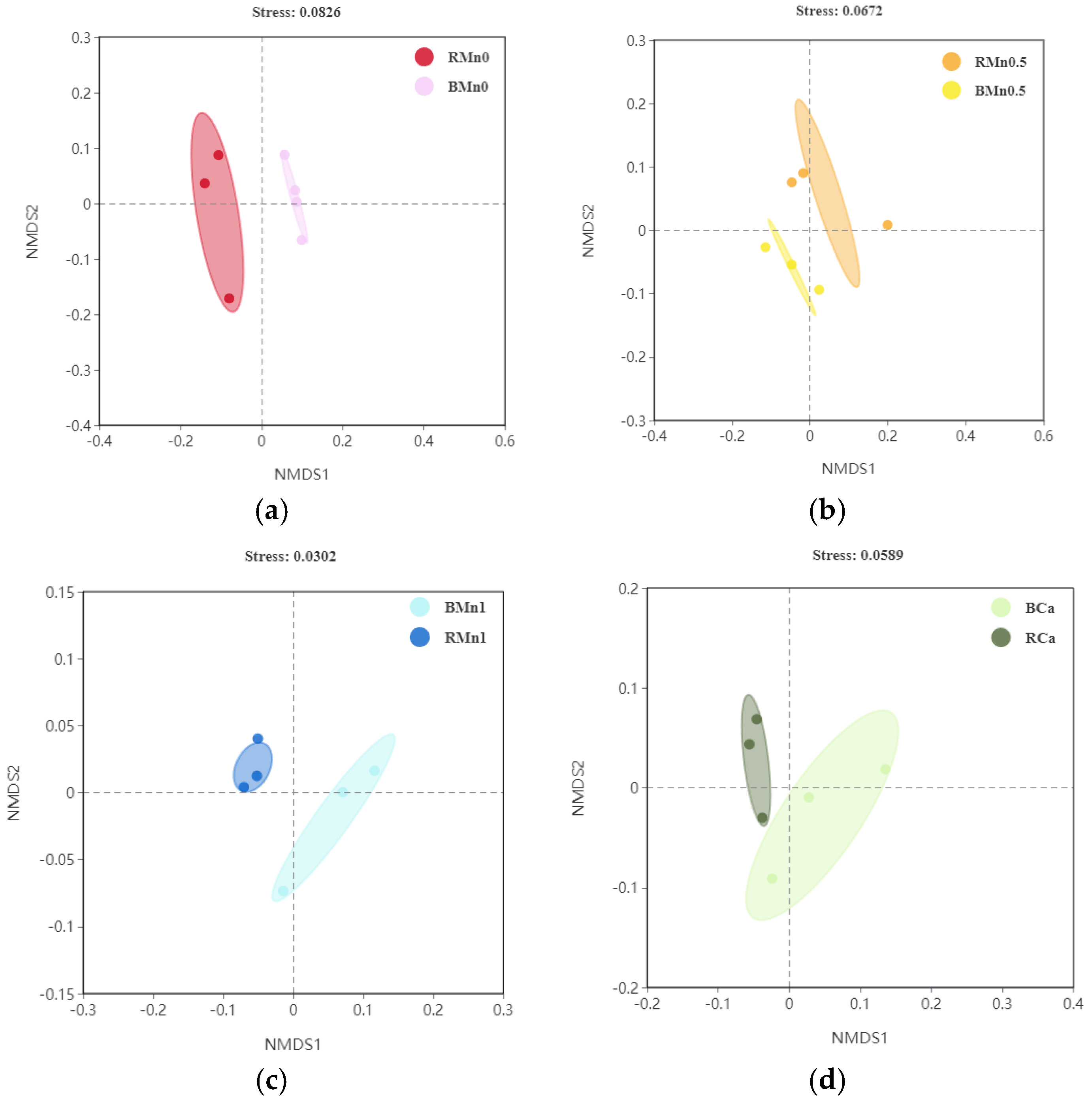
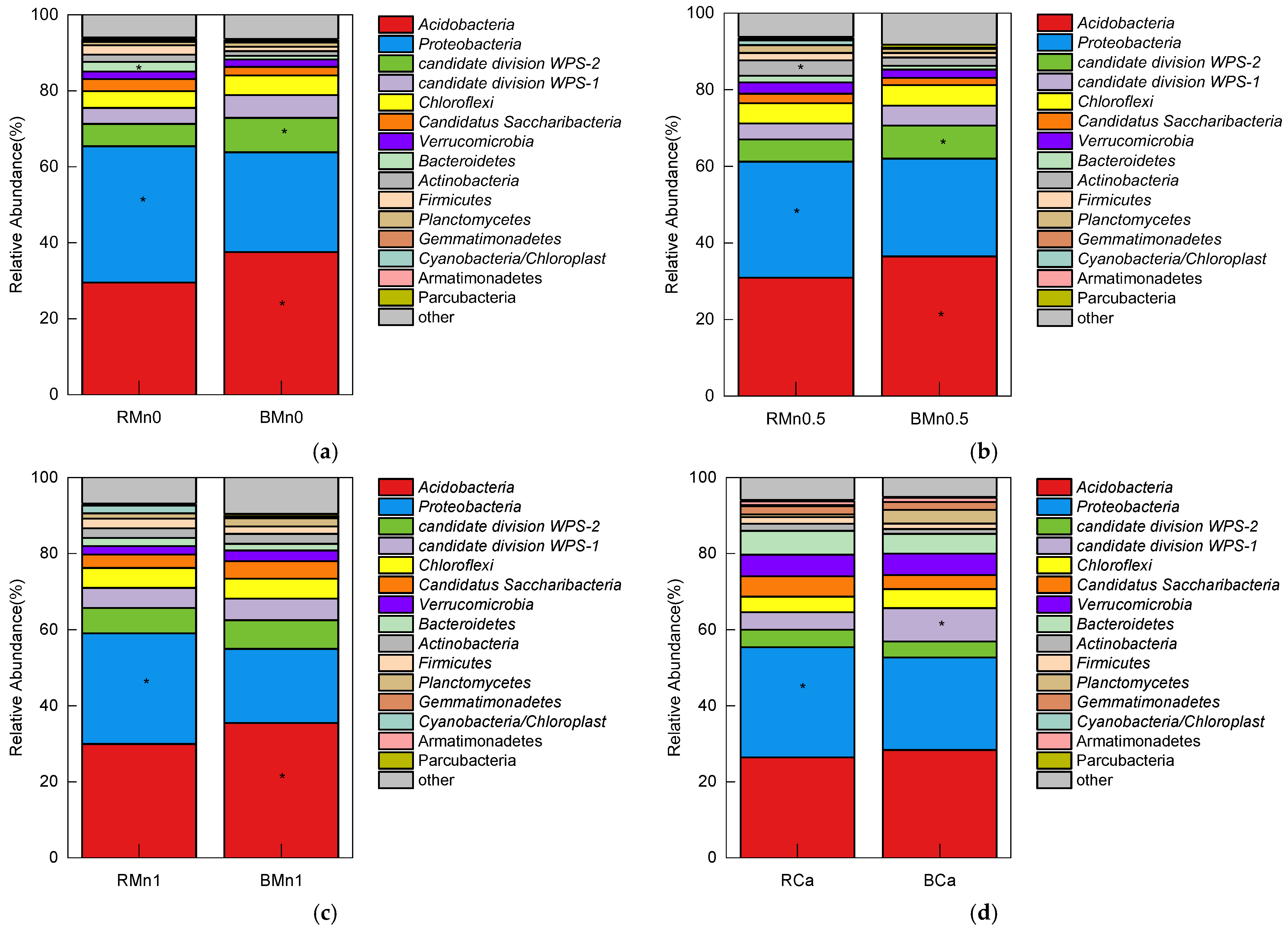
3.5. 16S rDNA Sequencing in the Rhizosphere and Bulk Soil
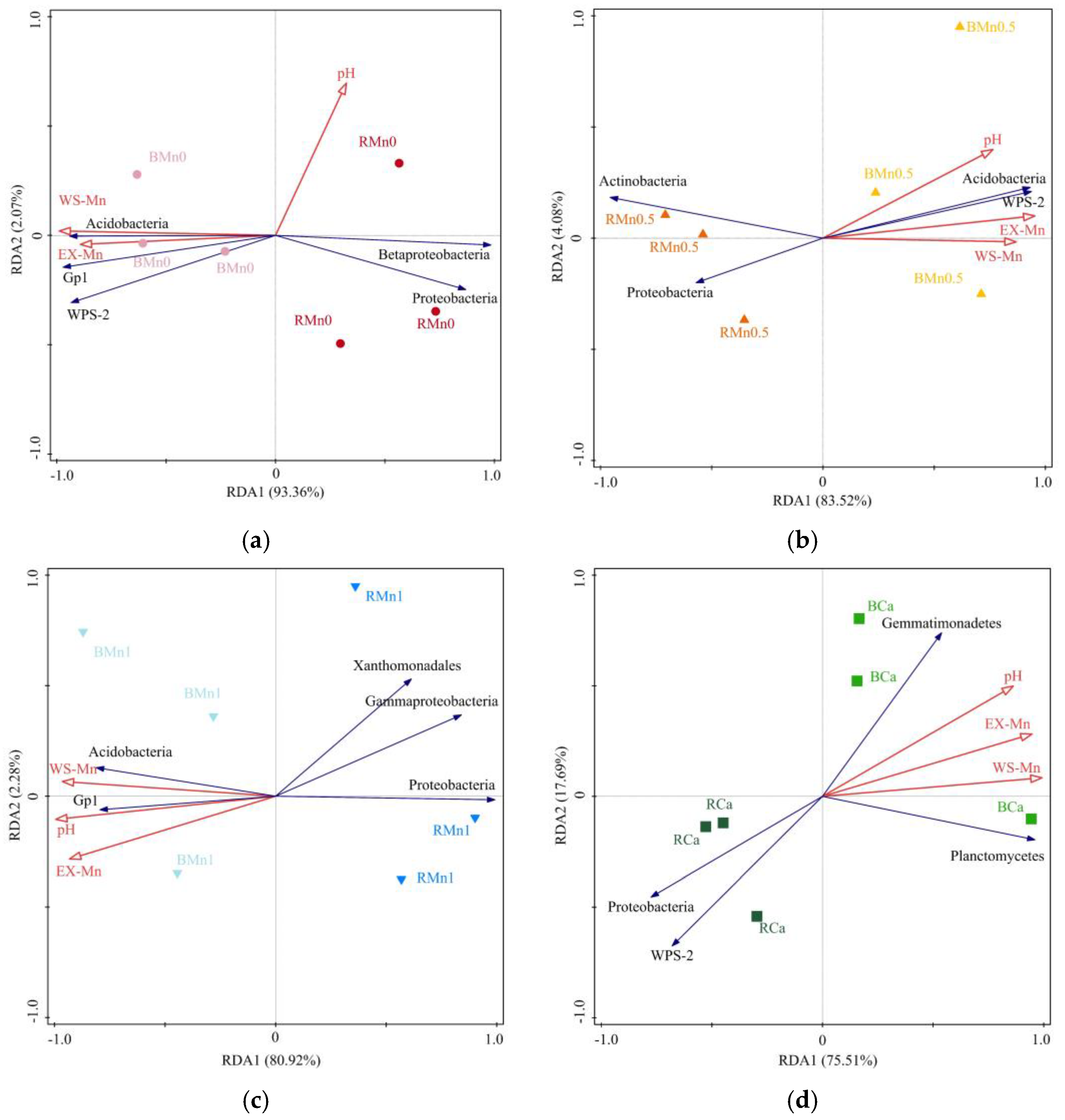
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, J.F.; Yamaji, N.; Shen, R.F.; Ma, J.F. The key to Mn homeostasis in plants: Regulation of Mn transporters. Trends Plant Sci. 2017, 22, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Li, M.S.; Luo, Y.P.; Su, Z.Y. Heavy metal concentrations in soils and plant accumulation in a restored manganese mineland in Guangxi, South China. Environ. Pollut. 2007, 147, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Wang, Z.; Liu, G.; Chen, Z. Advances in the mechanisms of plant tolerance to manganese toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Yang, S.; Long, G.X.; Zhao, Z.K.; Li, X.F.; Gu, M.H. Manganese toxicity in sugarcane plantlets grown on acidic soils of southern China. PLoS ONE 2016, 11, e148956. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fujii, Y.; Yamaji, N.; Masuda, S.; Takemoto, Y.; Kamiya, T.; Yusuyin, Y.; Iwasaki, K.; Kato, S.; Maeshima, M.; et al. Mn tolerance in rice is mediated by MTP8.1, a member of the cation diffusion facilitator family. J. Exp. Bot. 2013, 64, 4375–4387. [Google Scholar] [CrossRef]
- Takemoto, Y.; Tsunemitsu, Y.; Fujii-Kashino, M.; Mitani-Ueno, N.; Yamaji, N.; Ma, J.F.; Kato, S.; Iwasaki, K.; Ueno, D. The Tonoplast-localized transporter MTP8.2 contributes to manganese detoxification in the shoots and roots of Oryza sativa L. Plant Cell Physiol. 2017, 58, 1573–1582. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Xu, W.; Zhao, H.; Guo, F.; Wang, P.; Wang, Y.; Ni, D.; Wang, M.; Wei, C. Identification of MTP gene family in tea plant (Camellia sinensis L.) and characterization of CsMTP8.2 in manganese toxicity. Ecotox. Environ. Safe. 2020, 202, 110904. [Google Scholar] [CrossRef]
- Delhaize, E.; Gruber, B.D.; Pittman, J.K.; White, R.G.; Leung, H.; Miao, Y.; Jiang, L.; Ryan, P.R.; Richardson, A.E. A role for the AtMTP11 gene of Arabidopsis in manganese transport and tolerance. Plant J. 2007, 51, 198–210. [Google Scholar] [CrossRef]
- Yang, S.; Yi, K.; Chang, M.M.; Ling, G.Z.; Zhao, Z.K.; Li, X.F. Sequestration of Mn into the cell wall contributes to Mn tolerance in sugarcane (Saccharum officinarum L.). Plant Soil 2019, 436, 475–487. [Google Scholar] [CrossRef]
- Moreira, S.G.; Prochnow, L.I.; de Castro Kiehl, J.; Pauletti, V.; Martin-Neto, L. Chemical forms in soil and availability of manganese and zinc to soybean in soil under different tillage systems. Soil Till. Res. 2016, 163, 41–53. [Google Scholar] [CrossRef]
- Yu, P.; He, X.; Baer, M.; Beirinckx, S.; Tian, T.; Moya, Y.A.T.; Zhang, X.; Deichmann, M.; Frey, F.P.; Bresgen, V.; et al. Plant flavones enrich rhizosphere Oxalobacteraceae to improve maize performance under nitrogen deprivation. Nat. Plants 2021, 7, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Salas-González, I.; Reyt, G.; Flis, P.; Custódio, V.; Gopaulchan, D.; Bakhoum, N.; Dew, T.P.; Suresh, K.; Franke, R.B.; Dangl, J.L.; et al. Coordination between microbiota and root endodermis supports plant mineral nutrient homeostasis. Science 2021, 371, eabd0695. [Google Scholar] [CrossRef] [PubMed]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Naylor, D.; Coleman-Derr, D. Drought stress and root-associated bacterial communities. Front. Plant Sci. 2018, 8, 2223. [Google Scholar] [CrossRef]
- Duan, C.; Wang, Y.; Wang, Q.; Ju, W.; Zhang, Z.; Cui, Y.; Beiyuan, J.; Fan, Q.; Wei, S.; Li, S.; et al. Microbial metabolic limitation of rhizosphere under heavy metal stress: Evidence from soil ecoenzymatic stoichiometry. Environ. Pollut. 2022, 300, 118978. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Jiang, D.; Xiang, Z.; Wang, S.; Kang, C.; Zhang, W.; Ge, Y.; Wang, T.; Huang, L.; et al. Soil microbe inoculation alters the bacterial communities and promotes root growth of Atractylodes lancea under heat stress. Plant Soil 2022, 478, 371–389. [Google Scholar] [CrossRef]
- Miyata, N.; Tani, Y.; Sakata, M.; Iwahori, K. Microbial manganese oxide formation and interaction with toxic metal ions. J. Biosci. Bioeng. 2007, 104, 1–8. [Google Scholar] [CrossRef]
- Barboza, N.R.; Guerra-Sa, R.; Leao, V.A. Mechanisms of manganese bioremediation by microbes: An overview. J. Chem. Technol. Biot. 2016, 91, 2733–2739. [Google Scholar] [CrossRef]
- Yang, S.; Ling, G.; Li, Q.; Yi, K.; Tang, X.; Zhang, M.; Li, X. Manganese toxicity-induced chlorosis in sugarcane seedlings involves inhibition of chlorophyll biosynthesis. Crop J. 2022, 10, 1674–1682. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Alifragis, D.; Papaioannou, A. The influence of liming on soil chemical properties and on the alleviation of manganese and copper toxicity in Juglans regia, Robinia pseudoacacia, Eucalyptus sp. and Populus sp. plantations. J. Environ. Manag. 2015, 150, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, A.; Mumtaz, M.Z.; Wang, X.; Ahmad, M.; Saqib, M.; Maqbool, H.; Zaheer, A.; Wang, W.; Mustafa, A. Insights into manganese solubilizing Bacillus spp. for improving plant growth and manganese uptake in maize. Front. Plant Sci. 2021, 12, 719504. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alcalá, I.; Walker, D.J.; Bernal, M.P. Chemical and biological properties in the rhizosphere of Lupinus albus alter soil heavy metal fractionation. Ecotox. Environ. Saf. 2010, 73, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Millaleo, R.; Raoz, M.; Ulloa-Inostroza, E.; Duran, P.; de la Luz Mora, M. Early responses to manganese (Mn) excess and its relation to antioxidant performance and organic acid exudation in barley cultivars. J. Soil Sci. Plant Nut. 2018, 18, 1206–1223. [Google Scholar] [CrossRef]
- Grierson, S.R.P.F. Phosphorus mobilization in agroforestry: Organic anions, phosphatase activity and phosphorus fractions in the rhizosphere. Plant Soil 2004, 259, 209–219. [Google Scholar]
- Zhao, Q.; Li, L.; Li, C.; Li, M.; Amini, F.; Zhang, H. Factors affecting improvement of engineering properties of MICP-treated soil catalyzed by bacteria and urease. J. Mater. Civ. Eng. 2014, 26, 10. [Google Scholar] [CrossRef]
- Eichlerová, I.; Anajdr, J.; Baldrian, P. Laccase activity in soils: Considerations for the measurement of enzyme activity. Chemosphere 2012, 88, 1154–1160. [Google Scholar] [CrossRef]
- He, H.; Li, W.; Yu, R.; Ye, Z. Illumina-Based Analysis of Bulk and Rhizosphere Soil Bacterial Communities in Paddy Fields Under Mixed Heavy Metal Contamination. Pedosphere 2017, 27, 569–578. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, M.; Cao, Y.; Wang, J.; Tang, M.; Ban, Y. Comparisons of Soil Properties, Enzyme Activities and Microbial Communities in Heavy Metal Contaminated Bulk and Rhizosphere Soils of Robinia pseudoacacia L. in the Northern Foot of Qinling Mountain. Forests 2017, 8, 430. [Google Scholar] [CrossRef]
- Teheran-Sierra, L.G.; Funnicelli, M.I.G.; de Carvalho, L.A.L.; Ferro, M.I.T.; Soares, M.A.; Pinheiro, D.G. Bacterial communities associated with sugarcane under different agricultural management exhibit a diversity of plant growth-promoting traits and evidence of synergistic effect. Microbiol. Res. 2021, 247, 126729. [Google Scholar] [CrossRef]
- Drzewiecka, D. Significance and Roles of Proteus spp. Bacteria in Natural Environments. Microb. Ecol. 2016, 72, 741–758. [Google Scholar] [CrossRef]
- Rodrigues, J.L.M.; Pellizari, V.H.; Mueller, R.; Baek, K.; Jesus, E.D.C.; Paula, F.S.; Mirza, B.; Hamaoui, G.S.; Tsai, S.M.; Feigl, B.; et al. Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2013, 110, 988–993. [Google Scholar] [CrossRef]
- Nunes, D.R.U.; Plugge, C.M.; George, I.; van Elsas, J.D.; van Overbeek, L.S. The rhizosphere selects for particular groups of acidobacteria and verrucomicrobia. PLoS ONE 2013, 8, e82443. [Google Scholar] [CrossRef]
- Smit, A.; Kooijman, A.M. Impact of grazing on the input of organic matter and nutrients to the soil in a grass-encroached Scots pine forest. For. Ecol. Manag. 2001, 142, 99–107. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, G.; Yu, Z.; Ding, H.; Xu, Y.; Zhang, Z. Effect of drought stress and developmental stages on microbial community structure and diversity in peanut rhizosphere soil. Int. J. Mol. Sci. 2019, 20, 2265. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Chen, L.; Wang, D. Responses of soil microbial communities and their network interactions to saline-alkaline stress in Cd-contaminated soils. Environ. Pollut. 2019, 252, 1609–1621. [Google Scholar] [CrossRef]
- Supek, F.R.R.C.; Supekova, L.; Nelson, H.; Nelson, N. A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc. Natl. Acad. Sci. USA 1996, 93, 5105–5110. [Google Scholar] [CrossRef]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. BIOGENIC MANGANESE OXIDES: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Dick, G.J.; Torpey, J.W.; Beveridge, T.J.; Tebo, B.M. Direct identification of a bacterial manganese (II) oxidase, the multicopper oxidase MnxG, from spores of several different marine Bacillus species. Appl. Environ. Microbiol. 2008, 74, 1527–1534. [Google Scholar] [CrossRef]
- Chris, A.; Francis, E.C.B.M. Enzymatic manganese (II) oxidation by a marine α-Proteobacterium. Appl. Environ. Microb. 2001, 67, 4024–4029. [Google Scholar]
- Ridge, J.P.; Lin, M.; Larsen, E.I.; Fegan, M.; Mcewan, A.G.; Sly, L.I. A multicopper oxidase is essential for manganese oxidation and laccase-like activity in Pedomicrobium sp. ACM 3067. Environ. Microbiol. 2007, 9, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Miriyala, S.; Spasojevic, I.; Tovmasyan, A.; Salvemini, D.; Vujaskovic, Z.; Clair, D.S.; Batinic-Haberle, I. Manganese superoxide dismutase, MnSOD and its mimics. BBA-Mol. Basis Dis. 2012, 1822, 794–814. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Leadbetter, J.R. Bacterial chemolithoautotrophy via manganese oxidation. Nature 2020, 583, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Suksrichavalit, T.; Prachayasittikul, S.; Nantasenamat, C.; Isarankura-Na-Ayudhya, C.; Prachayasittikul, V. Copper complexes of pyridine derivatives with superoxide scavenging and antimicrobial activities. Eur. J. Med. Chem. 2009, 44, 3259–3265. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.Z.; Xiao, J.L.; Yang, S.; Li, D.L.; Tang, X.L.; Wang, X.X.; Zhang, M.Q.; Li, X.F. The alleviation of manganese toxicity by ammonium in sugarcane is related to pectin content, pectin methyl esterification, and nitric oxide. GCB Bioenergy 2022, 14, 585–596. [Google Scholar] [CrossRef]
- Geng, H.; Wang, F.; Yan, C.; Ma, S.; Zhang, Y.; Qin, Q.; Tian, Z.; Liu, R.; Chen, H.; Zhou, B.; et al. Rhizosphere microbial community composition and survival strategies in oligotrophic and metal(loid) contaminated iron tailings areas. J. Hazard. Mater. 2022, 436, 129045. [Google Scholar] [CrossRef]



| Treatment | Region | Shannon | Simpson | Chao | Shannoneven |
|---|---|---|---|---|---|
| Mn0 | Rhizosphere | 3.51 ± 0.06 ** | 0.049 ± 0.002 | 100.67 ± 5.81 * | 0.76 ± 0.003 * |
| Bulk | 3.16 ± 0.03 | 0.070 ± 0.002 ** | 77.33 ± 0.33 | 0.73 ± 0.007 | |
| Mn0.5 | Rhizosphere | 3.52 ± 0.05 * | 0.049 ± 0.002 | 96.33 ± 3.48 * | 0.78 ± 0.011 * |
| Bulk | 3.23 ± 0.05 | 0.067 ± 0.003 ** | 83.00 ± 2.89 | 0.73 ± 0.005 | |
| Mn1 | Rhizosphere | 3.42 ± 0.02 * | 0.051 ± 0.001 | 86.67 ± 3.28 * | 0.77 ± 0.009 |
| Bulk | 3.25 ± 0.10 | 0.066 ± 0.007 * | 76.00 ± 4.00 | 0.75 ± 0.013 | |
| Ca | Rhizosphere | 3.50 ± 0.00 | 0.047 ± 0.002 | 95.00 ± 1.73 * | 0.76 ± 0.002 |
| Bulk | 3.50 ± 0.03 | 0.046 ± 0.002 | 85.67 ± 1.86 | 0.76 ± 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Cai, Q.; Pan, L.; Tang, X.; Ling, G.; Wei, Y.; Li, X.; Yang, S. Changes in the Microbiome of Sugarcane (Saccharum spp. Hybrids.) Rhizosphere in Response to Manganese Toxicity. Life 2023, 13, 1956. https://doi.org/10.3390/life13101956
Li Q, Cai Q, Pan L, Tang X, Ling G, Wei Y, Li X, Yang S. Changes in the Microbiome of Sugarcane (Saccharum spp. Hybrids.) Rhizosphere in Response to Manganese Toxicity. Life. 2023; 13(10):1956. https://doi.org/10.3390/life13101956
Chicago/Turabian StyleLi, Qiuyue, Qiuliang Cai, Linjuan Pan, Xinlian Tang, Guizhi Ling, Yanyan Wei, Xiaofeng Li, and Shu Yang. 2023. "Changes in the Microbiome of Sugarcane (Saccharum spp. Hybrids.) Rhizosphere in Response to Manganese Toxicity" Life 13, no. 10: 1956. https://doi.org/10.3390/life13101956
APA StyleLi, Q., Cai, Q., Pan, L., Tang, X., Ling, G., Wei, Y., Li, X., & Yang, S. (2023). Changes in the Microbiome of Sugarcane (Saccharum spp. Hybrids.) Rhizosphere in Response to Manganese Toxicity. Life, 13(10), 1956. https://doi.org/10.3390/life13101956





