Evaluation of Salvia hispanica as a Therapeutic Agent against Sodium Arsenic-Induced Testicular Toxicity in a Male Rats Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- The chia seeds, scientifically known as Salvia hispanica, were obtained from the Local Company for Herbs and Medicinal Plants located in Cairo Governorate, Egypt.
- The powder form of sodium arsenite (NaAsO2) was acquired from Al-Gomhoria Company for Chemicals, located in Cairo, Egypt.
- The casein, which contains 85% protein as well as choline chloride, DL-methionine, vitamins, and a salt mixture, was procured from El-Fagr Company, located in Cairo, Egypt. Sunflower oil and maize starch were sourced from the local market in Tanta, Egypt.
- ▪
- A basal diet is a diet that provides the necessary amount of calories to produce basal heat as well as sufficient critical elements to fulfill fundamental requirements. Basal diet studies use a methodology in which the effects of a certain ingredient, which has yet to be identified, are first excluded from the diet and then introduced.
- ▪
- A standard diet refers to a typical diet that does not impose any dietary limitations. An ideal diet should include the whole spectrum of attributes associated with good health, namely: balance, nutritional adequacy, moderation in food consumption, diversity in food choices, and careful calorie management.
- ▪
- A baseline diet is essential for all animals before considering any supplements or unconventional dietary approaches.
- A total of 36 male albino rats of the “Sprague Dawley” strain, with an average weight of 200 ± 10 g, were procured from the experimental animal colony maintained by the Ministry of Health and Population in Helwan, Cairo, Egypt.
2.2. Methods
- (a)
- The chia (Salvia hispanica) seeds were subjected to a washing process using tap water in order to eliminate any extraneous matter. The chia seeds were measured using a laboratory electronic analytical balance (Explorer Pro, Swiss Company, Bern, Switzerland). The chia seeds were processed and placed in low-density polyethylene (LDPE) plastic bags with a wall thickness ranging from 30 to 60 microns. The chia seeds were dehydrated in a WTB binder Model 78,532 oven from Germany at a temperature of 63 °C for a duration of 30 min, using the low-temperature long time (LTLT) method. The drying trays were filled with chia seeds to a depth of 25 mm and then put in the oven at a room temperature of 26 °C. The duration required to obtain the treatment temperature of 63 °C was 11 min. The dried chia seeds were pulverized using a laboratory blender (Waring Commercial, manufactured in the New York, NY, United States), then filtered through a 0.2 mm screen and sealed in a vacuum-packed container. The powder was stored at a temperature of 5 °C until it was ready for examination [24]. The chia seed was milled using a grinder and measured according to three groups of particle size ranges: 200−600 μm (grinding time of 10 s), 100−400 μm (grinding time of 20 s) and 0−200 μm (grinding time of 30 s), respectively. Those ranges were selected based on the largest size of ground seed that was measured between 0 and 1000 μm and made up more than 60% of the sample [25].
- (b)
- The chemical composition of Salvia hispanica, including moisture, ash, crude protein, and fat content, was analyzed using the methodology outlined in the [26] guidelines. The total amount of carbs was determined using the method of difference, as outlined below:Carbohydrates% = 100% − the percentages of (moisture + protein + fat + ash)
- (c)
- The determination of amino acids in chia seeds involves the application of ion exchange liquid chromatography, which is a widely used technology for qualitative and quantitative compositional analysis in various fields. The fundamental concept underlying the biochrom systems has been further developed to yield fully automated, rapid, and very sensitive results. The method described by [27] is often known as classical amino acid analysis. The determination of antioxidant activity was conducted using the protocols outlined by [28].
- (d)
- The analyses of vitamins C and E were conducted using high-performance liquid chromatography (HPLC) based on the modified method developed by [29]. The detection of chromatographic studies for vitamin C was performed using an Agilent HPLC system (2000 ECOM, Chrastany u Prahy, CZ 252 19, Czech) with UV detection at a wavelength of 254 nm. In the study conducted by [30], the mobile phase used for the analysis was an analytical column YMC-Triart C18 with dimensions of 150 × 4.6 mm. The mobile phase composition was A/B 33/67, where A consisted of a mixture of 0.1 M potassium acetate and distilled water in a ratio of 50:50 with a pH of 4.9. The flow rate was set at 1 mL/min, and the analysis was performed at the ambient temperature.
- (e)
- The quantification of the total phenolic contents of Salvia hispanica was performed through the utilization of high-performance liquid chromatography (HPLC) employing an Agilent 1260 series instrument. The separation procedure was conducted utilizing an Eclipse C18 column with dimensions of 4.6 mm × 250 mm internal diameter and a particle size of 5 μm. The mobile phase was composed of water (A) and a solution of 0.05% trifluoroacetic acid in acetonitrile (B) at a flow rate of 0.9 mL/min. The mobile phase was sequentially programmed in a linear gradient according to the following protocol: In the first minute, the performance was graded at 82% A. From minutes 0 to 5, the performance maintained a grade of 80% A. During the time interval from 5 to 8 min, the performance received a grade of 60% A. Similarly, from 8 to 12 min and 12 to 15 min, the performance maintained a grade of 60% A. However, in the time span from 15 to 16 min, the performance improved and received a grade of 82% A. Finally, from 16 to 20 min, the performance continued to receive a grade of 82% A. The detector operating at several wavelengths was observed at a wavelength of 280 nm. Each of the sample solutions was injected with a volume of 5 μL. The temperature of the column was maintained at 40 °C [31].
2.3. Experimental Design
2.4. The Assessment of Biological Parameters
2.5. Biochemical Analysis
2.6. Antioxidant Enzymes and Malondialdehyde in Testes Tissue
2.7. Statistical Analysis
3. Result and Discussion
3.1. The Phenolic Compounds of Salvia hispanica Seeds Powder (Chia)
3.2. The Effects of Salvia hispanica Seeds Powder on the Weight of the Prostate and Testes in Male Rats with Induced Testicular Damage from Sodium Arsenite
3.3. The Impact of Salvia hispanica Seeds on Feed Intake (FI), Body Weight Gain Percentage (BWG%), and Feed Efficiency Ratio (FER) in Male Rats with Experimentally Induced Testicular Toxicity Caused by NaAsO2
3.4. The Effect of Salvia hispanica Seeds Powder on Sperm Parameters in Rats Suffering from NaAsO2-Induced Testicular Damage
3.5. The Impact of Salvia hispanica Seeds Powder on Testicular Hormone Levels in Male Rats with Experimentally Produced Testicular Damage Caused by NaAsO2
3.6. Effects of Salvia hispanica Seeds Powder on Thyroid Hormone Levels in Male Rats with NaAsO2-Induced Testicular Damage
3.7. The Effects of Salvia hispanica Seeds Powder on Antioxidant Enzymes and Malondialdehyde Levels in Male Rats with Experimentally Induced Testicular Damage from NaAsO2
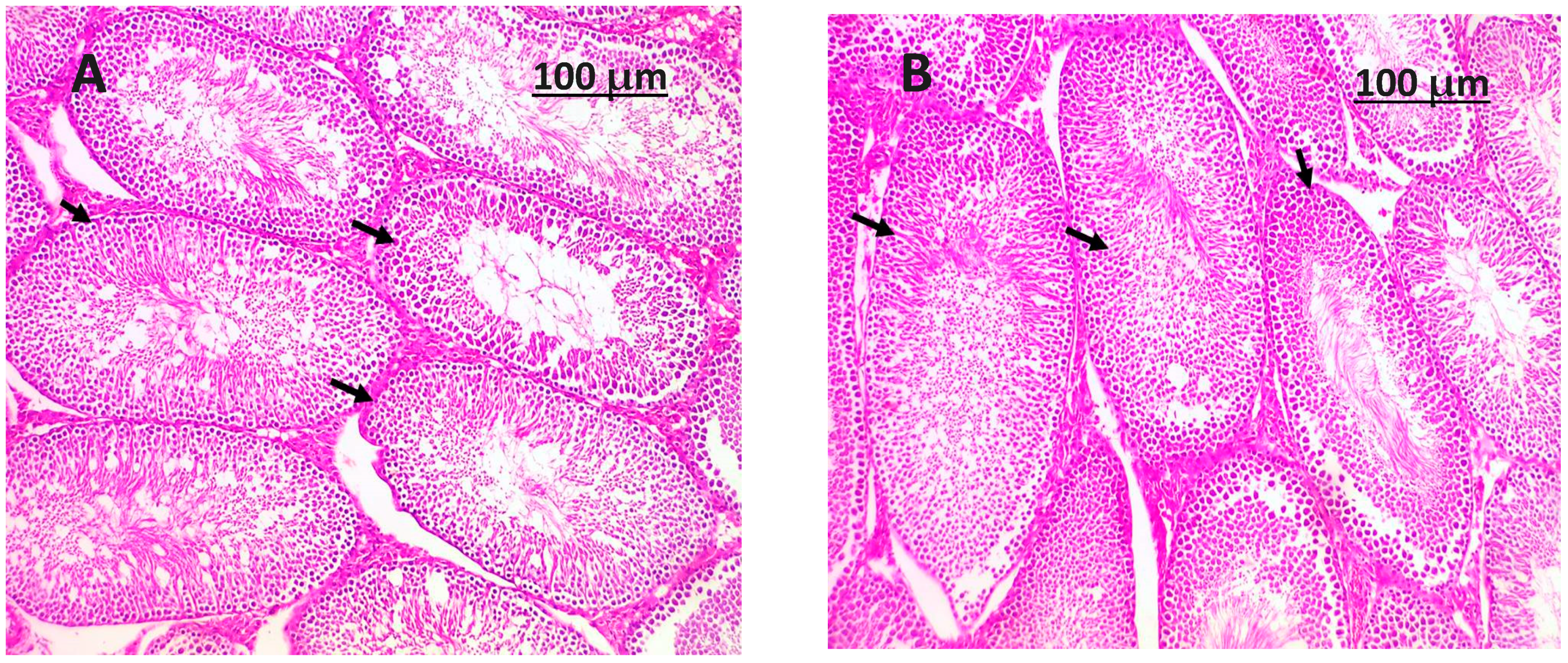
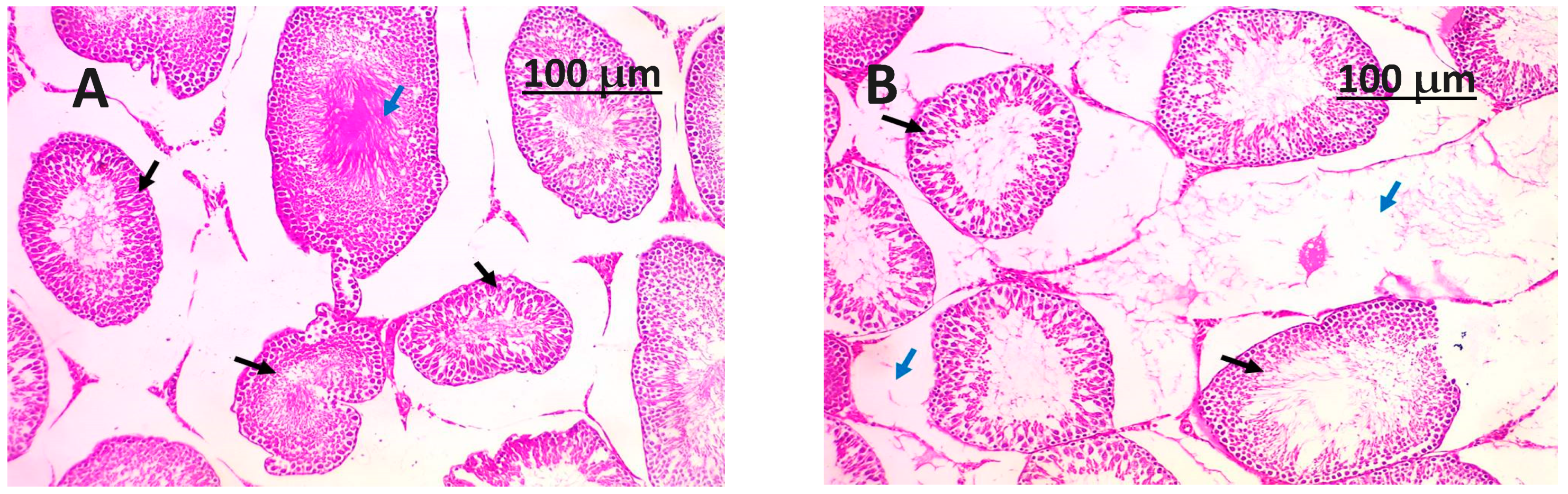
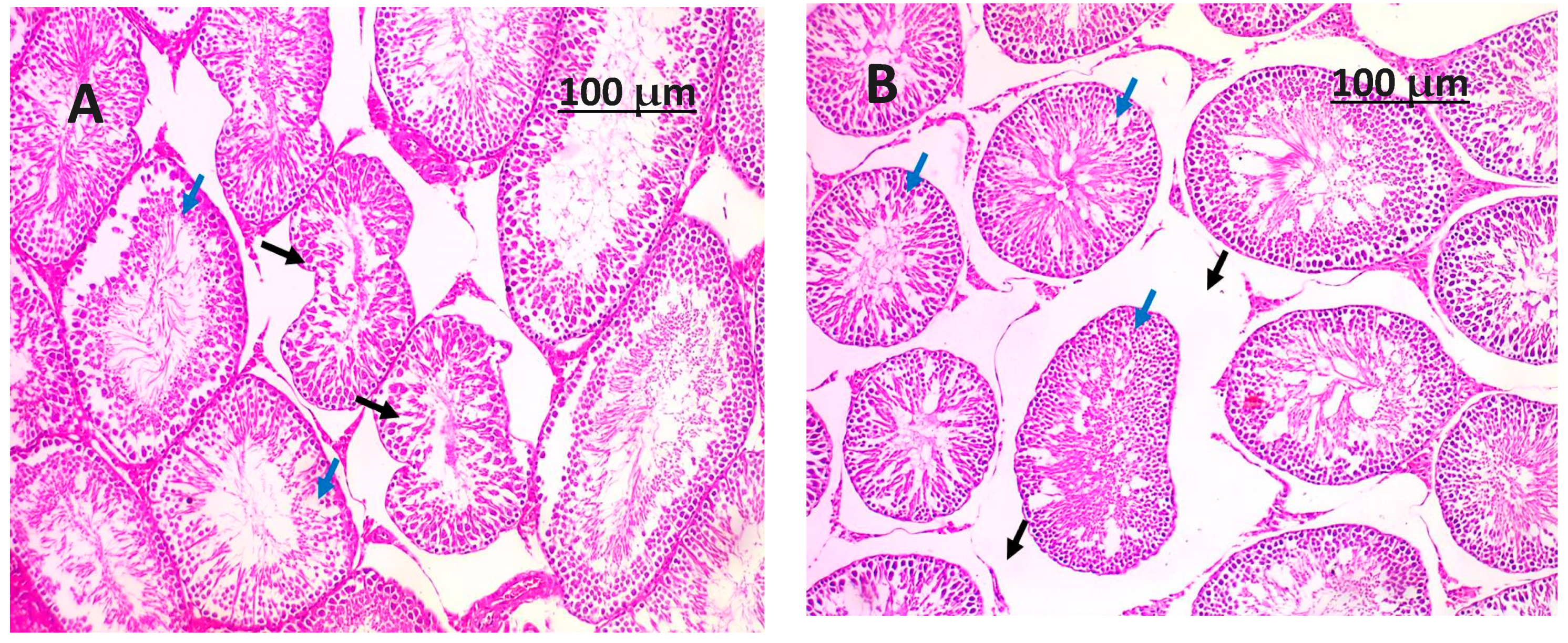
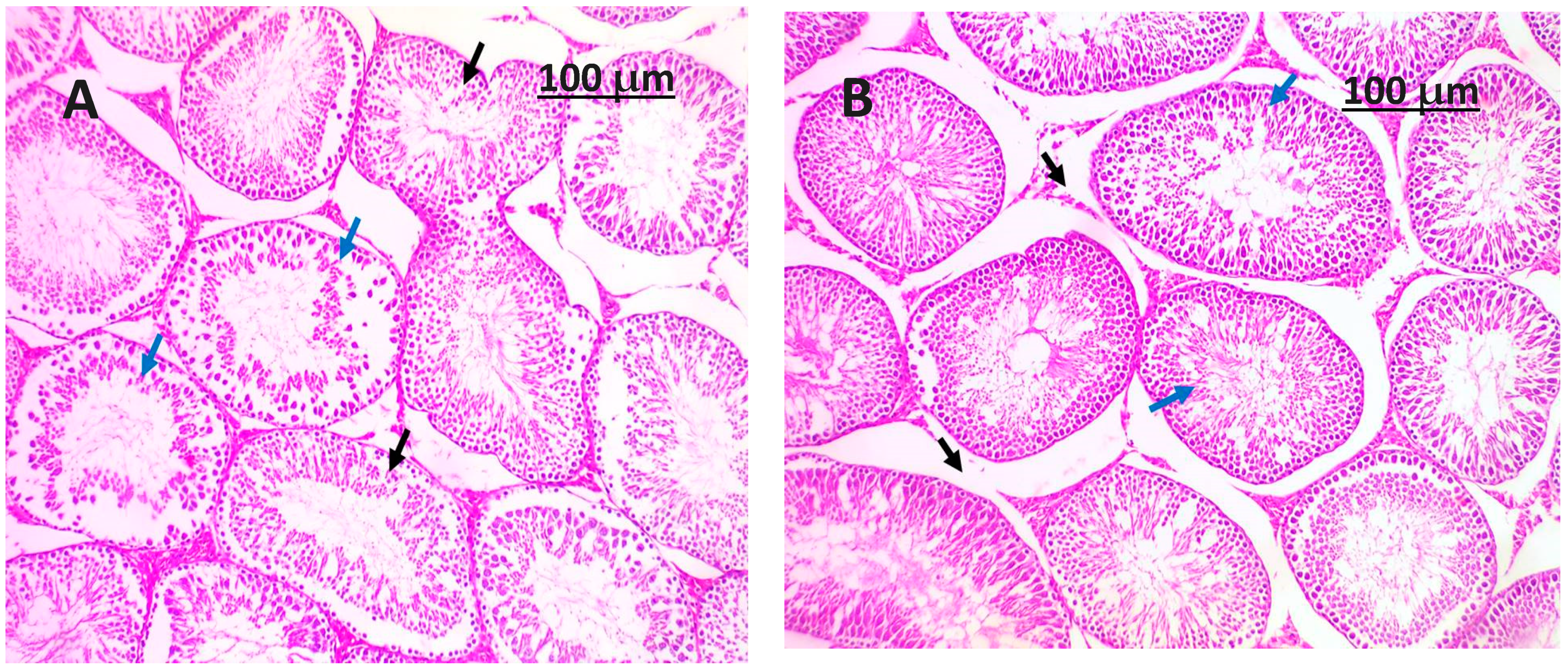
3.8. Histopathology Examination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alias, C.; Benassi, L.; Bertazzi, L.; Sorlini, S.; Volta, M.; Gelatti, U. Environmental exposure and health effects in a highly polluted area of Northern Italy: A narrative review. Environ. Sci. Pollut. Res. 2019, 26, 4555–4569. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.S.; Pacheco, T.L.; Guerra, M.T.; Barros, A.L.; Silva, P.V.; Missassi, G.; Kempinas, W.D.G. Reproductive disorders in female rats after prenatal exposure to betamethasone. J. Appl. Toxicol. 2017, 37, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Khan, R.; Khan, A.; Qamar, W.; Arafah, A.; Ahmad, A.; Ahmad, P. Fate of arsenic in living systems: Implications for sustainable and safe food chains. J. Hazard. Mater. 2021, 417, 126050. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Ahmad, M.; Saleemi, M.K.; Gul, S.T.; Ahmad, M.; Martyniuk, C.J.; Umar, S. Sodium arsenite toxicity on hematology indices and reproductive parameters in Teddy goat bucks and their amelioration with vitamin C. Environ. Sci. Pollut. Res. 2020, 27, 15223–15232. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.F.; Marchesi, S.C.; Ferraz, R.P.; Lima, G.D.D.A.; Oliveira, J.A.D.; Machado-Neves, M. Effects of sodium arsenate and arsenite on male reproductive functions in Wistar rats. J. Toxicol. Environ. Health Part A 2016, 79, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.F.; Bastos, D.S.S.; Sertorio, M.N.; Santos, F.C.; Ervilha, L.O.G.; de Oliveira, L.L.; Machado-Neves, M. Combined effects of arsenic exposure and diabetes on male reproductive functions. Andrology 2019, 7, 730–740. [Google Scholar] [CrossRef]
- Souza, A.C.F.; Ervilha, L.O.G.; Coimbra, J.L.P.; Bastos, D.S.S.; Guimarães, S.E.F.; Machado-Neves, M. Reproductive disorders in female rats after prenatal exposure to sodium arsenite. J. Appl. Toxicol. 2020, 40, 214–223. [Google Scholar] [CrossRef]
- Couto-Santos, F.; de Azevedo Viana, A.G.; Souza, A.C.F.; de Assis Dutra, A.A.; de Oliveira Mendes, T.A.; da Silva Ferreira, A.T.; Machado-Neves, M. Prepubertal arsenic exposure alters phosphoproteins profile, quality, and fertility of epididymal spermatozoa in sexually mature rats. Toxicology 2021, 460, 152886. [Google Scholar] [CrossRef]
- Zubair, M.; Ahmad, M.; Jamil, H.; Deeba, F. Toxic effects of arsenic on semen and hormonal profile and their amelioration with vitamin E in Teddy goat bucks. Andrologia 2016, 48, 1220–1228. [Google Scholar] [CrossRef]
- Deb, B.; Maity, M.; Maiti, S.; Pan, B.; Perveen, H.; Dash, M.; Chattopadhyay, S. Abrogation of sodium arsenite driven uterine antioxidant exhaustion and tissue impairment: Role of B and folate. J. Environ. Biol. 2018, 39, 581–591. [Google Scholar] [CrossRef]
- Rana, T.; Bera, A.K.; Bhattacharya, D.; Das, S.; Das, S.K. Evaluation of hemato-biochemical indices and oxidative stress in goats: A potential risk towards arsenic toxicity in contaminated zone of West Bengal, India. Comp. Clin. Pathol. 2021, 30, 277–284. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A. Goji berry (Lycium barbarum): Composition and health effects—A review. Pol. J. Food Nutr. Sci. 2016, 66, 67–75. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A.; Bueschke, M.; Kulczyński, B.; Gliszczyńska-Świgło, A.; Kmiecik, D.; Bilska, A.; Jędrusek-Golińska, A. Phenolic compounds and multivariate analysis of antiradical properties of red fruits. J. Food Meas. Charact. 2019, 13, 1739–1747. [Google Scholar] [CrossRef]
- Ayaz, A.; Akyol, A.; Inan-Eroglu, E.; Cetin, A.K.; Samur, G.; Akbiyik, F. Chia seed (Salvia hispanica L.) added yogurt reduces short-term food intake and increases satiety: Randomized controlled trial. Nutr. Res. Pract. 2017, 11, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Motyka, S.; Kusznierewicz, B.; Ekiert, H.; Korona-Głowniak, I.; Szopa, A. Comparative analysis of metabolic variations, antioxidant profiles and antimicrobial activity of Salvia hispanica (Chia) seed, sprout, leaf, flower, root and herb extracts. Molecules 2023, 28, 2728. [Google Scholar] [CrossRef] [PubMed]
- Marcinek, K.; Krejpcio, Z. Chia seeds (Salvia hispanica): Health promoting properties and therapeutic applications—A review. Rocz. Państwowego Zakładu Hig. 2017, 68, 123–129. [Google Scholar]
- Mohammed, O.; Bekhet, M.; El-Razek, A.; Amal, M.; Moharram, Y. Evaluation of chia (Salvia hispanica L.) seeds meal as a source of bioactive ingredients. Alex. Sci. Exch. J. 2019, 40, 177–189. [Google Scholar] [CrossRef]
- Knez-Hrnčič, M.; Ivanovski, M.; Cör, D.; Knez, Ž. Chia Seeds (Salvia hispanica L.): An overview—Phytochemical profile, isolation methods, and application. Molecules 2019, 25, 11. [Google Scholar] [CrossRef]
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michałowska, A. The chemical composition and nutritional value of chia Seeds-Current state of knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef]
- Grancieri, M.; Martino, H.S.D.; Gonzalez de Mejia, E. Protein digests and pure peptides from chia seed prevented adipogenesis and inflammation by inhibiting PPARγ and NF-κB pathways in 3T3L-1 adipocytes. Nutrients 2021, 13, 176. [Google Scholar] [CrossRef]
- Peláez, P.; Orona-Tamayo, D.; Montes-Hernández, S.; Valverde, M.E.; Paredes-López, O.; Cibrián-Jaramillo, A. Comparative transcriptome analysis of cultivated and wild seeds of Salvia hispanica (chia). Sci. Rep. 2019, 9, 9761. [Google Scholar] [CrossRef] [PubMed]
- Motyka, S.; Skała, E.; Ekiert, H.; Szopa, A. Health-promoting approaches of the use of chia seeds. J. Funct. Foods 2023, 103, 105480. [Google Scholar] [CrossRef]
- Mburu, M.W. The role of chia seeds oil in human health: A critical review. Eur. J. Agric. Food Sci. 2021, 3, 1–4. [Google Scholar] [CrossRef]
- Katunzi-Kilewela, A.; Rweyemamu, L.; Kibazohi, O.; Kaale, L. Effects of drying, packaging conditions and storage time on proximate composition of Chia seeds (Salvia hispanica). Tanzan. J. Sci. 2021, 47, 258–267. [Google Scholar]
- Ishak, I.; Hussain, N.; Coorey, R.; Abd Ghani, M. Optimization and characterization of chia seed (Salvia hispanica L.) oil extraction using supercritical carbon dioxide. J. CO2 Util. 2021, 45, 101430. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of Association of Official Chemists, 18th ed.; AOAC: Washington, DC, USA, 2010. [Google Scholar]
- Nitrayová, S.; Brestenský, M.; Heger, J.; Patráš, P.; Rafay, J.; Sirotkin, A. Amino acids and fatty acids profile of chia (Salvia hispanica L.) and flax (Linum usitatissimum L.) seed. Slovak J. Food Sci. 2014, 8, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Moukette, B.M.; Anatole, P.C.; Biapa, C.P.N.; Njimou, J.R.; Ngogang, J.Y. Free radicals quenching potential, protective properties against oxidative mediated ion toxicity and HPLC phenolic profile of a Cameroonian spice: Piper guineensis. Toxicol. Rep. 2015, 2, 792–805. [Google Scholar] [CrossRef]
- Sami, R.; Li, Y.; Qi, B.; Wang, S.; Zhang, Q.; Han, F.; Jiang, L. HPLC analysis of water-soluble vitamins (B2, B3, B6, B12, and C) and fat-soluble vitamins (E, K, D, A, and β-carotene) of okra (Abelmoschus esculentus). J. Chem. 2014, 2014, 831357. [Google Scholar] [CrossRef]
- Aljumayi, H.; Aljumayi, A.; Algarni, E.; Algheshairy, R.M.; Alharbi, H.F.; Azhar, W.; Qadhi, A. The effect of chia seed extracts against complete Freud’s adjuvant-induced rheumatoid arthritis in rats. J. Food Qual. 2022, 2022, 3507674. [Google Scholar] [CrossRef]
- Martínez-Cruz, O.; Paredes-López, O. Phytochemical profile and nutraceutical potential of chia seeds (Salvia hispanica L.) by ultra-high performance liquid chromatography. J. Chromatogr. A 2014, 1346, 43–48. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, M.K.; Kumar, M. Protective effect of Mentha piperita against arsenic-induced toxicity in liver of Swiss Albino mice. Basic Clin. Pharmacol. Toxicol. 2007, 100, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Jan, T.; Hussain, S.Z.; Saadiya, A.R. Shelf life studies of the optimized chia-corn based snacks developed by extrusion processing. Pharma Innov. Int. J. 2022, 11, 1549–1552. [Google Scholar]
- Ortuño, J.; Ball, E.; Linton, M.; Richmond, A.; Corcionivoschi, N.; Theodoridou, K.; Houdijk, J.G.M. Oral communications and invited talks accepted for presentation at the Virtual WPSA UK Branch Meeting held on the 15th and 16 April 2021. These summaries have been edited for clarity and style by the WPSA UK Programme Committee but have not been fully peer-reviewed. In British Poultry Abstracts; Taylor & Francis: Abingdon, UK, 2021; Volume 17, pp. 1–29. [Google Scholar]
- Muxiddinovna, I.M. Effects of energy drinks on biochemical and sperm parameters in albino rats. Cent. Asian J. Med. Nat. Sci. 2022, 3, 126–131. [Google Scholar]
- Çayan, S.; Uğuz, M.; Saylam, B.; Akbay, E. Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of coronavirus disease 2019 (COVID-19) in SARS-CoV-2 infected male patients: A cohort study. Aging Male 2020, 23, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Siddique, N.; Cheema, R.A. Association of serum follicle stimulating hormone and serum luteinizing hormone with secondary infertility in obese females in Pakistan. Pak. Armed Forces Med. J. 2021, 71, S193. [Google Scholar] [CrossRef]
- Quinlan, P.; Horvath, A.; Eckerström, C.; Wallin, A.; Svensson, J. Altered thyroid hormone profile in patients with Alzheimer’s disease. Psychoneuroendocrinology 2020, 121, 104844. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, X.; Fu, C.; Su, M.; Jiang, F.; Xu, D.; Jiang, Q. Thyroid stimulating hormone (TSH) is associated with general and abdominal obesity: A cohort study in school-aged girls during puberty in East China. Front. Endocrinol. 2020, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Baş, H.; Taşkesen, H.O.; Boz, M.A.; Sarıca, M.; Erensoy, K.; Dotas, V.; Symeon, G. The effects of varying combinations of dietary selenium, vitamin E, and zinc supplements on antioxidant enzyme activity, and developmental and histological traits in testicular tissues of 1-year-old native Turkish ganders. Sustainability 2023, 15, 12245. [Google Scholar] [CrossRef]
- Abdel-Aty, A.M.; Elsayed, A.M.; Salah, H.A.; Bassuiny, R.I.; Mohamed, S.A. Egyptian chia seeds (Salvia hispanica L.) during germination: Upgrading of phenolic profile, antioxidant, antibacterial properties and relevant enzymes activities. Food Sci. Biotechnol. 2021, 30, 723–734. [Google Scholar] [CrossRef]
- Ghafoor, K.; Al Juhaimi, F.; Özcan, M.M.; Uslu, N.; Ahmed, I.A.M.; Babiker, E.E. The effect of boiling, germination and roasting on bioactive properties, phenolic compounds, fatty acids and minerals of chia seed (Salvia hispanica L.) and oils. Int. J. Gastron. Food Sci. 2022, 27, 100447. [Google Scholar] [CrossRef]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.Y.O.; Lima, G.P.P. Phenolic compounds: Functional properties, impact of processing and bioavailability. Phenolic Compd. Biol. Act 2017, 8, 1–24. [Google Scholar]
- Morales-Olán, G.; Rojas-López, M.; Díaz-Reyes, J.; Rosas-Cárdenas, F.D.F.; Luna-Suárez, S. Effect of ethanol and methanol on the total phenolic content and antioxidant capacity of chia seeds (Salvia hispanica L.). Sains Malays. 2020, 49, 1283–1292. [Google Scholar]
- Salgado, V.D.S.C.N.; Zago, L.; Antunes, A.E.C.; Miyahira, R.F. Chia (Salvia hispanica L.) seed germination: A brief review. Plant Foods Hum. Nutr. 2022, 77, 485–494. [Google Scholar] [CrossRef]
- Hatamian, M.; Noshad, M.; Abdanan-Mehdizadeh, S.; Barzegar, H. Effect of roasting treatment on functional and antioxidant properties of chia seed flours. NFS J. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Khalid, W.; Arshad, M.S.; Aziz, A.; Rahim, M.A.; Qaisrani, T.B.; Afzal, F.; Anjum, F.M. Chia seeds (Salvia hispanica L.): A therapeutic weapon in metabolic disorders. Food Sci. Nutr. 2023, 11, 3–16. [Google Scholar] [CrossRef]
- Din, Z.U.; Alam, M.; Ullah, H.; Shi, D.; Xu, B.; Li, H.; Xiao, C. Nutritional, phytochemical and therapeutic potential of chia seed (Salvia hispanica L.). A mini-review. Food Hydrocoll. Health 2021, 1, 100010. [Google Scholar] [CrossRef]
- Özbek, T.; Şahin-Yeşilçubuk, N.; Demirel, B. Quality and nutritional value of functional strawberry marmalade enriched with chia seed (Salvia hispanica L.). J. Food Qual. 2019, 2019, 2391931. [Google Scholar] [CrossRef]
- Motyka, S.; Koc, K.; Ekiert, H.; Blicharska, E.; Czarnek, K.; Szopa, A. The current state of knowledge on Salvia hispanica and Salviae hispanicae semen (chia seeds). Molecules 2022, 27, 1207. [Google Scholar] [CrossRef]
- Char-Kiewicz, A.E.; Back-strand, J.R. Lead toxicity and pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef]
- Alwosais, E.Z.M.; Al-Ozairi, E.; Zafar, T.A.; Alkandari, S. Chia seed (Salvia hispanica L.) supplementation to the diet of adults with type 2 diabetes improved systolic blood pressure: A randomized controlled trial. Nutr. Health 2021, 27, 181–189. [Google Scholar] [CrossRef]
- Biswas, S.; Islam, F.; Imran, A.; Zahoor, T.; Noreen, R.; Fatima, M.; Asif Shah, M. Phytochemical profile, nutritional composition, and therapeutic potentials of chia seeds: A concise review. Cogent Food Agric. 2023, 9, 2220516. [Google Scholar] [CrossRef]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Prakash, A.; Tiwari, R. Environmental endocrine-disrupting chemical exposure: Role in non-communicable diseases. Front. Public Health 2020, 8, 553850. [Google Scholar] [CrossRef] [PubMed]
- Knapke, E.T.; Magalhaes, D.D.P.; Dalvie, M.A.; Mandrioli, D.; Perry, M.J. Environmental and occupational pesticide exposure and human sperm parameters: A Navigation Guide review. Toxicology 2022, 465, 153017. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, B.P.; Roychoudhury, S.; Sengupta, P.; Toman, R.; Dutta, S.; Kesari, K.K. Arsenic-induced sex hormone disruption: An insight into male infertility. In Oxidative Stress and Toxicity in Reproductive Biology and Medicine: A Comprehensive Update on Male Infertility Volume II; Springer International Publishing: Cham, Switzerland, 2022; pp. 83–95. [Google Scholar]
- Abdel-Emam, R.A.; Ahmed, E.A. Ameliorative effect of L-carnitine on chronic lead-induced reproductive toxicity in male rats. Vet. Med. Sci. 2021, 7, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.K.; Paliwal, A.; Saraf, P. Effects of heavy metals on reproduction owing to infertility. J. Biochem. Mol. Toxicol. 2021, 35, e22823. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Saravanan, A.; Elangovan, A.; Ramesh, S.; Annamalai, S.; Namachivayam, A.; Gopalakrishnan, A.V. An appraisal on molecular and biochemical signalling cascades during arsenic-induced hepatotoxicity. Life Sci. 2020, 260, 118438. [Google Scholar] [CrossRef] [PubMed]
- Rharbi, S.; Talbi, C.; Sijilmassi, B.; Triqui, Z.E.A.; Lamaoui, M.; Filali-Maltouf, A.; Chakhchar, A. Foliar application with salicylic acid alleviates cadmium toxicity in chia (Salvia hispanica L.). Sci. Afr. 2023, 21, e01773. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Mathai, M.; Zulli, A. Cytoprotective remedies for ameliorating nephrotoxicity induced by renal oxidative stress. Life Sci. 2023, 318, 121466. [Google Scholar] [CrossRef]
- Mohammadi, H.; Golbabaei, F.; Dehghan, S.F.; Imani, H.; Ramezani Tehrani, F.; Khodakarim Ardakani, S. The influence of vitamin E and omega-3 fatty acids on reproductive health indices among male workers exposed to electromagnetic fields. Am. J. Men’s Health 2022, 16, 15579883221074821. [Google Scholar] [CrossRef]
- Podar, D.; Maathuis, F.J. The role of roots and rhizosphere in providing tolerance to toxic metals and metalloids. Plant Cell Environ. 2022, 45, 719–736. [Google Scholar] [CrossRef]
- Sun, S.K.; Chen, J.; Zhao, F.J. Regulatory mechanisms of sulfur metabolism affecting tolerance and accumulation of toxic trace metals and metalloids in plants. J. Exp. Bot. 2023, 74, 3286–3299. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Aluminum, arsenic, beryllium, cadmium, chromium, cobalt, copper, iron, lead, mercury, molybdenum, nickel, platinum, thallium, titanium, vanadium, and zinc: Molecular aspects in experimental liver injury. Int. J. Mol. Sci. 2022, 23, 12213. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.; Motamedzadegan, A.; Raeisi, S.N.; Rahaiee, S. Antioxidant activity of nanoencapsulated chia (Salvia hispanica L.) seed extract and its application to manufacture a functional cheese. Food Sci. Nutr. 2023, 11, 1328–1341. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Rizwana Tripathi, A.D.; Kumar, T.; Sharma, K.P.; Patel, S.K.S. Nutritional and functional new perspectives and potential health benefits of quinoa and chia seeds. Antioxidants 2023, 12, 1413. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.S.; Kholief, T.; Mohamed, R.W.; Abd El-Rhman, A. The modulatory effects of black chia (Salvia hispanica) and garden cress (Lepidium sativum) seeds on Nε-CML formation in streptozotocin-injected rats. J. Herbmed Pharmacol. 2023, 12, 250–261. [Google Scholar] [CrossRef]
- Rehman, G.; Kumari, N.; Bano, F.; Tyagi, R.K. Thyroid hormone receptor beta: Relevance in human health and diseases. Endocr. Metab. Sci. 2023, 13, 100144. [Google Scholar] [CrossRef]
- Gładysz, A.K.; Stępniak, J.; Karbownik-Lewińska, M. Exogenous melatonin protects against oxidative damage to membrane lipids caused by some sodium/iodide symporter inhibitors in the thyroid. Antioxidants 2023, 12, 1688. [Google Scholar] [CrossRef]
- Sorrenti, S.; Baldini, E.; Pironi, D.; Lauro, A.; D’Orazi, V.; Tartaglia, F.; Ulisse, S. Iodine: Its role in thyroid hormone biosynthesis and beyond. Nutrients 2021, 13, 4469. [Google Scholar] [CrossRef]
- Mégier, C.; Dumery, G.; Luton, D. Iodine and thyroid maternal and fetal metabolism during pregnancy. Metabolites 2023, 13, 633. [Google Scholar] [CrossRef]
- Leemans, M.; Couderq, S.; Demeneix, B.; Fini, J.B. Pesticides with potential thyroid hormone-disrupting effects: A review of recent data. Front. Endocrinol. 2019, 10, 743. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Annunziata, G.; Camajani, E.; Caprio, M.; Sojat, A.S.; Muscogiuri, G. Role of Mediterranean diet in endocrine diseases: A joint overview by the endocrinologist and the nutritionist. J. Endocrinol. Investig. 2023, 1–17. [Google Scholar] [CrossRef]
- Mendelson, S.D. Herbal Treatment of Major Depression: Scientific Basis and Practical Use; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Gadallah, E.E.; El-Hamied, A.; El-Naggar, E.A.; Mustafa, M.A. Evaluation of quality attributes of chia (Salvia hispanica), moringa (Moringa oleifera) and flax (Linum usitatissimum) oils. Arch. Agric. Sci. J. 2023, 6, 58–78. [Google Scholar] [CrossRef]
- Chiang, J.H.; Ong, D.S.M.; Ng, F.S.K.; Hua, X.Y.; Tay, W.L.W.; Henry, C.J. Application of chia (Salvia hispanica) mucilage as an ingredient replacer in foods. Trends Food Sci. Technol. 2021, 115, 105–116. [Google Scholar] [CrossRef]
- Akaras, N.; Gur, C.; Kucukler, S.; Kandemir, F.M. Zingerone reduces sodium arsenite-induced nephrotoxicity by regulating oxidative stress, inflammation, apoptosis and histopathological changes. Chem. Biol. Interact. 2023, 374, 110410. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.A.; John, S.; Monica, S.J.; Saraswathi, K.; Arumugam, P. Screening of secondary metabolites, bioactive compounds, in vitro antioxidant, antibacterial, antidiabetic and anti-inflammatory activities of chia seeds (Salvia hispanica L.). Res. J. Pharm. Technol. 2021, 14, 6289–6294. [Google Scholar] [CrossRef]
- El-Gendy, M.S.; El-Gezawy, E.S.; Saleh, A.A.; Alhotan, R.A.; Al-Badwi, M.A.A.; Hussein, E.O.S.; El-Tahan, H.M.; Kim, I.H.; Cho, S.; Omar, S.M. Investigating the Chemical Composition of Lepidium sativum Seeds and Their Ability to Safeguard against Monosodium Glutamate-Induced Hepatic Dysfunction. Foods 2023, 12, 4129. [Google Scholar] [CrossRef]
- Saleh, A.A.; Soliman, M.M.; Yousef, M.F.; Eweedah, N.M.; El-Sawy, H.B.; Shukry, M.; Wadaan, M.A.M.; Kim, I.H.; Cho, S.; Eltahan, H.M. Effects of herbal supplements on milk production quality and specific blood parameters in heat-stressed early lactating cows. Front. Veter-Sci. 2023, 10, 1180539. [Google Scholar] [CrossRef]
- Selim, S.; Hussein, E.; Abdel-Megeid, N.S.; Melebary, S.J.; AL-Harbi, M.S.; Saleh, A.A. Growth Performance, Antioxidant Activity, Immune Status, Meat Quality, Liver Fat Content, and Liver Histomorphology of Broiler Chickens Fed Rice Bran Oil. Animals 2021, 11, 3410. [Google Scholar] [CrossRef]
- Mondor, M. Chia (Salvia hispanica) seed oil extraction by-product and its edible applications. Food Rev. Int. 2023, 1–20. [Google Scholar] [CrossRef]
| Salvia hispanica Seeds Powder (Chia Seeds) | |
|---|---|
| Phenolic Compounds | Conc. (µg/g) |
| Gallic acid | 833.35 ± 15.10 |
| Chlorogenic acid | 1717.79 ± 20.42 |
| Catechin | 3918.61 ± 12.65 |
| Coffeic acid | 9596.08 ± 18.32 |
| Rutin | 828.77 ± 11.45 |
| Ellagic acid | 2028.77 ± 16.27 |
| Vanillin | 199.55 ± 28.10 |
| Ferulic acid | 2085.90 ± 24.11 |
| Naringenin | 1449.25 ± 9.05 |
| Daidzein | 509.53 ± 23.08 |
| Querectin | 1584.25 ± 35.16 |
| Cinnamic acid | 9.94 ± 0.04 |
| Apigenin | 256.69 ± 10.27 |
| Hesperetin | 359.28 ± 18.23 |
| Groups | Parameters | |
|---|---|---|
| Prostate Weight (g/100 g b.w) | Testes Weight (g/100 g b.w) | |
| (Mean ± SE) | (Mean ± SE) | |
| G1 (−Ve) | 0.60 ± 0.012 a | 2.34 ± 0.015 a |
| G2 (+Ve) | 0.32 ± 0.014 d | 1.62 ± 0.012 d |
| G3 (5%/100 g diet) | 0.56 ± 0.012 b | 2.24 ± 0.014 b |
| G4 (10%/100 g diet) | 0.59 ± 0.008 a | 2.28 ± 0.008 b |
| G5 (15%/100 g diet) | 0.55 ± 0.006 b | 2.16 ± 0.011 bc |
| G6 (20%/100 g diet) | 0.52 ± 0.014 c | 2.02 ± 0.014 c |
| Groups | Parameters | ||
|---|---|---|---|
| FI (g/28 days) | BWG% | FER | |
| Mean ± SE | Mean ± SE | Mean ± SE | |
| G1 (−Ve) | 294.99 ± 0.86 a | 28.56 ± 0.88 a | 0.12 ± 0.005 a |
| G2 (+Ve) | 238.21 ± 1.17 f | 18.18 ± 0.84 d | 0.06 ± 0.007 d |
| G3 (5%/100 g diet) | 283.75 ± 1.75 c | 27.23 ± 1.15 ab | 0.11 ± 0.009 b |
| G4 (10%/100 g diet) | 288.29 ± 1.47 b | 28.85 ± 0.87 b | 0.10 ± 0.005 b |
| G5 (15%/100 g diet) | 275.23 ± 1.16 d | 23.95 ± 0.89 bc | 0.08 ± 0.007 bc |
| G6 (20%/100 g diet) | 262.44 ± 1.20 e | 21.99 ± 0.86 c | 0.07 ± 0.008 c |
| Groups | Parameters | |||
|---|---|---|---|---|
| Sperm Count (×106/mL) | Sperm Motility (%) | Progressive Motility (%) | Normal Form (%) | |
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |
| G1 (−Ve) | 67.42 ± 1.01 a | 78.34 ± 2.41 a | 65.00 ± 1.60 a | 75.06 ± 2.11 a |
| G2 (+Ve) | 32.15 ± 2.45 e | 36.63 ± 2.02 e | 31.14 ± 1.25 f | 37.03 ± 1.04 f |
| G3 (5%/100 g diet) | 62.92 ± 3.10 c | 70.61 ± 2.03 b | 58.36 ± 1.50 c | 68.42 ± 2.34 c |
| G4 (10%/100 g diet) | 65.67 ± 2.41 b | 74.35 ± 2.06 ab | 62.00 ± 2.14 b | 73.06 ± 2.11 b |
| G5 (15%/100 g diet) | 54.10 ± 3.16 cd | 58.72 ± 1.56 c | 46.00 ± 2.08 d | 58.14 ± 1.63 d |
| G6 (20%/100 g diet) | 51.30 ± 3.17 d | 54.64 ± 2.31 d | 40.23 ± 2.16 e | 51.23 ± 1.13 e |
| Groups | Parameters | ||
|---|---|---|---|
| FSH (ng/mL) | LH (ng/mL) | Testosterone H. (ng/mL) | |
| Mean ± SE | Mean ± SE | Mean ± SE | |
| G1 (−Ve) | 1.38 ± 0.02 a | 1.89 ± 0.05 a | 3.65 ± 0.05 a |
| G2 (+Ve) | 0.18 ± 0.01 c | 0.39 ± 0.03 f | 1.16 ± 0.01 f |
| G3 (5%/100 g diet) | 1.17 ± 0.03 bc | 1.47 ± 0.02 c | 2.92 ± 0.02 c |
| G4 (10%/100 g diet) | 1.22 ± 0.02 b | 1.55 ± 0.04 b | 3.05 ± 0.03 b |
| G5 (15%/100 g diet) | 1.01 ± 0.02 d | 1.31 ± 0.03 d | 2.63 ± 0.04 d |
| G6 (20%/100 g diet) | 0.85 ± 0.04 e | 1.20 ± 0.01 e | 2.40 ± 0.03 e |
| Groups | Parameters | ||
|---|---|---|---|
| T3 (ng/mL) | T4 (ug/dL) | TSH (µlU/mL) | |
| Mean ± SE | Mean ± SE | Mean ± SE | |
| G1 (−Ve) | 0.86 ± 0.04 a | 6.80 ± 0.48 a | 0.0015 ± 0.0001 a |
| G2 (+Ve) | 0.63 ± 0.02 e | 4.51 ± 0.17 f | 0.0009 ± 0.0000 f |
| G3 (5%/100 g diet) | 0.81 ± 0.01 b | 6.63 ± 0.03 c | 0.0012 ± 0.0002 c |
| G4 (10%/100 g diet) | 0.84 ± 0.03 ab | 6.76 ± 0.20 b | 0.0014 ± 0.0002 b |
| G5 (15%/100 g diet) | 0.73 ± 0.05 c | 6.12 ± 0.27 d | 0.0011 ± 0.0001 d |
| G6 (20%/100 g diet) | 0.70 ± 0.02 d | 5.65 ± 0.18 e | 0.0010 ± 0.0001 e |
| Groups | Parameters | |||
|---|---|---|---|---|
| GPX (ng/mg) | SOD (U/min/mg ) | CAT (U/min/mg ) | MDA (nMol/mg ) | |
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |
| G1 (−Ve) | 0.54 ± 0.02 a | 0.37 ± 0.01 a | 0.35 ± 0.02 a | 0.10 ± 0.01 e |
| G2 (+Ve) | 0.17 ± 0.01 f | 0.12 ± 0.02 e | 0.14 ± 0.01 f | 0.28 ± 0.04 a |
| G3 (5%/100 g diet) | 0.48 ± 0.03 c | 0.33 ± 0.004 b | 0.30 ± 0.04 c | 0.15 ± 0.02 d |
| G4 (10%/100 g diet) | 0.50 ± 0.04 b | 0.35 ± 0.01 ab | 0.33 ± 0.02 b | 0.14 ± 0.03 d |
| G5 (15%/100 g diet) | 0.35 ± 0.02 d | 0.31 ± 0.02 c | 0.29 ± 0.03 d | 0.20 ± 0.01 c |
| G6 (20%/100 g diet) | 0.24 ± 0.05 e | 0.27 ± 0.03 d | 0.24 ± 0.01 e | 0.24 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, S.M.; Zahran, N.N.; Alhotan, R.A.; Hussein, E.O.; Galik, B.; Saleh, A.A. Evaluation of Salvia hispanica as a Therapeutic Agent against Sodium Arsenic-Induced Testicular Toxicity in a Male Rats Model. Life 2024, 14, 109. https://doi.org/10.3390/life14010109
Omar SM, Zahran NN, Alhotan RA, Hussein EO, Galik B, Saleh AA. Evaluation of Salvia hispanica as a Therapeutic Agent against Sodium Arsenic-Induced Testicular Toxicity in a Male Rats Model. Life. 2024; 14(1):109. https://doi.org/10.3390/life14010109
Chicago/Turabian StyleOmar, Sara Mahmoud, Nasser Nesim Zahran, Rashed A. Alhotan, Elsayed Osman Hussein, Branislav Galik, and Ahmed Ali Saleh. 2024. "Evaluation of Salvia hispanica as a Therapeutic Agent against Sodium Arsenic-Induced Testicular Toxicity in a Male Rats Model" Life 14, no. 1: 109. https://doi.org/10.3390/life14010109
APA StyleOmar, S. M., Zahran, N. N., Alhotan, R. A., Hussein, E. O., Galik, B., & Saleh, A. A. (2024). Evaluation of Salvia hispanica as a Therapeutic Agent against Sodium Arsenic-Induced Testicular Toxicity in a Male Rats Model. Life, 14(1), 109. https://doi.org/10.3390/life14010109







