Laparoscopic Approach to Primary Splenic Cyst: Case Report and Review of the Literature
Abstract
1. Introduction
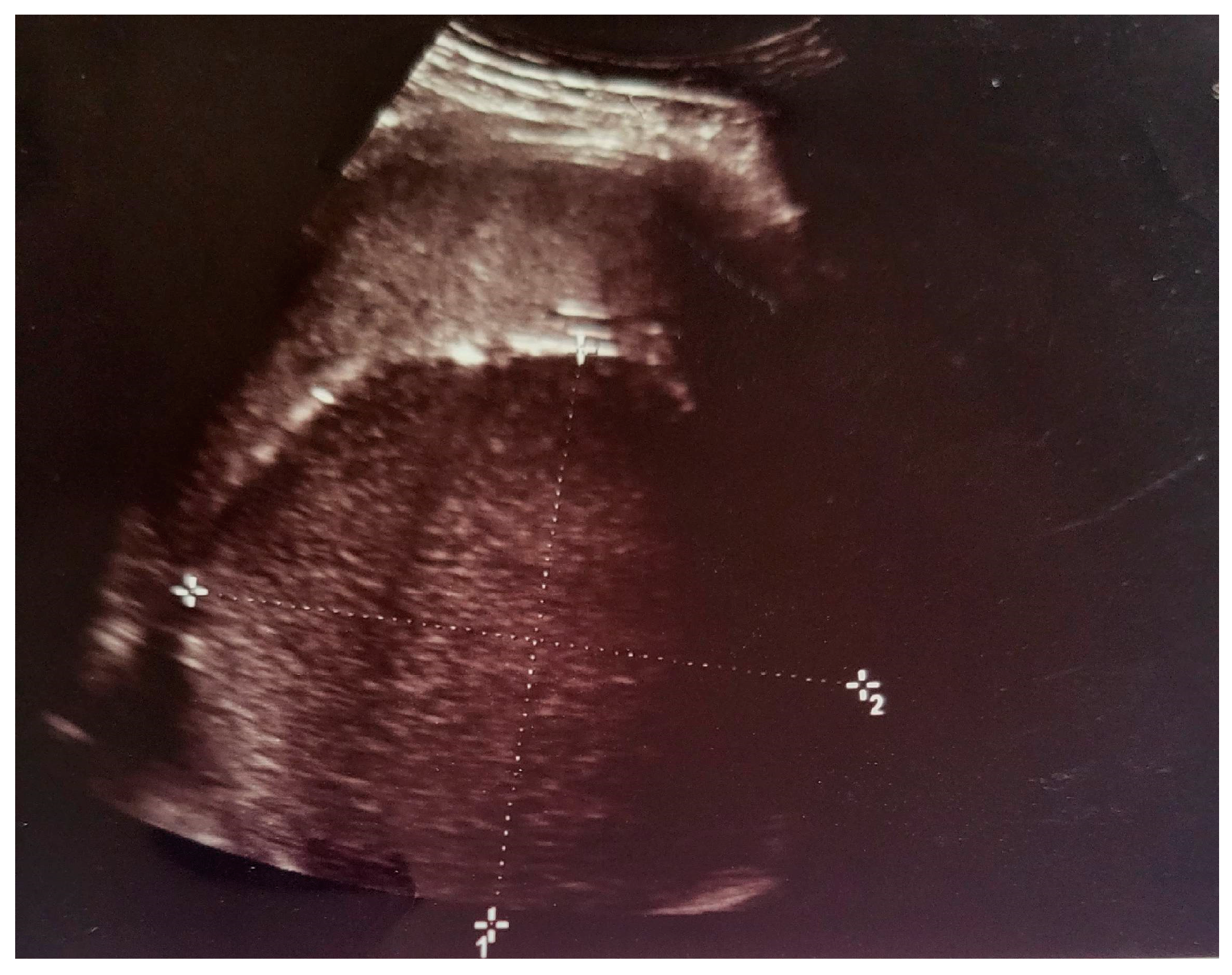
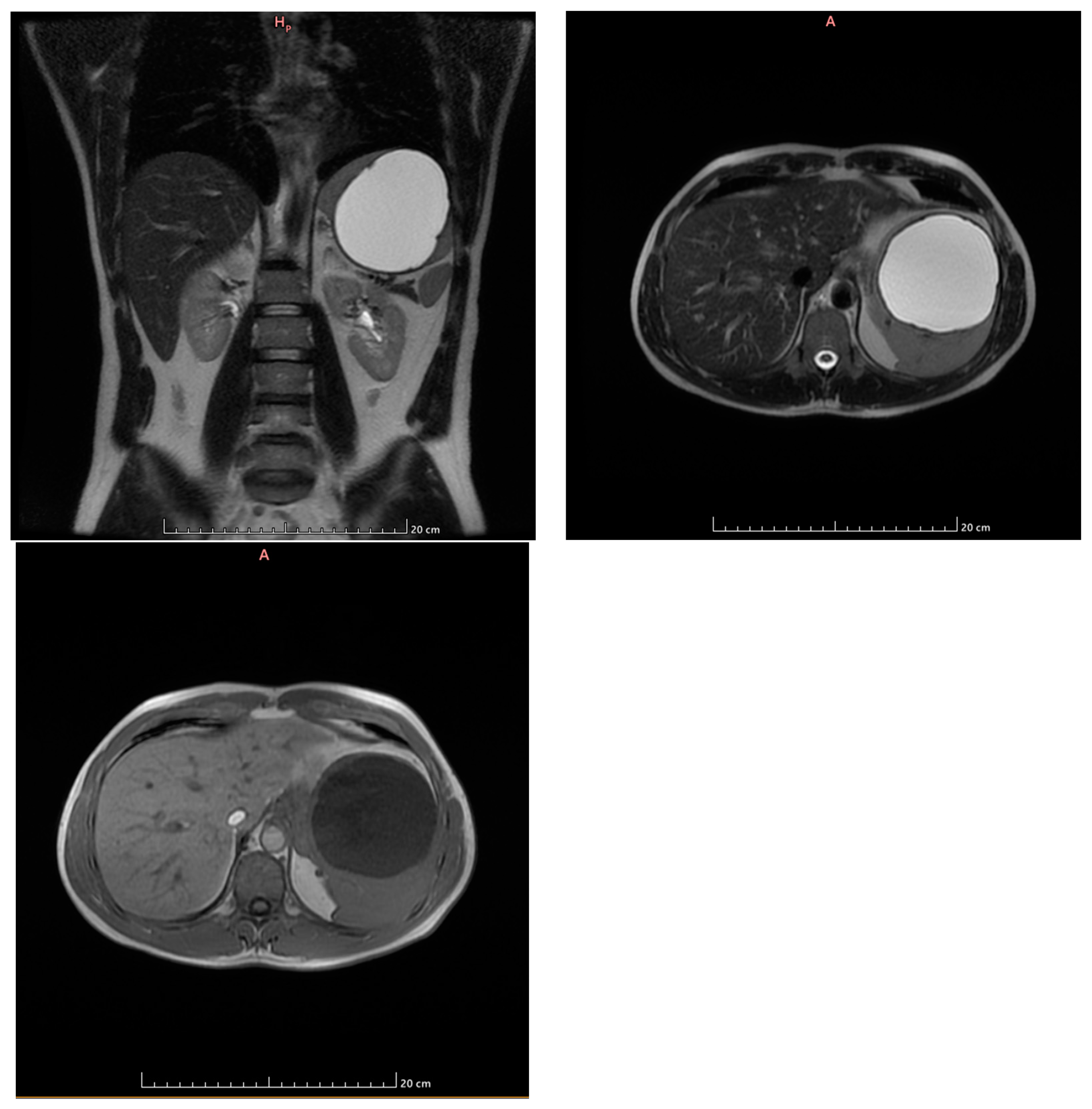
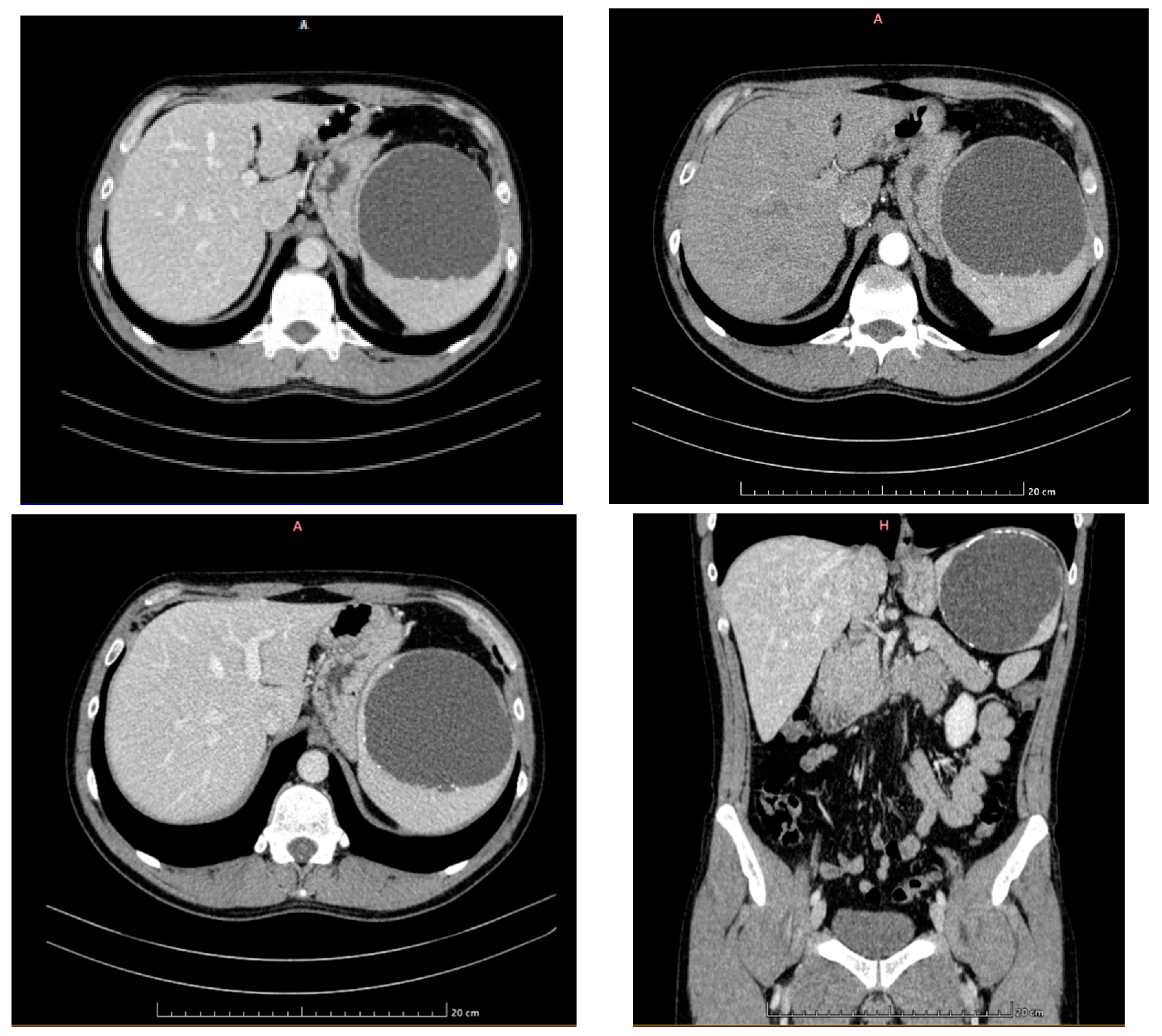
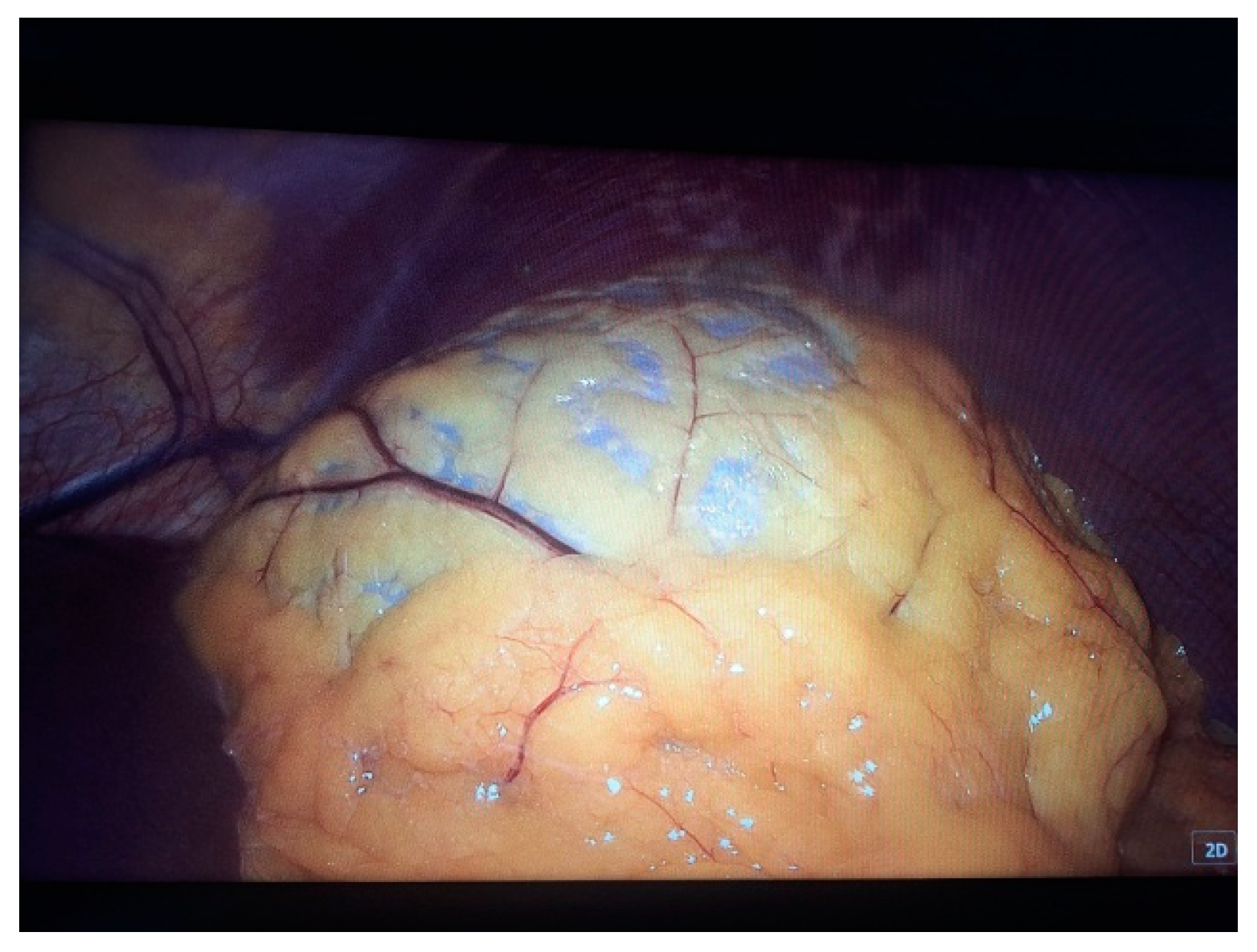
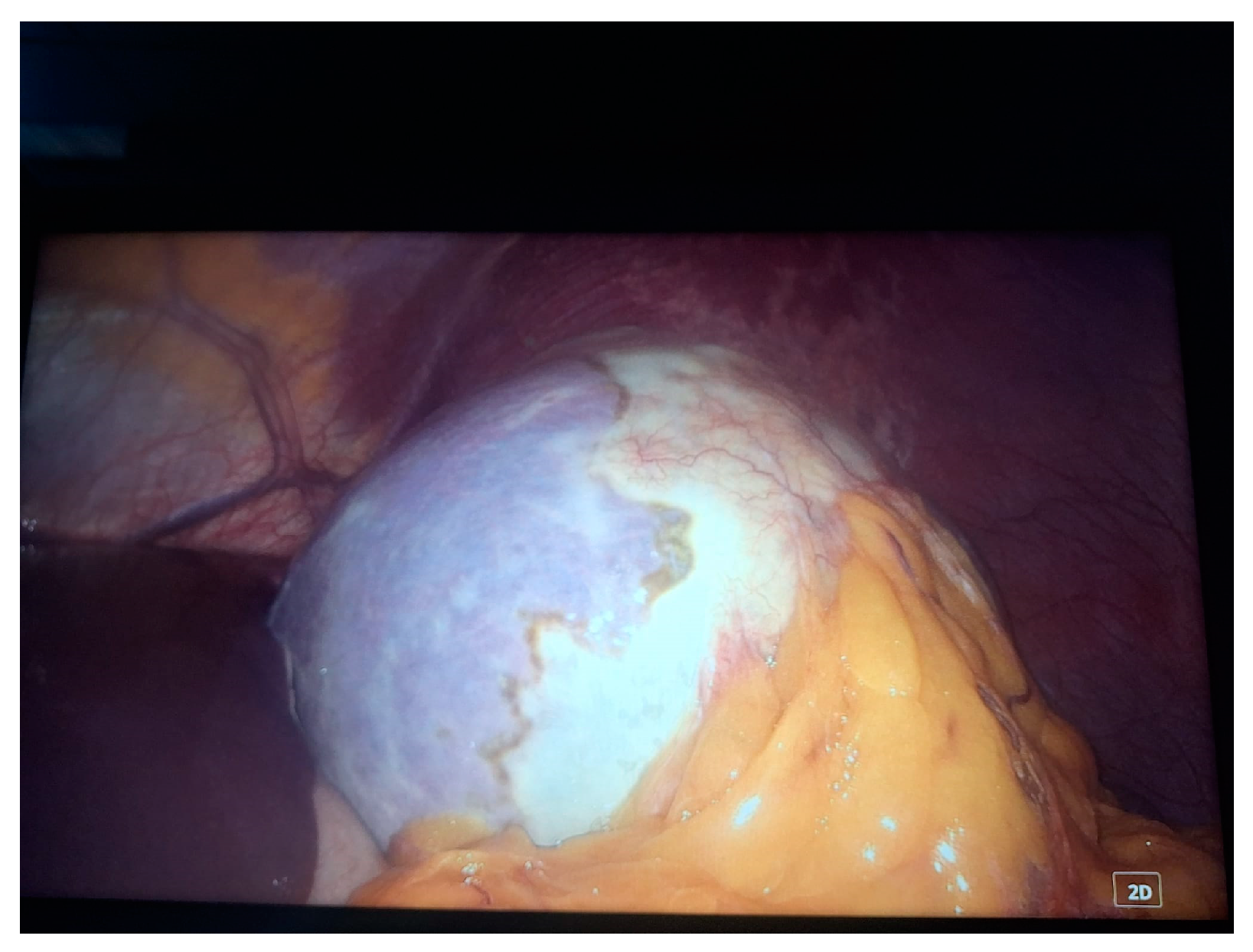
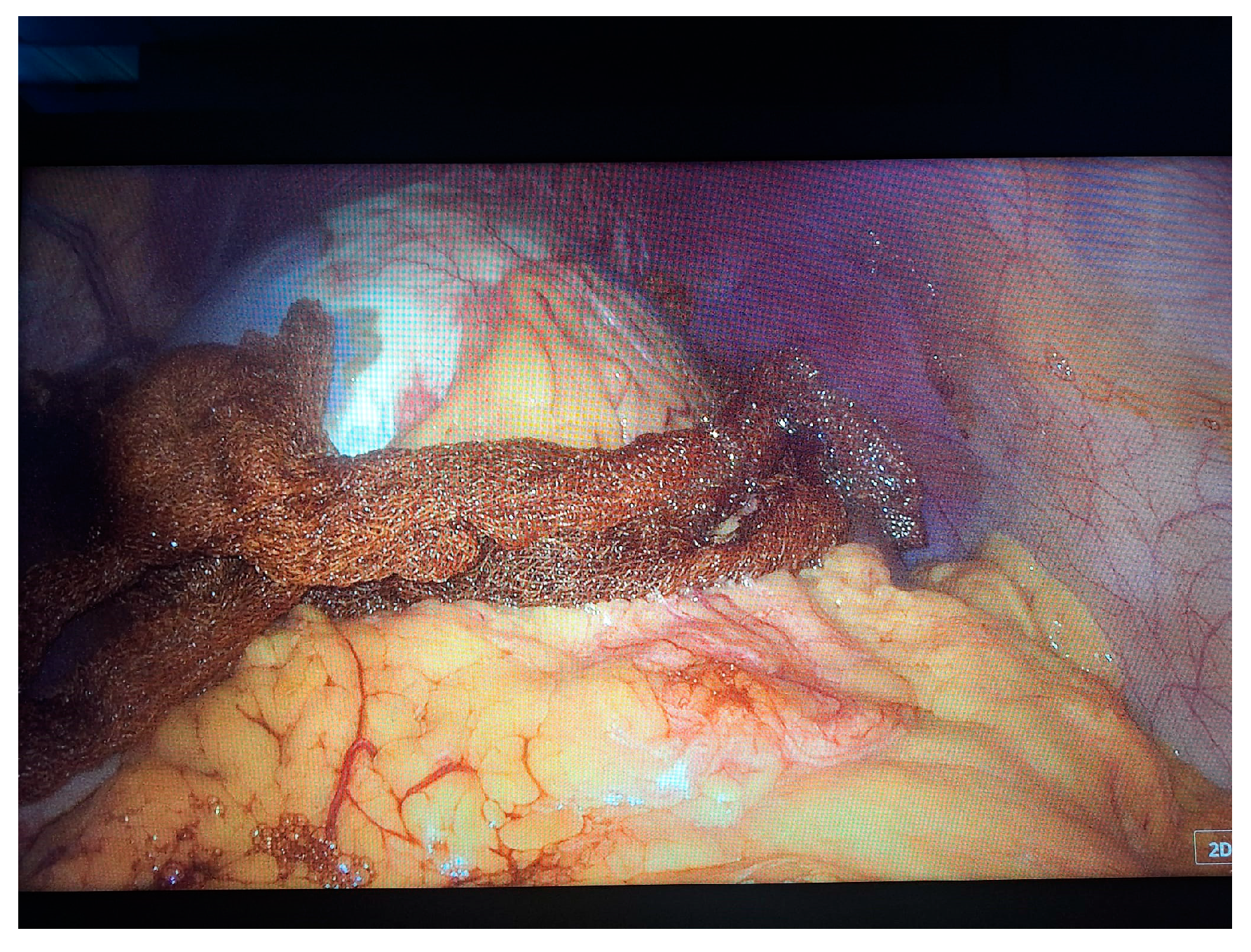
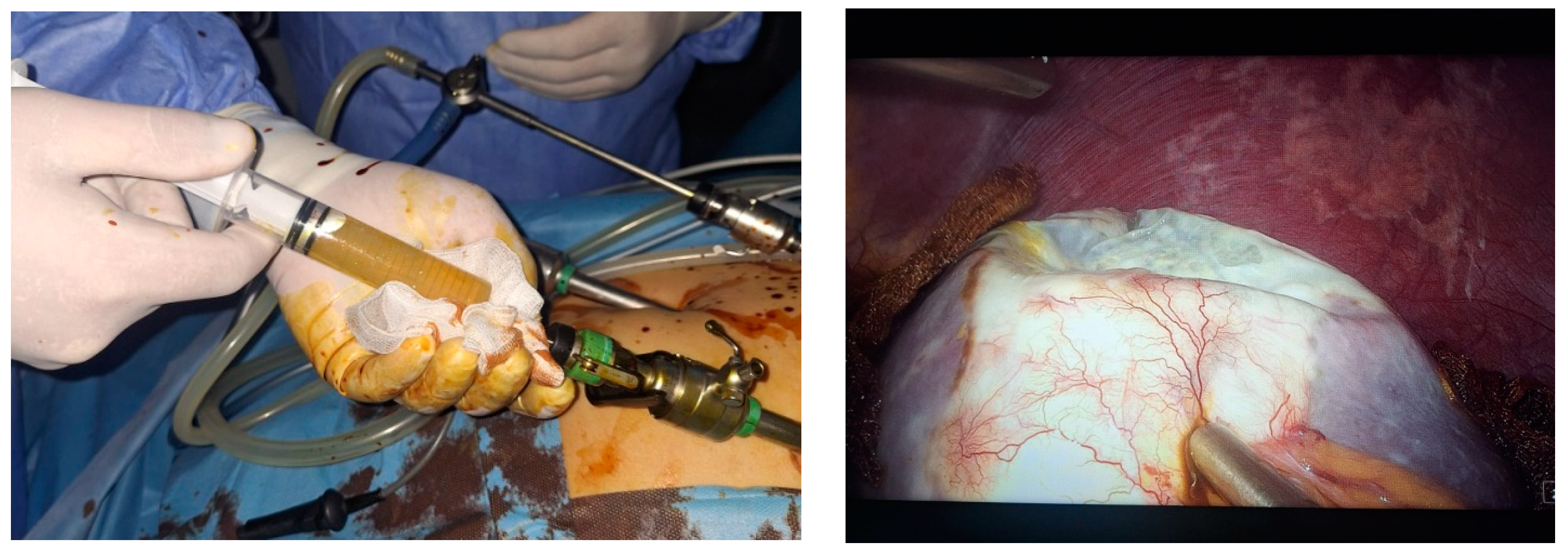
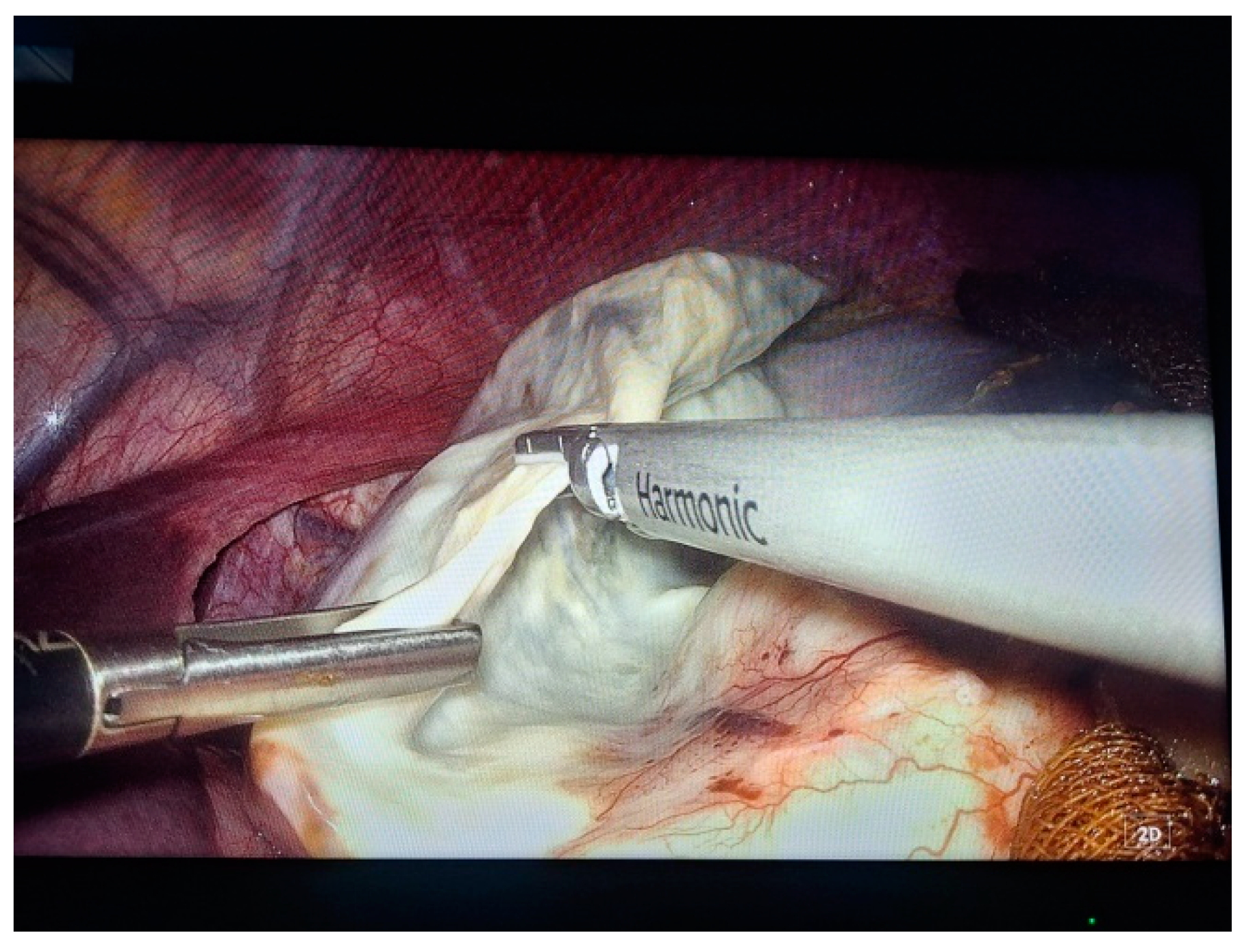
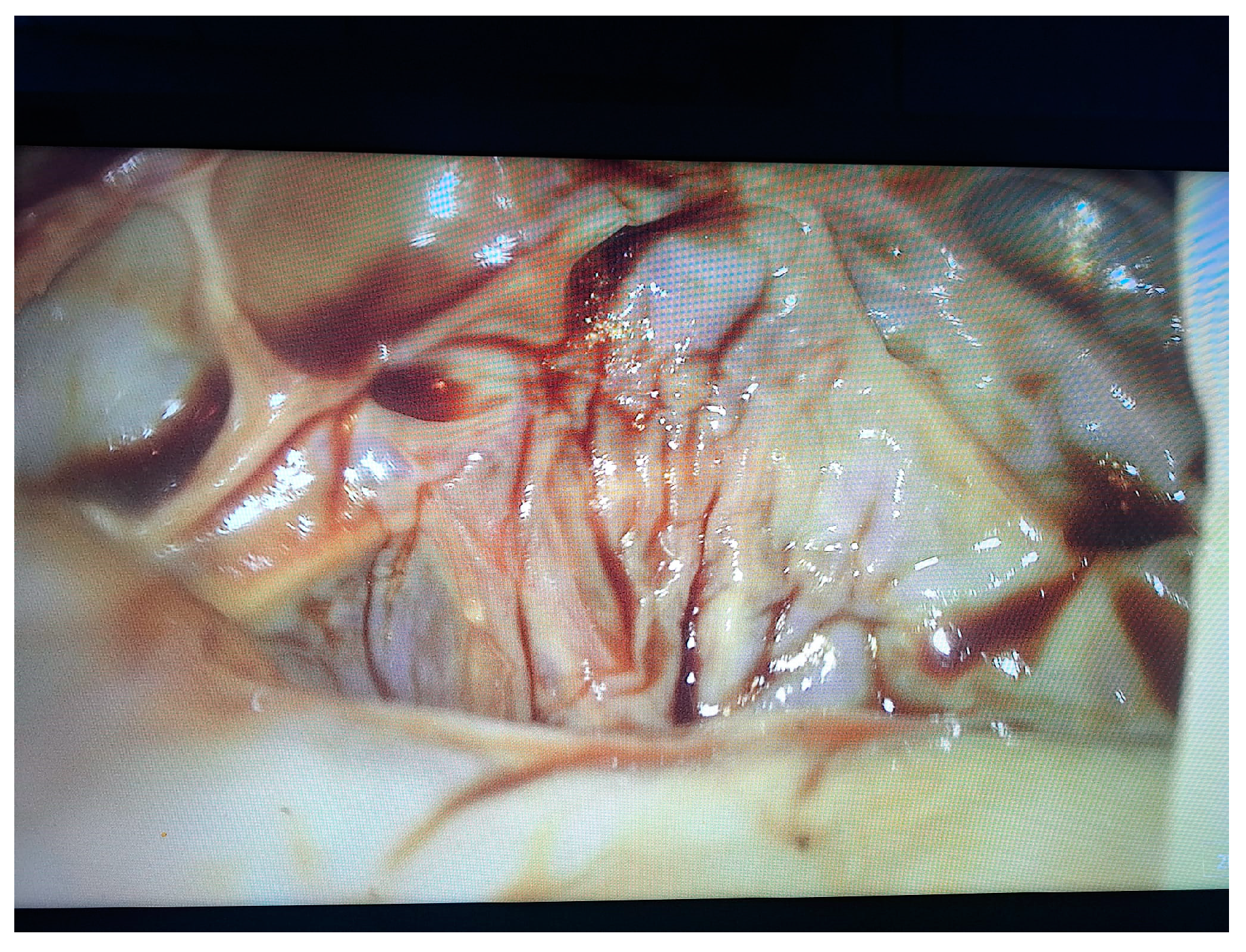
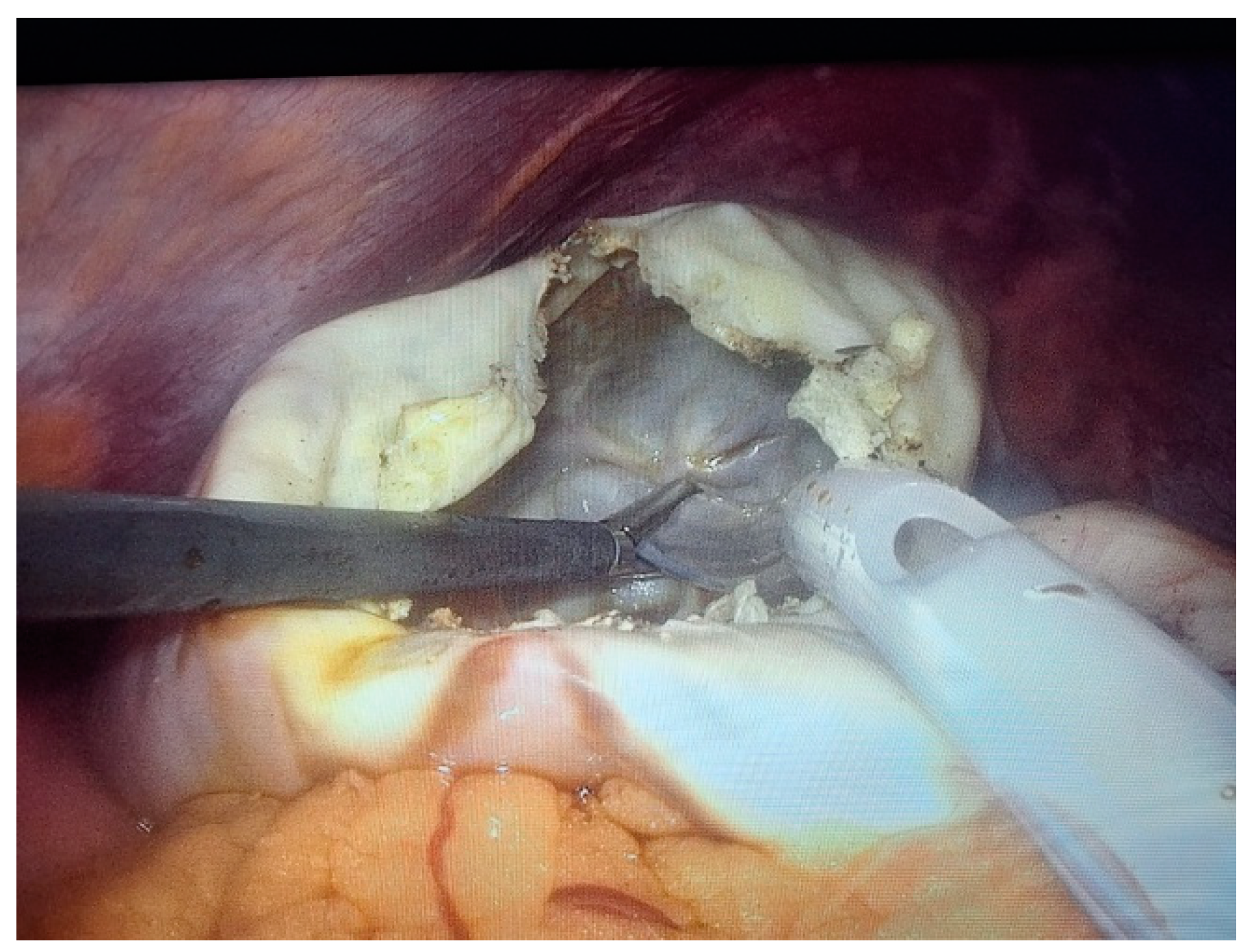
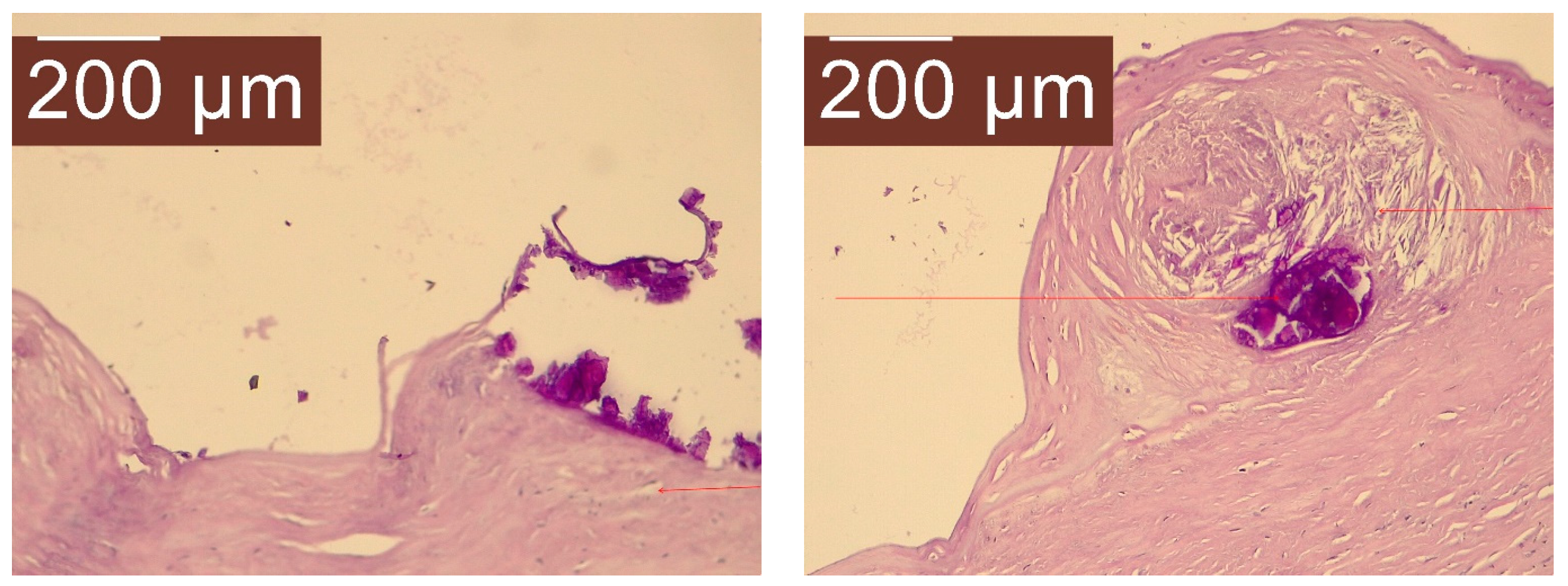
2. Case Presentation
- Capillary hemangioma hepatic segment VII of 8 mm diameter.
- Grade I splenomegaly with simple voluminous splenic cyst, 10 cm in diameter.
- Accessory spleen adjacent to the lower pole of the spleen.
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansen, M.B.; Moller, A.C. Splenic cysts. Surg. Laparosc. Endosc. Percutan. Tech. 2004, 14, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Chetty, R. A review of the cysts of the spleen. Diagn. Histopathol. 2016, 22, 479–484. [Google Scholar] [CrossRef]
- Kenney, C.D.; Hoeger, Y.E.; Yetasook, A.K.; Linn, J.G.; Denham, E.W.; Carbray, J. Management of Non-Parasitic Splenic Cysts: Does Size Really Matter? J. Gastrointest. Surg. 2014, 18, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.J.; Bynoe, R.P.; Greene, F.L.; Burke, M.L. Splenic salvage techniques in the management of pseudocysts of the spleen. South Med. J. 1986, 79, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Tagaya, N.; Oda, N.; Furihata, M.; Nemoto, T.; Suzuki, N.; Kubota, K. Experience with Laparoscopic Management of Solitary Symptomatic Splenic Cysts. Surg. Laparosc. Endosc. Percutaneous Tech. 2002, 12, 279–282. [Google Scholar] [CrossRef]
- Res, L.C.; Knook, M.T.T.; Hazelbag, H.M.; Guicherit, O.R. Spontaneous rupture of a non-parasitic splenic cyst. BMJ Case Rep. 2019, 12, e231473. [Google Scholar] [CrossRef]
- Gianom, D.; Wildisen, A.; Hotz, T.; Goti, F.; Decurtins, M. Open and laparoscopic treatment of nonparasitic splenic cysts. Dig. Surg. 2003, 20, 74–78. [Google Scholar] [CrossRef]
- Kumar, P.; Hasan, A.; Kumar, M.; Singh, V. Isolated hydatid cyst of spleen: A rare case with rare presentation. Int. J. Surg. Case Rep. 2016, 28, 279–281. [Google Scholar] [CrossRef]
- Gunes, O.; Bag, Y.M.; Turgut, E.; Gunes, A.; Sumer, F.; Kayaalp, C. Splenic surgery: A ten years experience of a tertiary center in Turkey. Ann. Ital. Chir. 2022, 92, 59–64. [Google Scholar]
- Gore, R.M.; Ecanow, J.S. Management of splenic “incidentalomas” found on ultrasound and computed tomography. Cancer Imaging 2015, 15 (Suppl. S1), O11. [Google Scholar] [CrossRef]
- Civardi, G.; Vallisa, D.; Bertè, R.; Giorgio, A.; Filice, C.; Caremani, M.; Caturelli, E.; Pompili, M.; De Sio, I.; Buscarini, E.; et al. Ultrasound-guided fine needle biopsy of the spleen: High clinical efficacy and low risk in a multicenter Italian study. Am. J. Hematol. 2001, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sammon, J.; Twomey, M.; Crush, L.; Maher, M.M.; O’Connor, O.J. Image-guided percutaneous splenic biopsy and drainage. Semin. Intervent. Radiol. 2012, 29, 301–310. [Google Scholar] [CrossRef] [PubMed]
- M’rad, S.; Oudni-M’rad, M.; Boubaker, G.; Bouazzi, L.; Gorcii, M.; Nouri, A.; Mezhoud, H.; Babba, H. Étude rétrospective de la distribution et de la fertilité des kystes hydatiques chez l’enfant en Tunisie [Retrospective study of the distribution and the fertility of hydatid cysts in the child in Tunisia]. Pathol. Biol. 2012, 60, 166–169. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.M.; Han, J.K.; Lee, J.Y.; Kim, K.W.; Cho, K.C.; Choi, B.I. Intrapancreatic accessory spleen: Findings on MR Imaging, CT, US and scintigraphy, and the pathologic analysis. Korean J. Radiol. 2008, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.A.; Wei, X.J.; Iyengar, P.; Moreira, A.L. Diagnostic pitfalls in fine needle aspiration biopsy of the spleen. Diagn. Cytopathol. 2008, 36, 69–75. [Google Scholar] [CrossRef]
- Lee, D.S.; Lee, S.K.; Seo, D.W. Long-term safety and efficacy of ethanol retention therapy via percutaneous approach and/or EUS guidance for symptomatic large hepatic cysts (with video). Endosc. Ultrasound. 2020, 9, 31–36. [Google Scholar] [CrossRef]
- Siniluoto, T.M.J.; Päivänsalo, M.J.; Lähde, S.T.; Alavaikko, M.J.; Lohela, P.K.; Typpö, A.B.T.; Suramo, I.J.I. Nonparasitic Splenic Cysts: Ultrasonographic Features and Follow-up. Acta Radiol. 1994, 35, 447–451. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Yunotani, S.; Edakuni, G.; Mori, M.; Iyama, A.; Miyazaki, K. Laparoscopic splenectomy for a giant splenic epidermoid cyst: Report of a case. Surg. Today 1999, 29, 1268–1272. [Google Scholar] [CrossRef]
- Yang, X.; Yu, J.; Liang, P.; Yu, X.; Cheng, Z.; Han, Z.; Liu, F. Ultrasound-guided percutaneous ethanol ablation for primary non-parasitic splenic cysts in 15 patients. Abdom. Radiol. 2016, 41, 538–544. [Google Scholar] [CrossRef]
- Targarona, E.M.; Martínez, J.; Ramos, C.; Becerra, J.A.; Trías, M. Conservative laparoscopic treatment of a posttraumatic splenic cyst. Surg. Endosc. 1995, 9, 71–72. [Google Scholar] [CrossRef]
- Farhangi, B.; Farhangi, A.; Firouzjahi, A.; Jahed, B. Huge epithelial nonparasitic splenic cyst: A case report and a review of treatment methods. Caspian J. Intern. Med. 2016, 7, 146–149. [Google Scholar]
- Morgenstern, L. Nonparasitic splenic cysts: Pathogenesis, classification, and treatment. J. Am. Coll. Surg. 2002, 194, 306–314. [Google Scholar] [CrossRef]
- Karfis, E.A.; Roustanis, E.; Tsimoyiannis, E.C. Surgical management of nonparasitic splenic cysts. JSLS 2009, 13, 207–212. [Google Scholar] [PubMed]
- Comitalo, J.B. Laparoscopic treatment of splenic cysts. JSLS 2001, 5, 313–316. [Google Scholar]
- Schier, F.; Waag, K.L.; Ure, B. Laparoscopic unroofing of splenic cysts results in a high rate of recurrences. J. Pediatr. Surg. 2007, 42, 1860–1863. [Google Scholar] [CrossRef] [PubMed]
- Nowak, B.; Fielding, G.A.; Pachter, H.L. Treatment of splenic cysts. Laparosc. Surg. 2021, 5, 11. [Google Scholar] [CrossRef]
- Liu, G.; Fan, Y. Feasibility and Safety of Laparoscopic Partial Splenectomy: A Systematic Review. World J. Surg. 2019, 43, 1505–1518. [Google Scholar] [CrossRef]
- Golmohammadzadeh, H.; Maddah, G.; Shams Hojjati, Y.; Abdollahi, A.; Shabahang, H. Splenic cysts: Analysis of 16 cases. Caspian J. Intern. Med. 2016, 7, 217–221. [Google Scholar]
- Al Khafaji, B.; Younis, M.U. Laparoscopic splenic cyst fenestration-a viable spleen preserving option. J. Surg. Case Rep. 2017, 2017, rjx154. [Google Scholar] [CrossRef]
- Fahel, E.; Amaral, P.C.; Filho, E.M.; Ettinger, J.E.; Souza, E.L.; Fortes, M.F.; Alcântara, R.S.; Regis, A.B.; Neto, M.P.; Sousa, M.M.; et al. Videolaparoscopic approach of the splenic cyst: A case report. JSLS 2000, 4, 23–26. [Google Scholar]
- Dan, D.; Bascombe, N.; Harnanan, D.; Hariharan, S.; Naraynsingh, V. Laparoscopic management of a massive splenic cyst. Asian J. Surg. 2010, 33, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, A.M.; Târcoveanu, E.; Ciuntu, B.; Fotea, V.; Georgescu, S.; Andreea, L.; Ciobanu, D.L.; Lupascu, C.; Bradea, C. Laparoscopic approach for nonparasitic splenic cysts and splenic abcesses. Ann. Ital. Chir. 2022, 93, 671–679. [Google Scholar] [PubMed]
- Geraghty, M.; Khan, I.Z.; Conlon, K.C. Large primary splenic cyst: A laparoscopic technique. J. Minim. Access Surg. 2009, 5, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Krichen, I.; Maazoun, K.; Kitar, M.; Kamal, N.M.; Khan, U.; Khalif, M.Y.; Rasha, A.; Assiri, H.; Bokari, K.A. Huge Non-parasitic Mesothelial Splenic Cyst in a Child: A Case Report and Literature Review. Clin. Med. Insights Pediatr. 2021, 15, 11795565211021597. [Google Scholar] [CrossRef]
- Gasparetto, A.; Alonso, J.; Temple, M.; Parra, D.; Chiramel, G.; Chand, R.; Amaral, J. Safety and Effectiveness of Sclerotherapy for Nonparasitic Splenic Cysts: A Systematic Review and Meta-Analysis. J. Vasc. Interv. Radiol. 2023, 34, 2110–2119.e1. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiutiuca, R.C.; Nastase Puscasu, A.I.; Stoenescu, N.; Moscalu, M.; Bradea, C.; Eva, I.; Lupascu, C.D.; Ivan, L.; Palaghia, M.M.; Prisecariu, D.I.; et al. Laparoscopic Approach to Primary Splenic Cyst: Case Report and Review of the Literature. Life 2024, 14, 120. https://doi.org/10.3390/life14010120
Tiutiuca RC, Nastase Puscasu AI, Stoenescu N, Moscalu M, Bradea C, Eva I, Lupascu CD, Ivan L, Palaghia MM, Prisecariu DI, et al. Laparoscopic Approach to Primary Splenic Cyst: Case Report and Review of the Literature. Life. 2024; 14(1):120. https://doi.org/10.3390/life14010120
Chicago/Turabian StyleTiutiuca, Razvan Calin, Alina Ioana Nastase Puscasu, Nicoleta Stoenescu, Mihaela Moscalu, Costel Bradea, Iuliana Eva, Cristian Dumitru Lupascu, Luminita Ivan, Madalina Maria Palaghia, Denisa Ioana Prisecariu, and et al. 2024. "Laparoscopic Approach to Primary Splenic Cyst: Case Report and Review of the Literature" Life 14, no. 1: 120. https://doi.org/10.3390/life14010120
APA StyleTiutiuca, R. C., Nastase Puscasu, A. I., Stoenescu, N., Moscalu, M., Bradea, C., Eva, I., Lupascu, C. D., Ivan, L., Palaghia, M. M., Prisecariu, D. I., Târcoveanu, E., Vâță, A., Bejan, V., & Vasilescu, A. M. (2024). Laparoscopic Approach to Primary Splenic Cyst: Case Report and Review of the Literature. Life, 14(1), 120. https://doi.org/10.3390/life14010120









