Impact of Blood-Count-Derived Inflammatory Markers in Psoriatic Disease Progression
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Biomarkers
2.4. Study Outcome
2.5. Statistical Analysis
3. Results
3.1. Patients’ Clinical Profile
3.2. Serological Markers and Disease Severity
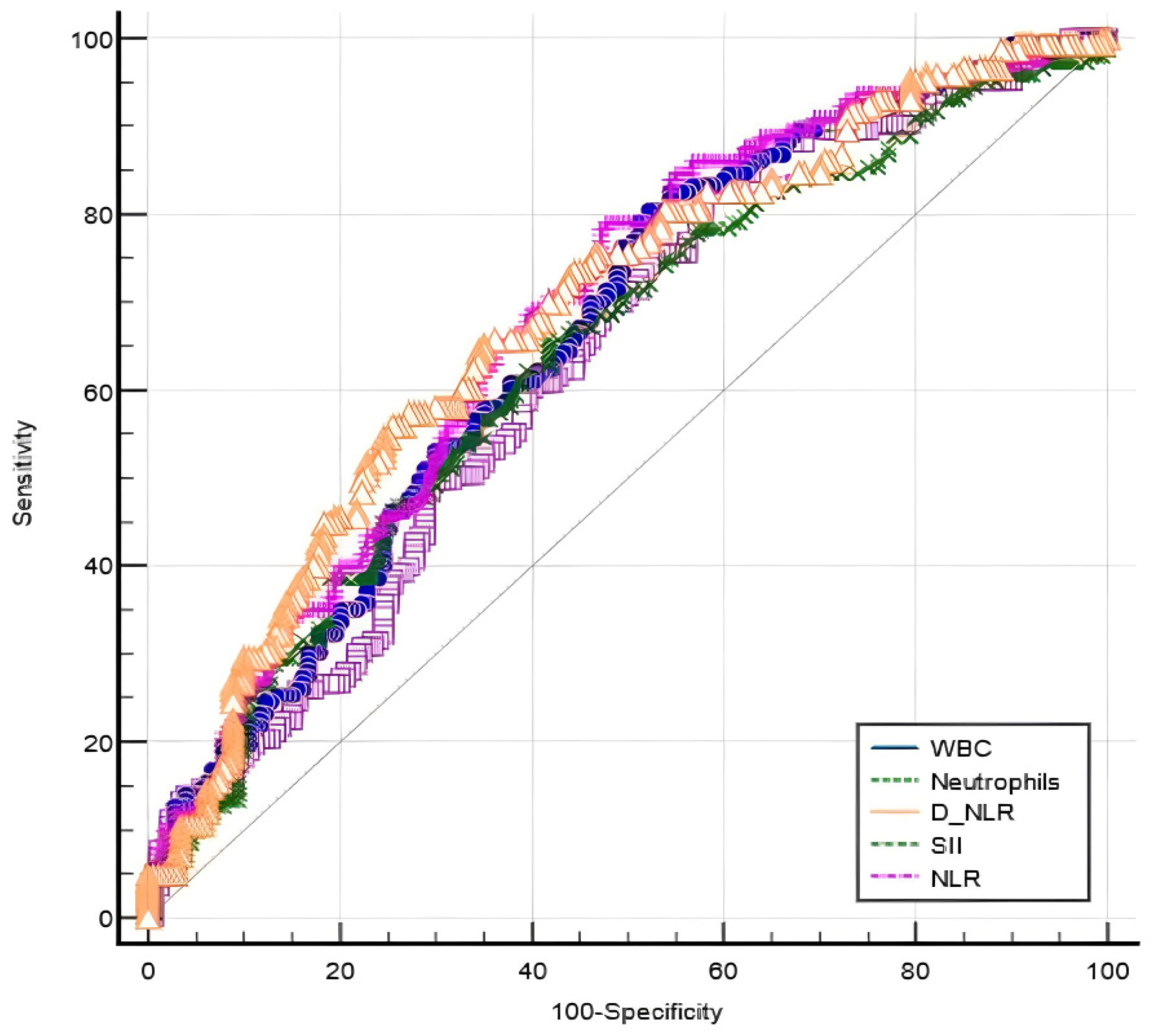
3.3. Performance of Blood-Count-Derived Inflammatory Markers in Evaluating Disease Severity
3.4. The Dependability of Blood-Count-Derived Inflammatory Markers for Predicting Disease Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scarpa, R.; Ayala, F.; Caporaso, N.; Olivieri, I. Psoriasis, psoriatic arthritis, or psoriatic disease? J. Rheumatol. 2006, 33, 210–212. [Google Scholar]
- Yamazaki, F. Psoriasis: Comorbidities. J. Dermatol. 2021, 48, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Tada, Y.; Hau, C.; Tatsuta, A.; Yamamoto, M.; Kamata, M.; Karakawa, M.; Asano, Y.; Mitsui, H.; Sugaya, M.; et al. Adiponectin as an anti-inflammatory factor in the pathogenesis of psoriasis: Induction of elevated serum adiponectin levels following therapy: Correspondence. Br. J. Dermatol. 2011, 164, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Li, D.; Xie, B.B.; Hu, L.H.; Huang, J.; Jia, X.X.; Tang, Y.L.; Liu, G.H.; Shen, N.N.; Yu, X.B. IL-17A and TNF-α inhibitors induce multiple molecular changes in psoriasis. Front. Immunol. 2022, 13, 1015182. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Mazzilli, S.; Di Prete, M.; Dattola, A.; Cosio, T.; Lettieri Barbato, D.; Costanza, G.; Lanna, C.; Manfreda, V.; Gaeta Schumak, R.; et al. The Role of Glutathione-S Transferase in Psoriasis and Associated Comorbidities and the Effect of Dimethyl Fumarate in This Pathway. Front. Med. 2022, 9, 760852. [Google Scholar] [CrossRef]
- Trevisan, G.; Stinco, G.; Giansante, C.; Fiotti, N.; Vidimari, P.; Kokelj, F. Psoriasis and endothelins. Acta Derm. Venereol. Suppl. 1994, 186, 139–140. [Google Scholar]
- Boehncke, W.H.; Boehncke, S.; Tobin, A.M.; Kirby, B. The ‘psoriatic march’: A concept of how severe psoriasis may drive cardiovascular comorbidity: The ‘psoriatic march’. Exp. Dermatol. 2011, 20, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Berth-Jones, J.; Grotzinger, K.; Rainville, C.; Pham, B.; Huang, J.; Daly, S.; Herdman, M.; Firth, P.; Hotchkiss, K. A study examining inter- and intrarater reliability of three scales for measuring severity of psoriasis: Psoriasis Area and Severity Index, Physician’s Global Assessment and Lattice System Physician’s Global Assessment: Validation of PASI, PGA and LS-PGA. Br. J. Dermatol. 2006, 155, 707–713. [Google Scholar] [CrossRef]
- Henseler, T.; Schmitt-Rau, K. A comparison between BSA, PASI, PLASI and SAPASI as measures of disease severity and improvement by therapy in patients with psoriasis. Int. J. Dermatol. 2008, 47, 1019–1023. [Google Scholar] [CrossRef]
- Cancelliere, R.; Cosio, T.; Campione, E.; Corvino, M.; D’Amico, M.P.; Micheli, L.; Signori, E.; Contini, G. Label-free electrochemical immunosensor as a reliable point-of-care device for the detection of Interleukin-6 in serum samples from patients with psoriasis. Front. Chem. 2023, 11, 1251360. [Google Scholar] [CrossRef]
- Gambichler, T.; Hessam, S.; Cramer, P.; Abu Rached, N.; Bechara, F.G. Complete blood collection-based systemic inflammation biomarkers for patients with hidradenitis suppurativa. Acad Dermatol. Venereol. 2022, 36, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Öksüm Solak, E.; Baran Ketencioglu, B.; Cinar, S.L.; Kartal, D.; Borlu, M. The role of new inflammatory markers in determining disease activation and severity in patients with hidradenitis suppurativa. Int. J. Dermatol. 2023, 62, 1076–1081. [Google Scholar] [CrossRef]
- Bakic, M.; Klisic, A.; Karanikolic, V. Comparative Study of Hematological Parameters and Biomarkers of Immunity and Inflammation in Patients with Psoriasis and Atopic Dermatitis. Medicina 2023, 59, 1622. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, X.; Qian, H.; Liang, G.; Xiang, R.; Zhao, C.; Li, Z.; Li, S.; Jing, K.; Wang, Y.; et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are positively correlated with disease activity of bullous pemphigoid. Arch. Dermatol. Res. 2023, 315, 2383–2391. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.D.; Lim, J.; McSorley, S.T.; Horgan, P.G.; McMillan, D.C. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: Systematic review and meta-analysis. Sci. Rep. 2017, 7, 16717. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.-Q.; Deng, Q.-W.; He, B.-S.; Pan, Y.-Q.; Wang, F.; Sun, H.-L.; Chen, J.; Liu, X.; Wang, S.-K. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med. Oncol. 2014, 31, 305. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Fois, A.G.; Sotgia, S.; Mangoni, A.A.; Zinellu, E.; Pirina, P.; Negri, S.; Carru, C.; Zinellu, A. Neutrophil to lymphocyte ratio and clinical outcomes in COPD: Recent evidence and future perspectives. Eur. Respir. Rev. 2018, 27, 170113. [Google Scholar] [CrossRef]
- Mertoglu, C.; Gunay, M. Neutrophil-Lymphocyte ratio and Platelet-Lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S127–S131. [Google Scholar] [CrossRef]
- Zinellu, A.; Collu, C.; Nasser, M.; Paliogiannis, P.; Mellino, S.; Zinellu, E.; Traclet, J.; Ahmad, K.; Mangoni, A.A.; Carru, C.; et al. The Aggregate Index of Systemic Inflammation (AISI): A Novel Prognostic Biomarker in Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2021, 10, 4134. [Google Scholar] [CrossRef]
- Xiu, J.; Lin, X.; Chen, Q.; Yu, P.; Lu, J.; Yang, Y.; Chen, W.; Bao, K.; Wang, J.; Zhu, J.; et al. The aggregate index of systemic inflammation (AISI): A novel predictor for hypertension. Front. Cardiovasc. Med. 2023, 10, 1163900. [Google Scholar] [CrossRef]
- Krenn-Pilko, S.; Langsenlehner, U.; Stojakovic, T.; Pichler, M.; Gerger, A.; Kapp, K.S.; Langsenlehner, T. The elevated preoperative derived neutrophil-to-lymphocyte ratio predicts poor clinical outcome in breast cancer patients. Tumor Biol. 2016, 37, 361–368. [Google Scholar] [CrossRef]
- Proctor, M.J.; McMillan, D.C.; Morrison, D.S.; Fletcher, C.D.; Horgan, P.G.; Clarke, S.J. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br. J. Cancer 2012, 107, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.W.; O’Callaghan, C.J.; Hu, N.; Ding, K.; A Yothers, G.; Catalano, P.J.; Shi, Q.; Gray, R.G.; O’Connell, M.J.; ACCENT Collaborative Group; et al. Innovative estimation of survival using log-normal survival modelling on ACCENT database. Br. J. Cancer 2013, 108, 784–790. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Wang, M.; Gu, C.; Liu, J. Platelet-to-Monocyte Ratio as a Novel Promising Agent for the Prognosis of Hepatitis B Virus-Associated Decompensated Cirrhosis. Can. J. Gastroenterol. Hepatol. 2023, 2023, 6646156. [Google Scholar] [CrossRef] [PubMed]
- Alfhili, M.A.; Alotaibi, G.A.; Alfaifi, M.; Almoghrabi, Y.; Alsughayyir, J. Association of Platelet-Monocyte Ratio with Dyslipidemia in Saudi Arabia: A Large, Population-Based Study. Life 2023, 13, 1685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, Y.; Shen, H.; Shou, L.; Fang, Q.; Zheng, X.; Zhu, M.; Huang, X.; Huang, J.; Li, L.; et al. The value of a new prognostic model developed by lymphocyte-monocyte ratio and platelet-monocyte ratio in peripheral T-cell lymphoma. Cancer Cell Int. 2021, 21, 573. [Google Scholar] [CrossRef]
- Feng, F.; Tian, Y.; Liu, S.; Zheng, G.; Liu, Z.; Xu, G.; Guo, M.; Lian, X.; Fan, D.; Zhang, H. Combination of PLR, MLR, MWR, and Tumor Size Could Significantly Increase the Prognostic Value for Gastrointestinal Stromal Tumors. Medicine 2016, 95, e3248. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, F.; Shafique, K.; Mirza, S.; Ayoob, Z.; Vart, P.; Rao, S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int. Arch. Med. 2012, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Satta, R.; Deligia, G.; Farina, G.; Bassu, S.; Mangoni, A.A.; Carru, C.; Zinellu, A. Associations between the neutrophil-to-lymphocyte and the platelet-to-lymphocyte ratios and the presence and severity of psoriasis: A systematic review and meta-analysis. Clin. Exp. Med. 2019, 19, 37–45. [Google Scholar] [CrossRef]
- Kim, D.S.; Shin, D.; Lee, M.S.; Kim, H.J.; Kim, D.Y.; Kim, S.M.; Lee, M.G. Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J. Dermatol. 2016, 43, 305–310. [Google Scholar] [CrossRef]
- Adamstein, N.H.; MacFadyen, J.G.; Rose, L.M.; Glynn, R.J.; Dey, A.K.; Libby, P.; Tabas, I.A.; Mehta, N.N.; Ridker, P.M. The neutrophil–lymphocyte ratio and incident atherosclerotic events: Analyses from five contemporary randomized trials. Eur. Heart J. 2021, 42, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Kvist-Hansen, A.; Kaiser, H.; Wang, X.; Krakauer, M.; Gørtz, P.M.; McCauley, B.D.; Zachariae, C.; Becker, C.; Hansen, P.R.; Skov, L. Neutrophil Pathways of Inflammation Characterize the Blood Transcriptomic Signature of Patients with Psoriasis and Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 10818. [Google Scholar] [CrossRef] [PubMed]
- Tiucă, O.M.; Morariu, S.H.; Mariean, C.R.; Tiucă, R.A.; Nicolescu, A.C.; Cotoi, O.S. Predictive Performances of Blood-Count-Derived Inflammatory Markers for Liver Fibrosis Severity in Psoriasis Vulgaris. Int. J. Mol. Sci. 2023, 24, 16898. [Google Scholar] [CrossRef] [PubMed]
- Asahina, A.; Kubo, N.; Umezawa, Y.; Honda, H.; Yanaba, K.; Nakagawa, H. Neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and mean platelet volume in Japanese patients with psoriasis and psoriatic arthritis: Response to therapy with biologics. J. Dermatol. 2017, 44, 1112–1121. [Google Scholar] [CrossRef]
- Chiang, C.C.; Cheng, W.J.; Korinek, M.; Lin, C.Y.; Hwang, T.L. Neutrophils in Psoriasis. Front. Immunol. 2019, 10, 2376. [Google Scholar] [CrossRef] [PubMed]
- Schonthaler, H.B.; Guinea-Viniegra, J.; Wculek, S.K.; Ruppen, I.; Ximénez-Embún, P.; Guío-Carrión, A.; Navarro, R.; Hogg, N.; Ashman, K.; Wagner, E.F. S100A8-S100A9 Protein Complex Mediates Psoriasis by Regulating the Expression of Complement Factor C3. Immunity 2013, 39, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Cao, T.; Jin, L.; Li, B.; Fang, H.; Zhang, J.; Zhang, Y.; Hu, J.; Wang, G. Increased Lipocalin-2 Contributes to the Pathogenesis of Psoriasis by Modulating Neutrophil Chemotaxis and Cytokine Secretion. J. Investig. Dermatol. 2016, 136, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Yazici, C.; Köse, K.; Utaş, S.; Tanrikulu, E.; Taşlidere, N. A novel approach in psoriasis: First usage of known protein oxidation markers to prove oxidative stress. Arch. Dermatol. Res. 2016, 308, 207–212. [Google Scholar] [CrossRef]
- Bakic, M.; Klisic, A.; Kocic, G.; Kocic, H.; Karanikolic, V. Oxidative stress and metabolic biomarkers in patients with psoriasis. J. Med. Biochem. 2023, 42, 1–9. [Google Scholar]
- Klisic, A.; Bakic, M.; Karanikolic, V. Comparative Analysis of Redox Homeostasis Biomarkers in Patients with Psoriasis and Atopic Dermatitis. Antioxidants 2023, 12, 1875. [Google Scholar] [CrossRef]
- Yamanaka, K.; Yamamoto, O.; Honda, T. Pathophysiology of psoriasis: A review. J. Dermatol. 2021, 48, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Fang, H.; Dang, E.; Xue, K.; Zhang, J.; Li, B.; Qiao, H.; Cao, T.; Zhuang, Y.; Shen, S.; et al. Neutrophil Extracellular Traps Promote Inflammatory Responses in Psoriasis via Activating Epidermal TLR4/IL-36R Crosstalk. Front. Immunol. 2019, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Pereira, P.; Santos-Silva, A.; Rebelo, I.; Figueiredo, A.; Quintanilha, A.; Teixeira, F. The inflammatory response in mild and in severe psoriasis. Br. J. Dermatol. 2004, 150, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M. Platelets in inflammation and atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.; Rocha, B.D.O.; Paixão, C.S.; Oliveira, M.D.F.S.P.D.; Mota, L.M.H.D.; Carvalho, L.P.D. Monocyte subpopulations study in patients with plaque psoriasis. Med. Hypotheses 2017, 104, 101–103. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
| Marker | Formula |
|---|---|
| NLR | Neutrophil count/lymphocyte count [×103/μL] |
| MLR | Monocyte count/lymphocyte count [×103/μL] |
| d-NLR | Neutrophil count/(WBC − neutrophil count) [×103/μL] |
| PLR | Platelet count/lymphocyte count [×103/μL] |
| PMR | Platelet count/monocyte count [×103/μL] |
| SII | (Neutrophil count × platelet count)/lymphocyte count [×103/μL] |
| SIRI | (Neutrophil count × monocyte count)/lymphocyte count [×103/μL] |
| AISI | (Neutrophil count × monocyte count × platelet count)/lymphocyte count [×103/μL] |
| Variables * | All Patients | Mild Disease (n = 180) | Moderate Disease (n = 143) | Severe Disease (n = 43) | p-Value |
|---|---|---|---|---|---|
| Age | 54.48 ± 16.48 | 53.86 ± 17.46 | 57.51 ± 12.89 | 54.35 ± 16.18 | 0.43 |
| Gender | |||||
| Male | 219 | 101 (56%) | 91 (64%) | 27 (63%) | 0.36 |
| Female | 147 | 79 (44%) | 52 (36%) | 16 (37%) | |
| WBC | 7.49 [7.15–7.83] | 6.77 [6.38–7.26] | 8.08 [7.60–8.73] | 7.98 [7.50–8.42] | <0.001 |
| Platelets | 238.25 [230.93–243.69] | 231.70 [220-.95–240.22] | 243.20 [221.87–265.52] | 245 [236.51–259.56] | 0.11 |
| Neutrophils | 4.42 [4.22–4.69] | 3.80 [3.44–4.25] | 4.68 [4.39–5.26] | 4.96 [4.57–5.45] | <0.001 |
| Lymphocytes | 2.10 [1.97–2.23] | 2.22 [1.99–2.30] | 2.16 [1.91–2.34] | 1.97 [1.79–2.20] | 0.12 |
| Monocytes | 0.51 [0.48–0.53] | 0.49 [0.45–0.52] | 0.52 [0.47–0.56] | 0.53 [0.48–0.57] | 0.59 |
| PLR | 114.96 [110.45–121.10] | 108.07 [100.89–115.10] | 110.75 [96.66–126.26] | 129.79 [117.02–135.15] | 0.002 |
| NLR | 2.05 [1.90–2.19] | 1.73 [1.59–1.88] | 2.23 [1.93–2.66] | 2.53 [2.20–2.79] | <0.001 |
| d-NLR | 1.55 [1.44–1.65] | 1.37 [1.29–1.49] | 1.45 [1.36–1.82] | 1.80 [1.62–1.95] | 0.001 |
| MLR | 0.23 [0.22–0.25] | 0.22 [0.21–0.25] | 0.25 [0.23–0.28] | 0.24 [0.22–0.26] | 0.04 |
| PMR | 475.02 [450.39–508.40] | 479.08 [440.36–526.90] | 467.59 [400–539.75] | 476.42 [437.62–524.45] | 0.75 |
| SII | 478.52 [453.65–521.85] | 404.61 [357.44–446.03] | 541.21 [464.89–602.06] | 560.46 [518.95–646.30] | <0.001 |
| SIRI | 1.03 [0.93–1.09] | 0.86 [0.79–0.92] | 1.23 [1.01–1.44] | 1.27 [1.07–1.37] | 0.001 |
| AISI | 255.83 [229–273.18] | 196.96 [168.52–221.22] | 287.76 [258.18–331.08] | 293.10 [272.01–335.63] | 0.001 |
| ESR | 15 [12.8–17] | 12.80 [11.80–15.80] | 16.20 [12.20–20.98] | 18 [14–20] | 0.17 |
| Parameter * | Mild Psoriasis | Moderate Psoriasis | Severe Psoriasis | |||
|---|---|---|---|---|---|---|
| vs. Moderate Psoriasis | vs. Severe Psoriasis | vs. Mild Psoriasis | vs. Severe Psoriasis | vs. Mild Psoriasis | vs. Moderate Psoriasis | |
| WBC | 0.004 | 0.32 | 0.004 | 0.06 | 0.32 | 0.06 |
| Neutrophils | 0.003 | 1 | 0.003 | 0.01 | 1 | 0.01 |
| PLR | 0.07 | 0.32 | 0.07 | 1 | 0.32 | 1 |
| NLR | 0.01 | 0.96 | 0.01 | 0.11 | 0.86 | 0.11 |
| d-NLR | 0.02 | 1 | 0.02 | 0.007 | 1 | 0.007 |
| MLR | 1 | 1 | 1 | 1 | 1 | 1 |
| SII | 0.009 | 0.34 | 0.009 | 0.12 | 0.34 | 0.12 |
| SIRI | 0.18 | 1 | 0.18 | 0.26 | 1 | 0.26 |
| AISI | 0.06 | 1 | 0.06 | 0.37 | 1 | 0.37 |
| Marker * | r | p-Value | Marker | r | v-Value |
|---|---|---|---|---|---|
| WBC | 0.25 | <0.001 | MLR | 0.13 | 0.01 |
| Neutrophil count | 0.26 | <0.001 | SII | 0.29 | <0.001 |
| PLR | 0.14 | 0.005 | SIRI | 0.28 | <0.001 |
| NLR | 0.30 | <0.001 | AISI | 0.28 | <0.001 |
| d-NLR | 0.18 | <0.001 | ESR | 0.15 | <0.001 |
| Moderate vs. Mild Disease | |||||||
|---|---|---|---|---|---|---|---|
| Parameter * | AUC (95% CI) | p-Value | Cut-Off | Se (%) | Sp (%) | Youden Index J | p-Value * |
| WBC | 0.637 [0.582–0.689] | <0.001 | 6.25 | 82.52 | 42.22 | 0.25 | 0.11 |
| Neutrophil count | 0.662 [0.607–0.713] | <0.001 | 3.64 | 80.42 | 47.78 | 0.28 | 0.28 |
| NLR | 0.687 [0.633–0.737] | <0.001 | 2.35 | 55.94 | 74.44 | 0.30 | - |
| d-NLR | 0.640 [0.585–0.692] | <0.001 | 1.49 | 65.73 | 58.33 | 0.24 | <0.001 |
| SII | 0.683 [0.629–0.733] | <0.001 | 408.8 | 79.02 | 52.22 | 0.31 | 0.81 |
| Severe vs. moderate disease | |||||||
| Parameter * | AUC (95% CI) | p-value | Cut-Off | Se (%) | Sp (%) | Youden Index J | p-value |
| Neutrophil count | 0.527 [0.453–0.601] | 0.55 | 5.66 | 76.74 | 38.46 | 0.15 | - |
| d-NLR | 0.598 [0.524–0.669] | 0.03 | 2.18 | 90.70 | 31.47 | 0.22 | - |
| Parameter * | OR | 95% CI | p-Value |
|---|---|---|---|
| Mild psoriasis | |||
| d-NLR | 6.15 | 2.53–14.91 | <0.001 |
| NLR | 0.24 | 0.12–0.48 | <0.001 |
| SII | 0.99 | 0.99–1.02 | 0.043 |
| Moderate psoriasis | |||
| d-NLR | 0.16 | 0.07–0.39 | <0.001 |
| NLR | 4.13 | 2.11–8.11 | <0.001 |
| SII | 1 | 1–1.03 | 0.046 |
| Severe psoriasis | |||
| d-NLR | 0.69 | 0.47–1.01 | 0.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiucă, O.M.; Morariu, S.H.; Mariean, C.R.; Tiucă, R.A.; Nicolescu, A.C.; Cotoi, O.S. Impact of Blood-Count-Derived Inflammatory Markers in Psoriatic Disease Progression. Life 2024, 14, 114. https://doi.org/10.3390/life14010114
Tiucă OM, Morariu SH, Mariean CR, Tiucă RA, Nicolescu AC, Cotoi OS. Impact of Blood-Count-Derived Inflammatory Markers in Psoriatic Disease Progression. Life. 2024; 14(1):114. https://doi.org/10.3390/life14010114
Chicago/Turabian StyleTiucă, Oana Mirela, Silviu Horia Morariu, Claudia Raluca Mariean, Robert Aurelian Tiucă, Alin Codrut Nicolescu, and Ovidiu Simion Cotoi. 2024. "Impact of Blood-Count-Derived Inflammatory Markers in Psoriatic Disease Progression" Life 14, no. 1: 114. https://doi.org/10.3390/life14010114
APA StyleTiucă, O. M., Morariu, S. H., Mariean, C. R., Tiucă, R. A., Nicolescu, A. C., & Cotoi, O. S. (2024). Impact of Blood-Count-Derived Inflammatory Markers in Psoriatic Disease Progression. Life, 14(1), 114. https://doi.org/10.3390/life14010114






