Objective Non-Invasive Bio-Parametric Evaluation of Regenerated Skin: A Comparison of Two Acellular Dermal Substitutes
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Methods
2.2. Evaluation Tools
- -
- Corneometer CM825® to determine the moisture index, which represents the hydration level of the stratum corneum of the skin.
- -
- Tewameter TM300® to assess the transepidermal water loss (TEWL).
- -
- Visioscan® VC98 USB to determine skin texture using the SELS (Surface Evaluation of the Living Skin) parameters.
- -
- Mexameter MX18® to evaluate the melanin and erythema index which provide a reproducible estimate of the content of hemoglobin and melanin, respectively.
- -
- Skin-Colorimeter CL 400® to assess the color of the skin using the CIELAB system.
- -
- Glossymeter GL200® to evaluate the skin gloss.
- -
- Cutometer® dual MPA 580 (probe of 2 mm diameter) to determine the elasticity, using the R-parameters.
2.3. Statistical Analysis
3. Results
- -
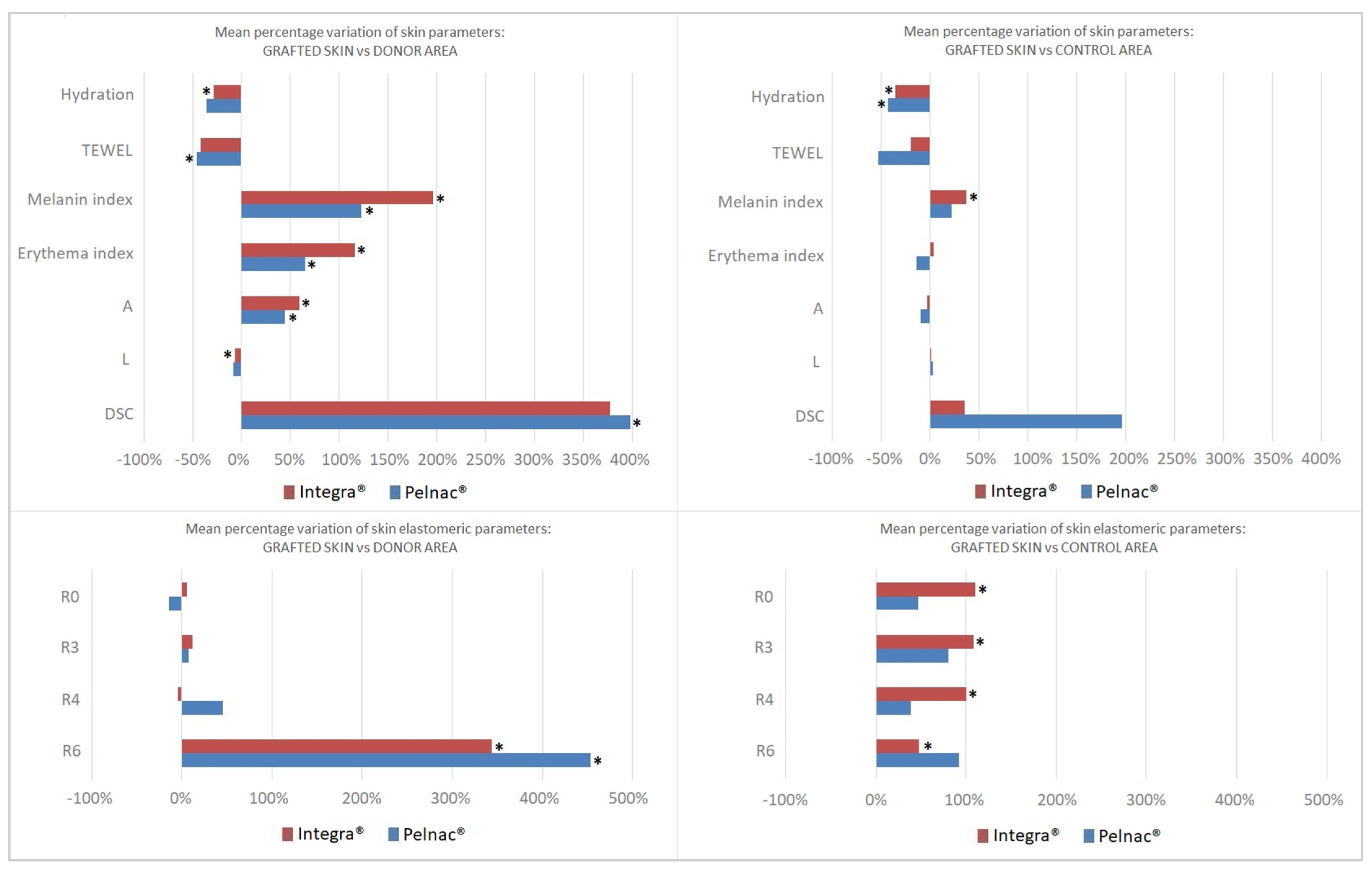
- R0 represents the passive behavior of the skin to external forces which depends on the thickness of the skin and/or its structure, with higher values indicating that the skin is more flexible [29,30]. The skin grafted over Integra® showed higher values vs. the control area, while Pelnac® showed no difference.
- -
- R1 represents the ability of the skin to return to its original state after a deformation. No differences were seen with both DSs.
- -
- R2 represents gross elasticity, with higher values indicating that the skin is more elastic. No differences were seen in both groups.
- -
- R3, R4 and R9 are the parameters most indicative of human skin fatigue [31]. After applying multiple stress deformations, the skin progressively loses the ability to restore its initial position. In our study, the skin reconstructed with the bovine-derived substitute showed higher values of these parameters when compared to the control area, while both DSs showed no difference vs. the donor area.
- -
- R5 represents the net elasticity, with higher values indicating that the skin is more elastic. No differences were seen in both groups.
- -
- R6 represents the portion of visco-elasticity on the elastic part of the curve (the smaller the value the higher the elasticity). Integra® showed higher values of this parameter compared to both the donor and control area, while Pelnac® just vs. the donor site.
- -
- R7 represents the portion of elasticity compared to the complete curve, with higher values indicating that the skin is more elastic. No differences were seen with both DSs.
- -
- R8 represents the ability of the skin to return to a normal state. No differences were seen in both groups.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuchs, E.; Raghavan, S. Getting under the Skin of Epidermal Morphogenesis. Nat. Rev. Genet. 2002, 3, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Tissot, F.S.; Boulter, E.; Estrach, S.; Féral, C.C. The Body’s Tailored Suit: Skin as a Mechanical Interface. Eur. J. Cell Biol. 2016, 95, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Amano, S. Characterization and Mechanisms of Photoageing-related Changes in Skin. Damages of Basement Membrane and Dermal Structures. Exp. Dermatol. 2016, 25, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.E.; Spencer, J.M. Clinical Aspects of Full-Thickness Wound Healing. Clin. Dermatol. 2007, 25, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lucich, E.A.; Rendon, J.L.; Valerio, I.L. Advances in Addressing Full-Thickness Skin Defects: A Review of Dermal and Epidermal Substitutes. Regen. Med. 2018, 13, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.-J.; Waughlock, N.; Kao, D.; Lin, C.-H.; Lin, C.-H.; Hsu, C.-C. Efficient Design of Split Anterolateral Thigh Flap in Extremity Reconstruction. Plast. Reconstr. Surg. 2011, 128, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Trignano, E.; Serra, P.L.; Grieco, F.; Rodio, M.; Rampazzo, S.; Pili, N.; Trignano, C.; Rubino, C. Heel Reconstruction with ALT Free Flap in a 4-Year-Old Patient after a Severe Lawnmower Injury. A Case Report. Case Rep. Plast. Surg. Hand Surg. 2023, 10, 2157280. [Google Scholar] [CrossRef]
- Grauberger, J.N.; Gibreel, W.O.; Moran, S.L.; Carlsen, B.T.; Bakri, K. Long-Term Clinical and Patient-Reported Outcomes in Free Flap Reconstruction of the Weight-Bearing Heel Pad and Non-Weight-Bearing Achilles Tendon Regions. Microsurgery 2020, 40, 835–845. [Google Scholar] [CrossRef]
- Maruccia, M.; Fallico, N.; Cigna, E.; Ciudad, P.; Nicoli, F.; Trignano, E.; Nacchiero, E.; Giudice, G.; Ribuffo, D.; Chen, H. Suprafascial versus Traditional Harvesting Technique for Free Antero Lateral Thigh Flap: A Case-control Study to Assess the Best Functional and Aesthetic Result in Extremity Reconstruction. Microsurgery 2017, 37, 851–857. [Google Scholar] [CrossRef]
- Ciudad, P.; Kaciulyte, J.; Torto, F.L.; Vargas, M.I.; Bustamante, A.; Chen, H.; Maruccia, M.; Zulueta, J.; Trignano, E.; Bolletta, A. The Profunda Artery Perforator Free Flap for Lower Extremity Reconstruction. Microsurgery 2022, 42, 13–21. [Google Scholar] [CrossRef]
- Innocenti, A.; Menichini, G.; Innocenti, M. Six-Years Experience in Major Scalp Defect Reconstruction with Free Flap: Analysis of the Results. Acta Biomed. Atenei Parm. 2022, 92, e2021301. [Google Scholar] [CrossRef]
- Di Summa, P.G.; Watfa, W.; Campisi, C.; Giordano, S.; Oranges, C.M.; Elahi-Rausis, L.; Bauquis, O.; Hahnloser, D.; Demartines, N.; Raffoul, W. Free Versus Pedicled Anterolateral Thigh Flap for Abdominal Wall Reconstruction. Anticancer Res. 2019, 39, 6759–6768. [Google Scholar] [CrossRef] [PubMed]
- Trignano, E.; Tettamanzi, M.; Rampazzo, S.; Trignano, C.; Boccaletti, R.; Fadda, G.M.; Sanna, F.; Bussu, F.; Cossu, A.; Rubino, C. Squamous Cell Carcinoma of the Scalp: A Combination of Different Therapeutic Strategies. Case Rep. Plast. Surg. Hand Surg. 2023, 10, 2210670. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, R.V.; James, S.L.; James, S.E. A Review of Tissue-Engineered Skin Bioconstructs Available for Skin Reconstruction. J. R. Soc. Interface 2010, 7, 229–258. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.F.; Yannas, I.V.; Quinby, W.C.; Bondoc, C.C.; Jung, W.K. Successful Use of a Physiologically Acceptable Artificial Skin in the Treatment of Extensive Burn Injury. Ann. Surg. 1981, 194, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V.; Burke, J.F.; Orgill, D.P.; Skrabut, E.M. Wound Tissue Can Utilize a Polymeric Template to Synthesize a Functional Extension of Skin. Science 1982, 215, 174–176. [Google Scholar] [CrossRef]
- Kyriakidis, C.; Lali, F.; Greco, K.V.; García-Gareta, E. Chronic Leg Ulcers: Are Tissue Engineering and Biomaterials Science the Solution? Bioengineering 2021, 8, 62. [Google Scholar] [CrossRef]
- Rehim, S.A.; Singhal, M.; Chung, K.C. Dermal Skin Substitutes for Upper Limb Reconstruction. Hand Clin. 2014, 30, 239–252. [Google Scholar] [CrossRef]
- Guo, X.; Mu, D.; Gao, F. Efficacy and Safety of Acellular Dermal Matrix in Diabetic Foot Ulcer Treatment: A Systematic Review and Meta-Analysis. Int. J. Surg. 2017, 40, 1–7. [Google Scholar] [CrossRef]
- Marcasciano, M.; Mazzocchi, M.; Kaciulyte, J.; Spissu, N.; Casella, D.; Ribuffo, D.; Dessy, L.A. Skin Cancers and Dermal Substitutes: Is It Safe? Review of the Literature and Presentation of a 2-stage Surgical Protocol for the Treatment of Non-melanoma Skin Cancers of the Head in Fragile Patients. Int. Wound J. 2018, 15, 756–768. [Google Scholar] [CrossRef]
- Bashir, M.M.; Sohail, M.; Shami, H.B. Traumatic Wounds of the Upper Extremity. Hand Clin. 2018, 34, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Haney, N.M.; Huang, M.M.; Liu, J.L.; Hawksworth, D.J.; Burnett, A.L. Acellular Dermal Matrix Tissues in Genitourinary Reconstructive Surgery: A Review of the Literature and Case Discussions. Sex. Med. Rev. 2021, 9, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, M.C.T.; Van Leeuwen, M.C.E.; Van Vucht, N.E.; Van Zuijlen, P.P.M.; Middelkoop, E. Dermal Substitution in Acute Burns and Reconstructive Surgery: A 12-Year Follow-Up. Plast. Reconstr. Surg. 2010, 125, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Osada, A.; Isago, T.; Sakurai, H. Comparison of Contraction among Three Dermal Substitutes: Morphological Differences in Scaffolds. Burns 2017, 43, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, T.D.S.; Machado, R.G.; Visoni, S.B.C.; Pereima, M.J.; Leonardi, D.F.; Trentin, A.G. Dermal Substitutes Support the Growth of Human Skin-Derived Mesenchymal Stromal Cells: Potential Tool for Skin Regeneration. PLoS ONE 2014, 9, e89542. [Google Scholar] [CrossRef] [PubMed]
- Wosgrau, A.C.C.; Jeremias, T.D.S.; Leonardi, D.F.; Pereima, M.J.; Di Giunta, G.; Trentin, A.G. Comparative Experimental Study of Wound Healing in Mice: Pelnac versus Integra. PLoS ONE 2015, 10, e0120322. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, G.; Brenta, F.; Bleve, M.; Pellegatta, T.; Malovini, A.; Faga, A.; Perugini, P. Long-Term In Vivo Assessment of Bioengineered Skin Substitutes: A Clinical Study. J. Tissue Eng. Regen. Med. 2015, 9, 460–468. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Busato, A.; Mannucci, S.; Zingaretti, N.; Cottone, G.; Amendola, F.; De Francesco, M.; Merigo, F.; Riccio, V.; Vaienti, L.; et al. Artificial Dermal Substitutes for Tissue Regeneration: Comparison of the Clinical Outcomes and Histological Findings of Two Templates. J. Int. Med. Res. 2020, 48, 030006052094550. [Google Scholar] [CrossRef]
- Dobrev, H. Use of Cutometer to Assess Dermal Oedema in Erysipelas of the Lower Legs. Ski. Res. Technol. 1998, 4, 155–159. [Google Scholar] [CrossRef]
- Jachowicz, J.; McMullen, R.; Prettypaul, D. Alteration of Skin Mechanics by Thin Polymer Films. Ski. Res. Technol. 2008, 14, 312–319. [Google Scholar] [CrossRef]
- Dobrev, H. Use of Cutometer to Assess Epidermal Hydration: Cutometer and Epidermal Hydration. Ski. Res. Technol. 2000, 6, 239–244. [Google Scholar] [CrossRef] [PubMed]
- De Vries, H.J.C.; Mekkes, J.R.; Middelkoop, E.; Hinrichs, W.L.J.; Wildevuur, C.R.H.; Westerhof, W. Dermal Substitutes for Full-Thickness Wounds in a One-Stage Grafting Model. Wound Repair Regen. 1993, 1, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Lamme, E.N.; De Vries, H.J.; Van Veen, H.; Gabbiani, G.; Westerhof, W.; Middelkoop, E. Extracellular Matrix Characterization during Healing of Full-Thickness Wounds Treated with a Collagen/Elastin Dermal Substitute Shows Improved Skin Regeneration in Pigs. J. Histochem. Cytochem. 1996, 44, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- De Vries, H.J.C.; Middelkoop, E.; Mekkes, J.R.; Dutrieux, R.P.; Wildevuur, C.H.R.; Westerhof, W. Dermal Regeneration in Native Non-Cross-Linked Collagen Sponges with Different Extracellular Matrix Molecules. Wound Repair Regen. 1994, 2, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Dzwigałowska, A.; Sołyga-Żurek, A.; Dębowska, R.M.; Eris, I. Preliminary Study in the Evaluation of Anti-aging Cosmetic Treatment Using Two Complementary Methods for Assessing Skin Surface. Ski. Res. Technol. 2013, 19, 155–161. [Google Scholar] [CrossRef]
- Clarys, P.; Clijsen, R.; Taeymans, J.; Barel, A.O. Hydration Measurements of the Stratum Corneum: Comparison between the Capacitance Method (Digital Version of the C Orneometer CM 825®) and the Impedance Method (S Kicon-200 EX®). Ski. Res. Technol. 2012, 18, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Barel, A.O.; Clarys, P. Study of the Stratum Corneum Barrier Function by Transepidermal Water Loss Measurements: Comparison between Two Commercial Instruments: Evaporimeter® and Tewameter®. Ski. Pharmacol. Physiol. 1995, 8, 186–195. [Google Scholar] [CrossRef]
- Stroumza, N.; Bosc, R.; Hersant, B.; Hermeziu, O.; Meningaud, J.-P. Intérêt du cutomètre pour l’évaluation de l’efficacité des traitements cutanés en chirurgie plastique et maxillo-faciale. Rev. Stomatol. Chir. Maxillo-Faciale Chir. Orale 2015, 116, 77–81. [Google Scholar] [CrossRef]
- Moiemen, N.; Yarrow, J.; Hodgson, E.; Constantinides, J.; Chipp, E.; Oakley, H.; Shale, E.; Freeth, M. Long-Term Clinical and Histological Analysis of Integra Dermal Regeneration Template. Plast. Reconstr. Surg. 2011, 127, 1149–1154. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, M.; Xu, Y.; Ni, X.-D.; Cang, Z.-Q.; Yuan, S.-M. Treatment of Large Scars in Children Using Artificial Dermis and Scalp Skin Grafting. J. Craniofacial Surg. 2019, 30, 891–896. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, T.G.; Kim, J.Y.; Lee, M.C.; Chung, Y.K.; Lee, W.J. Acellular Dermal Matrix to Treat Full Thickness Skin Defects: Follow-Up Subjective and Objective Skin Quality Assessments. Arch. Craniofacial Surg. 2014, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Ly, B.C.K.; Dyer, E.B.; Feig, J.L.; Chien, A.L.; Del Bino, S. Research Techniques Made Simple: Cutaneous Colorimetry: A Reliable Technique for Objective Skin Color Measurement. J. Investig. Dermatol. 2020, 140, 3–12.e1. [Google Scholar] [CrossRef] [PubMed]
- Van Lankveld, W.G.J.M.; Vonk, M.C.; Teunissen, H.; Van Den Hoogen, F.H.J. Appearance Self-Esteem in Systemic Sclerosis--Subjective Experience of Skin Deformity and Its Relationship with Physician-Assessed Skin Involvement, Disease Status and Psychological Variables. Rheumatology 2007, 46, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Ezerskaia, A.; Ras, A.; Bloemen, P.; Pereira, S.F.; Urbach, H.P.; Varghese, B. High Sensitivity Optical Measurement of Skin Gloss. Biomed. Opt. Express 2017, 8, 3981. [Google Scholar] [CrossRef]
- Busche, M.N.; Thraen, A.-C.J.; Gohritz, A.; Rennekampff, H.-O.; Vogt, P.M. Burn Scar Evaluation Using the Cutometer® MPA 580 in Comparison to “Patient and Observer Scar Assessment Scale” and “Vancouver Scar Scale”. J. Burn. Care Res. 2018, 39, 516–526. [Google Scholar] [CrossRef]
- Blichmann, C.W.; Serup, J. Assessment of Skin Moisture. Measurement of Electrical Conductance, Capacitance and Transepidermal Water Loss. Acta Derm. Venereol. 1988, 68, 284–290. [Google Scholar]
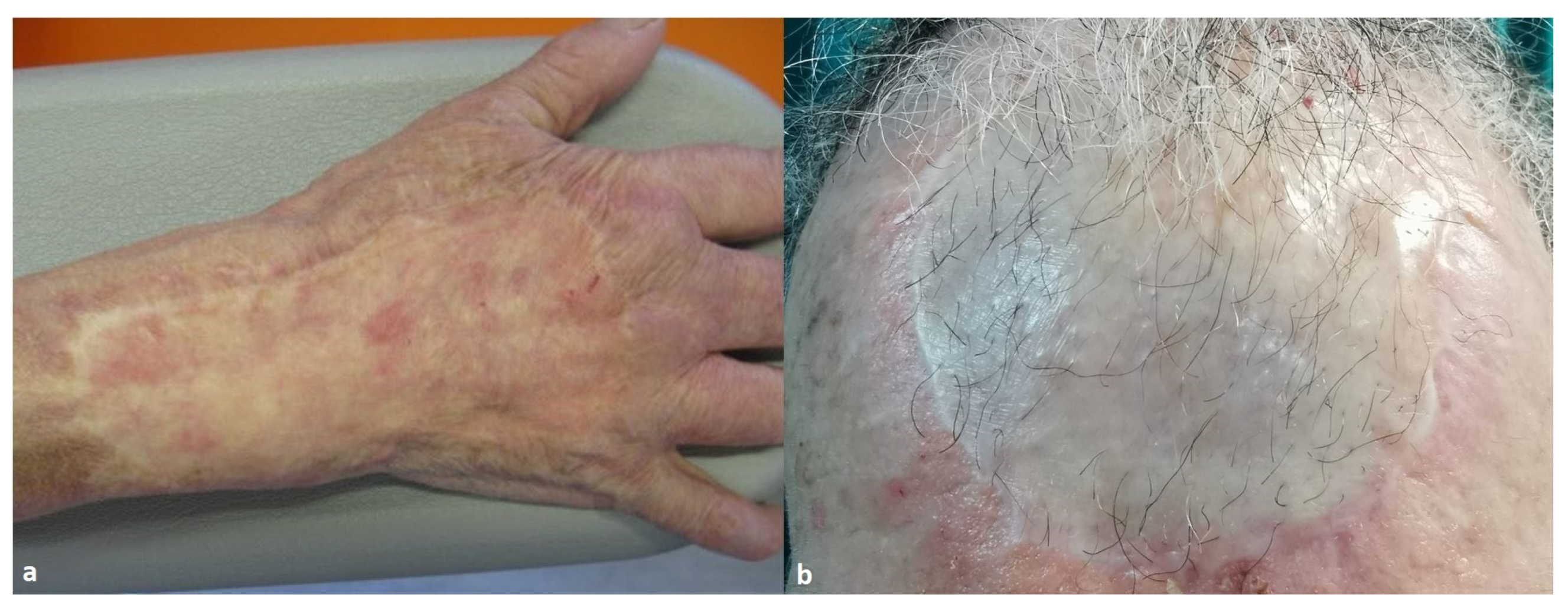
| Integra® | Pelnac® | Total | |
|---|---|---|---|
| AGE (mean ± SD in years) | 69.8 ± 18.1 | 60.0 ± 28.3 | 65.2 ± 23.1 |
| (range) | (30–86) | (18–91) | (18–91) |
| SEX (male:female) | 7:1 | 6:1 | 13:2 |
| PRIMARY LESION | |||
| Trauma | 0 | 2 | 2 |
| Tumour resection (BCC, SCC, Melanoma, Dermatofibrosarcoma) | 6 | 5 | 11 |
| Scar revision | 1 | 0 | 1 |
| Infusion leak | 1 | 0 | 1 |
| SITE OF INJURY | |||
| Scalp | 4 | 3 | 7 |
| Forehead | 2 | 2 | 4 |
| Upper limb | 1 | 2 | 3 |
| Lower extremity | 1 | 0 | 1 |
| SKIN DONOR SITE (Type of Skin Graft) | |||
| Thigh (STSG) | 3 | 2 | 5 |
| Groin (FTSG) | 1 | 1 | 2 |
| Belly (FTSG) | 3 | 1 | 4 |
| Clavicular region (FTSG) | 0 | 2 | 2 |
| Arm (FTSG) | 1 | 1 | 2 |
| FOLLOW-UP PERIOD (mean ± SD in months) | 38.3 ± 16.0 | 16.9 ± 4.7 | 28.3 ± 16.1 |
| (range) | (12–57) | (12–24) | (12–57) |
| Integra® | Pelnac® | |||||||
|---|---|---|---|---|---|---|---|---|
| Device | Parameter | Reconstructed Skin | Donor Area | Control Area | Reconstructed Skin | Donor Area | Control Area | |
| Corneometer | CM825® | Moisture index | 26.07 ± 11.3 | 38.51 ± 8.19 | 46.37 ± 16.7 | 29.96 ± 20.1 | 48.41 ± 7.93 | 58.28 ± 14.1 |
| p-value | 0.0471 | 0.0404 | 0.0762 | 0.0344 | ||||
| Tewameter | TM300® | TEWL | 7.037 ± 4.23 | 15.02 ± 12.3 | 11.23 ± 4.96 | 7.171 ± 4.52 | 13.18 ± 7.73 | 17.7 ± 14.2 |
| p-value | 0.0987 | 0.1422 | 0.0116 | 0.0503 | ||||
| Visioscan® VC98 | SEsc (Scaliness) | 1.503 ± 0.93 | 0.568 ± 0.34 | 1.058 ± 0.87 | 1.02 ± 0.74 | 0.374 ± 0.47 | 0.67 ± 0.59 | |
| p-value | 0.070 | 0.362 | 0.120 | 0.414 | ||||
| SEr (Skin roughness) | 3.331 ± 1.39 | 10.61 ± 19.7 | 3.123 ± 1.50 | 6.685 ± 7.71 | 3.337 ± 1.97 | 4.014 ± 2.75 | ||
| p-value | 0.342 | 0.824 | 0.262 | 0.407 | ||||
| SEw (Wrinkles) | 90.23 ± 29.5 | 116.4 ± 72.0 | 148 ± 93.7 | 181.6 ± 89.7 | 117.6 ± 42.3 | 127.1 ± 41.1 | ||
| p-value | 0.326 | 0.103 | 0.097 | 0.128 | ||||
| SEsm (Skin smoothness) | 210.2 ± 106 | 291.5 ± 132 | 333.4 ± 192 | 270.3 ± 175 | 298.0 ± 49.6 | 289.1 ± 76.9 | ||
| p-value | 0.203 | 0.088 | 0.675 | 0.805 | ||||
| Mexameter MX18® | Skin-Colorimeter CL 400® & CIELAB system | Melanin index | 151.4 ± 41.7 | 90.28 ± 42.8 | 116.8 ± 44.9 | 198.9 ± 132 | 91.89 ± 48.6 | 163.7 ± 93.7 |
| p-value | 0.001 | 0.009 | 0.0499 | 0.1548 | ||||
| Erythema index | 308.9 ± 65.0 | 184.0 ± 90.0 | 302.6 ± 75.2 | 305.9 ± 91.9 | 184.7 ± 20.7 | 387.2 ± 136 | ||
| p-value | 0.002 | 0.741 | 0.010 | 0.168 | ||||
| L* | 64.81 ± 3.27 | 69.90 ± 3.89 | 64.58 ± 3.93 | 63.10 ± 6.67 | 68.76 ± 2.90 | 61.64 ± 6.57 | ||
| p-value | 0.002 | 0.914 | 0.062 | 0.574 | ||||
| a* | 8.095 ± 1.85 | 5.722 ± 1.97 | 8.967 ± 2.82 | 8.6 ± 1.43 | 6.475 ± 2.27 | 11.01 ± 3.28 | ||
| p-value | 0.017 | 0.395 | 0.038 | 0.207 | ||||
| b* | 10.12 ± 2.63 | 11.42 ± 2.26 | 11.87 ± 2.86 | 11.11 ± 3.07 | 9.792 ± 2.38 | 11.40 ± 1.22 | ||
| p-value | 0.232 | 0.059 | 0.396 | 0.868 | ||||
| ITA | 54.75 ± 11.6 | 59.5 ± 7.92 | 50.25 ± 11.1 | 47.57 ± 21.5 | 62.14 ± 8.35 | 42.42 ± 17.9 | ||
| p-value | 0.204 | 0.367 | 0.174 | 0.513 | ||||
| Glossymeter GL200® | Direct glossiness | 8.141 ± 6.69 | 5.207 ± 1.34 | 8.195 ± 3.30 | 11.76 ± 5.10 | 5.318 ± 0.46 | 8.264 ± 2.94 | |
| p-value | 0.273 | 0.977 | 0.018 | 0.262 | ||||
| Diffuse Scattering Correction (DSC) | 6.692 ± 5.90 | 2.932 ± 4.21 | 5.615 ± 3.39 | 9.83 ± 5.37 | 1.962 ± 0.49 | 6.254 ± 3.36 | ||
| p-value | 0.178 | 0.555 | 0.010 | 0.270 | ||||
| Diffused light | 27.2 ± 2.78 | 37.61 ± 5.12 | 27.55 ± 3.26 | 20.7 ± 5.92 | 33.3 ± 3.50 | 21.31 ± 6.25 | ||
| p-value | 0.001 | 0.838 | 0.002 | 0.833 | ||||
| Cutometer® | R0 (Total elongation) | 0.085 ± 0.04 | 0.116 ± 0.05 | 0.042 ± 0.01 | 0.092 ± 0.03 | 0.151 ± 0.05 | 0.076 ± 0.02 | |
| p-value | 0.353 | 0.039 | 0.121 | 0.421 | ||||
| R1 (Return to original skin) | 0.012 ± 0.00 | 0.017 ± 0.00 | 0.009 ± 0.00 | 0.011 ± 0.00 | 0.019 ± 0.00 | 0.016 ± 0.00 | ||
| p-value | 0.079 | 0.197 | 0.206 | 0.381 | ||||
| R2 (Gross elasticity) | 0.819 ± 0.10 | 0.845 ± 0.07 | 0.798 ± 0.04 | 0.886 ± 0.08 | 0.858 ± 0.09 | 0.785 ± 0.10 | ||
| p-value | 0.670 | 0.674 | 0.662 | 0.120 | ||||
| R3 (Tiring effect) | 0.095 ± 0.05 | 0.123 ± 0.05 | 0.047 ± 0.01 | 0.117 ± 0.02 | 0.154 ± 0.06 | 0.081 ± 0.03 | ||
| p-value | 0.436 | 0.040 | 0.335 | 0.119 | ||||
| R4 (Tiring effect) | 0.028 ± 0.01 | 0.029 ± 0.00 | 0.014 ± 0.00 | 0.024 ± 0.01 | 0.028 ± 0.00 | 0.024 ± 0.00 | ||
| p-value | 0.827 | 0.025 | 0.649 | 0.975 | ||||
| R5 (Net elasticity) | 0.744 ± 0.25 | 0.531 ± 0.14 | 0.661 ± 0.22 | 0.655 ± 0.29 | 0.488 ± 0.21 | 0.530 ± 0.15 | ||
| p-value | 0.153 | 0.247 | 0.340 | 0.344 | ||||
| R6 (Viscoelasticity) | 1.022 ± 0.51 | 0.337 ± 0.13 | 0.686 ± 0.24 | 0.630 ± 0.25 | 0.171 ± 0.09 | 0.451 ± 0.22 | ||
| p-value | 0.014 | 0.048 | 0.004 | 0.168 | ||||
| R7 (Skin firmness) | 0.356 ± 0.06 | 0.402 ± 0.12 | 0.383 ± 0.09 | 0.525 ± 0.18 | 0.418 ± 0.17 | 0.367 ± 0.10 | ||
| p-value | 0.430 | 0.338 | 0.468 | 0.191 | ||||
| R8 (Total recovery) | 0.072 ± 0.04 | 0.098 ± 0.05 | 0.032 ± 0.00 | 0.080 ± 0.04 | 0.131 ± 0.06 | 0.060 ± 0.03 | ||
| p-value | 0.451 | 0.053 | 0.210 | 0.355 | ||||
| R9 (Tiring effect) | 0.009 ± 0.01 | 0.006 ± 0.00 | 0.005 ± 0.00 | 0.024 ± 0.02 | 0.003 ± 0.00 | 0.004 ± 0.00 | ||
| p-value | 0.505 | 0.276 | 0.119 | 0.127 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rampazzo, S.; Ferrari, M.; Sotgiu, M.A.; Piu, G.; Solinas, M.G.; Usai, N.; Bulla, A.; Serra, P.L.; Grieco, F.; Montella, A.; et al. Objective Non-Invasive Bio-Parametric Evaluation of Regenerated Skin: A Comparison of Two Acellular Dermal Substitutes. Life 2024, 14, 121. https://doi.org/10.3390/life14010121
Rampazzo S, Ferrari M, Sotgiu MA, Piu G, Solinas MG, Usai N, Bulla A, Serra PL, Grieco F, Montella A, et al. Objective Non-Invasive Bio-Parametric Evaluation of Regenerated Skin: A Comparison of Two Acellular Dermal Substitutes. Life. 2024; 14(1):121. https://doi.org/10.3390/life14010121
Chicago/Turabian StyleRampazzo, Silvia, Marco Ferrari, Maria Alessandra Sotgiu, Gabriella Piu, Maria Giuliana Solinas, Noemi Usai, Antonio Bulla, Pietro Luciano Serra, Federica Grieco, Andrea Montella, and et al. 2024. "Objective Non-Invasive Bio-Parametric Evaluation of Regenerated Skin: A Comparison of Two Acellular Dermal Substitutes" Life 14, no. 1: 121. https://doi.org/10.3390/life14010121
APA StyleRampazzo, S., Ferrari, M., Sotgiu, M. A., Piu, G., Solinas, M. G., Usai, N., Bulla, A., Serra, P. L., Grieco, F., Montella, A., Mazzarello, V., & Rubino, C. (2024). Objective Non-Invasive Bio-Parametric Evaluation of Regenerated Skin: A Comparison of Two Acellular Dermal Substitutes. Life, 14(1), 121. https://doi.org/10.3390/life14010121











