Update on Urinary Tract Infection Antibiotic Resistance—A Retrospective Study in Females in Conjunction with Clinical Data
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
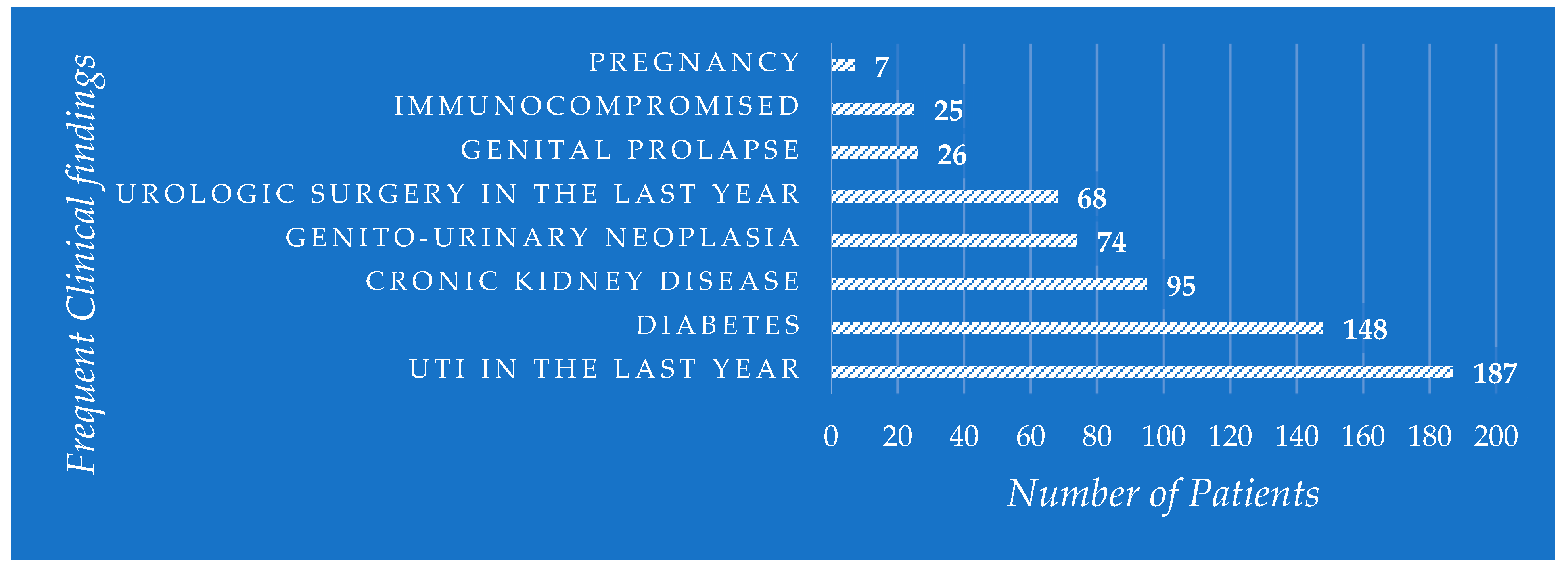
4.1. The Incidence of Uropathogens in UTIs and Relation to Patients’ Age
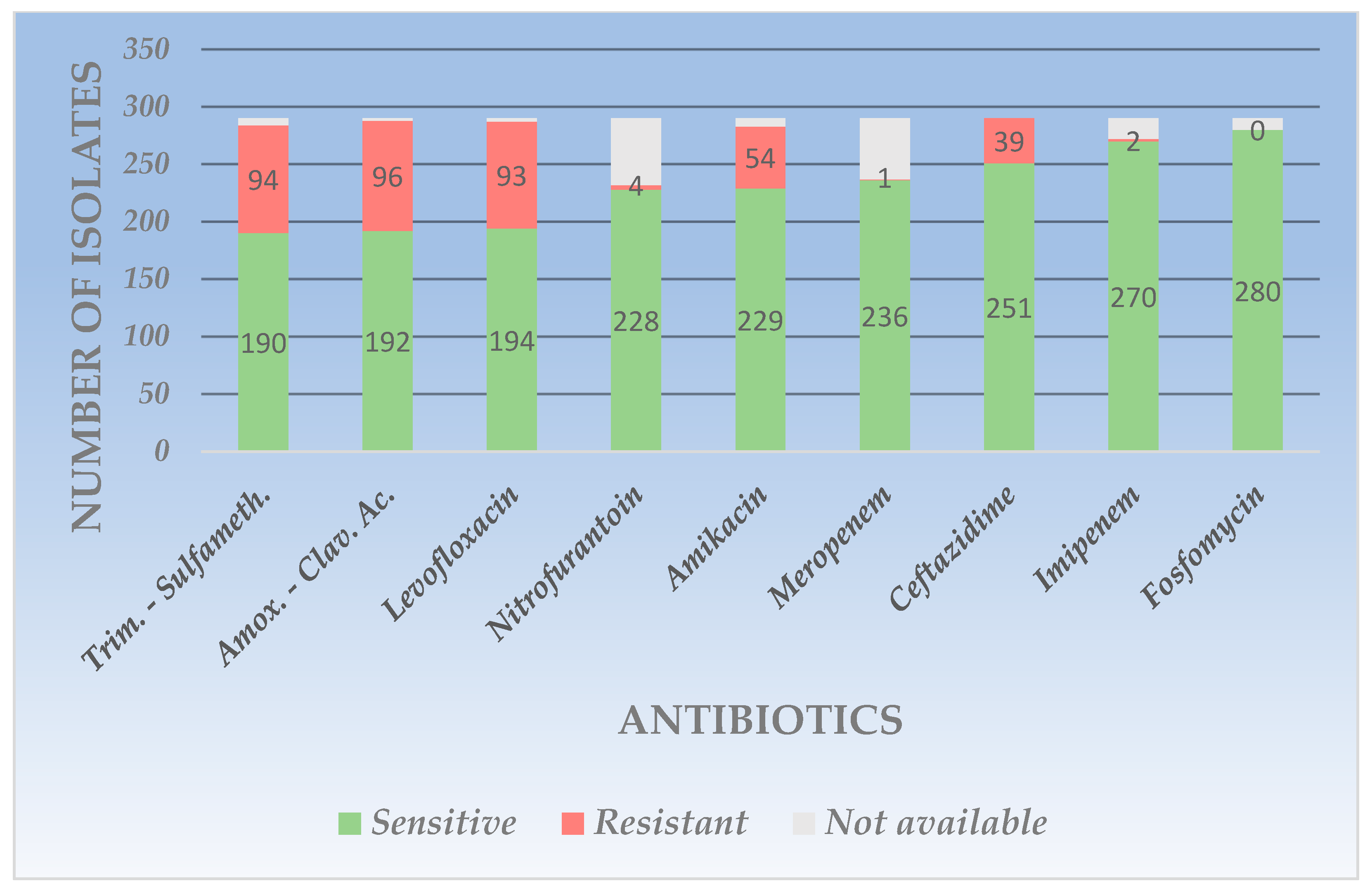
4.2. The Resistance Profile of Gram-Negative Uropathogens
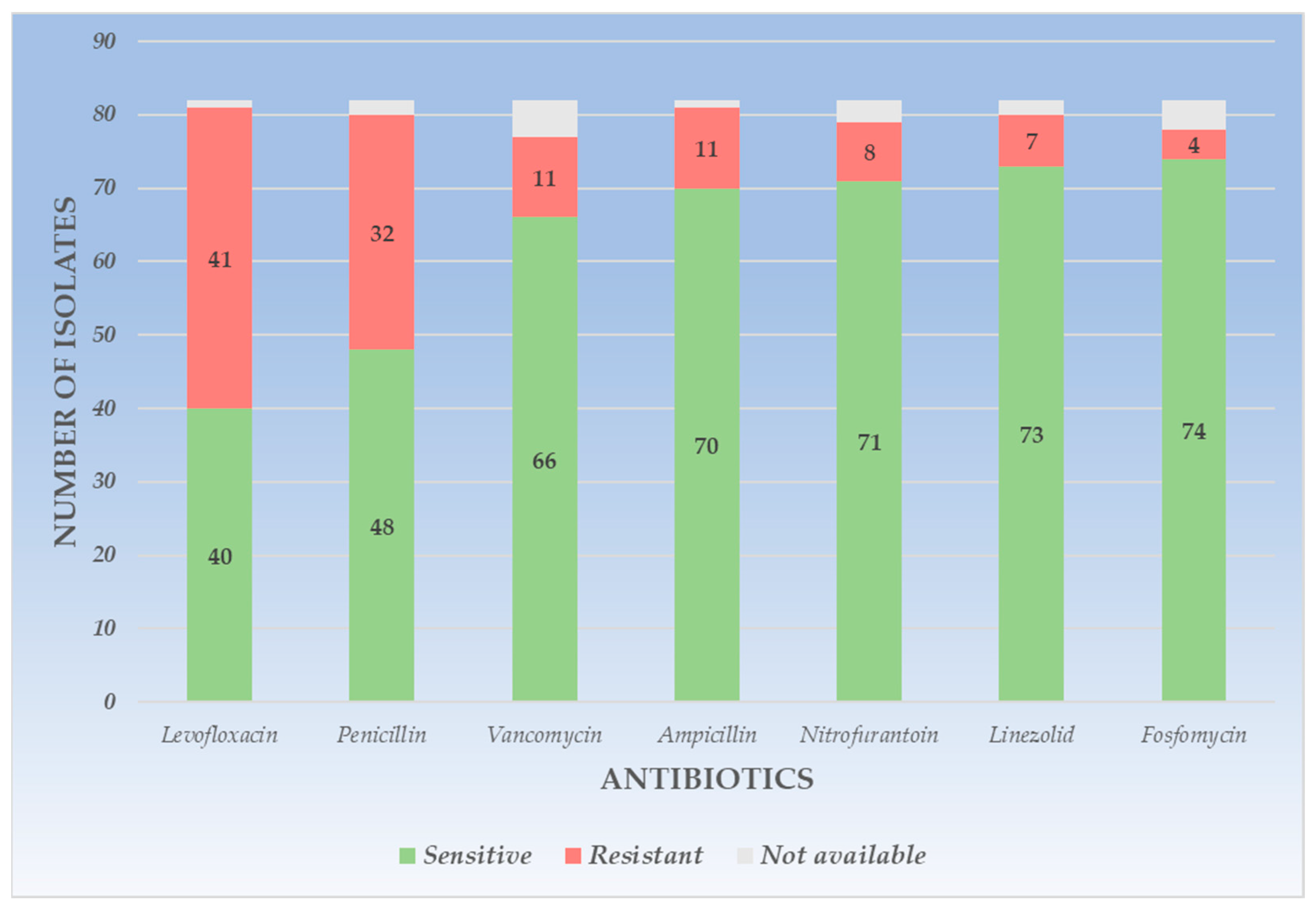
4.3. The Resistance Profile of Gram-Positive Uropathogens
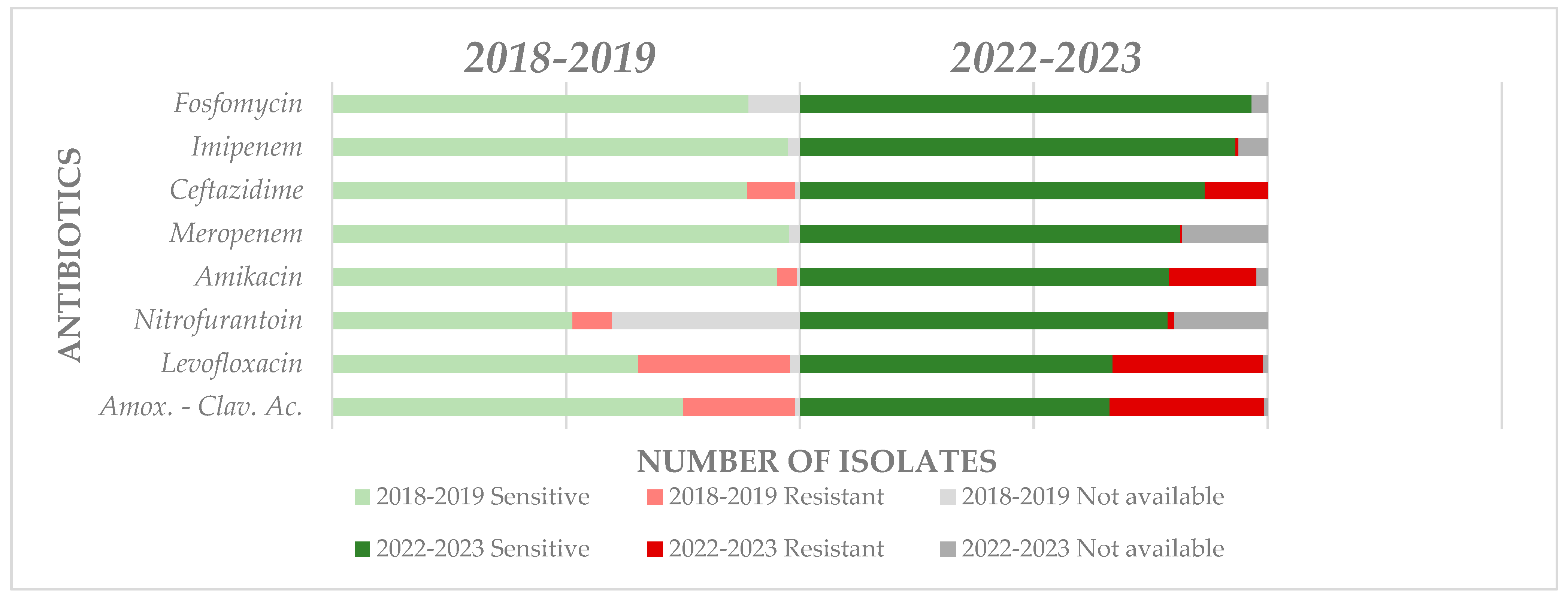
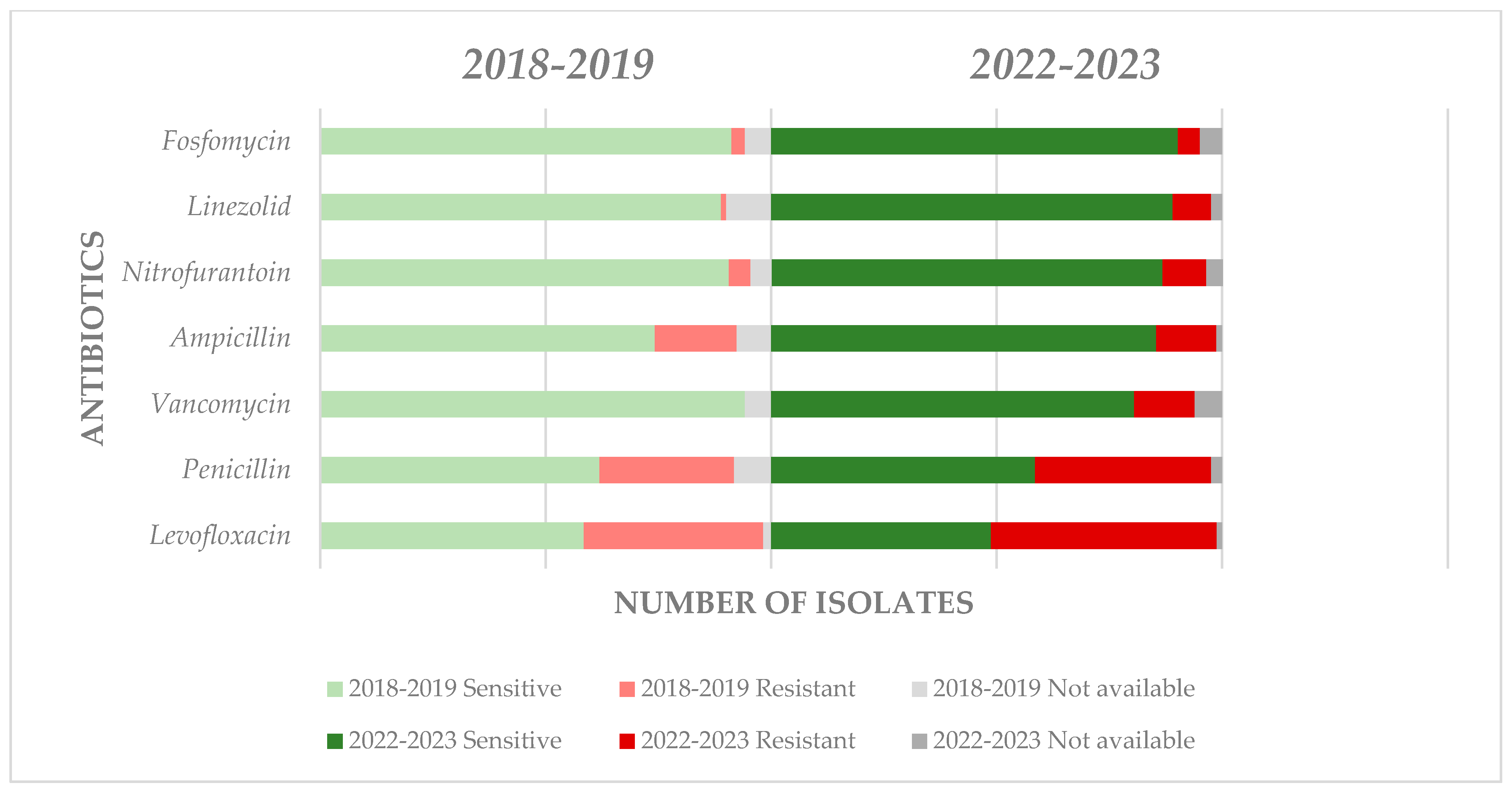
4.4. Antimicrobial Resistance Evolution in the Short and Middle Term
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foxman, B.; Brown, P. Epidemiology of urinary tract infections: Transmission and risk factors, incidence, and costs. Infect. Dis. Clin. N. Am. 2003, 17, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Paul, R. State of the globe: Rising antimicrobial resistance of pathogens in urinary tract infection. J. Glob. Infect. Dis. 2018, 10, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Tandogdu, Z.; Wagenlehner, F.M.E. Global epidemiology of urinary tract infections. Curr. Opin. Infect. Dis. 2016, 29, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med. 2002, 113, 5S–13S. [Google Scholar] [CrossRef] [PubMed]
- Abou Heidar, N.F.; Degheili, J.A.; Yacoubian, A.A.; Khauli, R.B. Management of urinary tract infection in women: A practical approach for everyday practice. Urol. Ann. 2019, 11, 339–346. [Google Scholar] [PubMed]
- Manges, A.R.; Natarajan, P.; Solberg, O.D.; Dietrich, P.S.; Riley, L.W. The changing prevalence of drug-resistant Escherichia coli clonal groups in a community: Evidence for community outbreaks of urinary tract infections. Epidemiol. Infect. 2006, 134, 425–431. [Google Scholar] [CrossRef]
- Mirsoleymani, S.R.; Salimi, M.; Shareghi, B.M.; Ranjbar, M.; Mehtarpoor, M. Bacterial pathogens and antimicrobial resistance patterns in pediatric urinary tract infections: A four-year surveillance study (2009–2012). Int. J. Pediatr. 2014, 2014, 126142. [Google Scholar] [CrossRef]
- Akram, M.; Shahid, M.; Khan, A. Etiology and antibiotic resistance pattern of community acquired urinary tract infection in JNMC Hospital India. Ann. Clin. Microbiol. Antimicrob. 2007, 6, 4. [Google Scholar] [CrossRef]
- Tibyangye, J.; Okech, M.; Nyabayo, J.; Nakavuma, J. In vitro antibacterial activity of Ocimum suave essential oils against uropathogens isolated from patients in selected hospitals in Bushenyi district, Uganda. Br. Microbiol. Res. J. 2015, 8, 489–498. [Google Scholar] [CrossRef]
- Seifu, W.D.; Gebissa, A.D. Prevalence and antibiotic susceptibility of Uropathogens from cases of urinary tract infections (UTI) in Shashemene referral hospital, Ethiopia. BMC Infect. Dis. 2018, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Gebretensaie, Y.; Atnafu, A.; Girma, S.; Alemu, Y.; Desta, K. Prevalence of bacterial urinary tract infection, associated risk factors, and antimicrobial resistance pattern in Addis Ababa, Ethiopia: A cross-sectional study. Infect. Drug Resist. 2023, 16, 3041–3050. [Google Scholar] [CrossRef] [PubMed]
- Bono, M.J.; Leslie, S.W.; Reygaert, W.C. Urinary Tract Infection; Updated 2022 November 28; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470195/ (accessed on 28 October 2023).
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Wiedemann, B.; Heisig, A.; Heisig, P. Uncomplicated urinary tract infections and antibiotic resistance—Epidemiological and mechanistic aspects. Antibiotics 2014, 3, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, A.H.; Alameer, K.M.; Abuageelah, B.M.; Alharbi, R.H.; Mobarki, M.; Musawi, S.; Haddad, M.; Matabi, A.; Dhayhi, N. Epidemiology and antimicrobial resistance patterns of urinary tract infections: A cross-sectional study from Southwestern Saudi Arabia. Medicina 2023, 59, 1411. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 28 October 2023).
- Available online: https://www.ecdc.europa.eu/en/news-events/who-and-ecdc-report-antimicrobial-resistance-remains-health-threat-europe (accessed on 28 October 2023).
- Bonkat, G.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Kranz, J.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; et al. EAU Guidelines on Urological Infections; European Association of Urology: Arnhem, The Netherlands, 2023. [Google Scholar]
- Li, X.; Fan, H.; Zi, H.; Hu, H.; Li, B.; Huang, J.; Luo, P.; Zeng, X. Global and regional burden of bacterial antimicrobial resistance in urinary tract infections in 2019. J. Clin. Med. 2022, 11, 2817. [Google Scholar] [CrossRef] [PubMed]
- Mareș, C.; Petca, R.C.; Popescu, R.I.; Petca, A.; Geavlete, B.F.; Jinga, V. Uropathogens’ antibiotic resistance evolution in a female population: A sequential multi-year comparative analysis. Antibiotics 2023, 12, 948. [Google Scholar] [CrossRef]
- Ghiga, I.; Pitchforth, E.; Stålsby Lundborg, C.; Machowska, A. Family doctors’ roles and perceptions on antibiotic consumption and antibiotic resistance in Romania: A qualitative study. BMC Prim. Care 2023, 24, 93. [Google Scholar] [CrossRef]
- Petca, R.-C.; Mareș, C.; Petca, A.; Negoiță, S.; Popescu, R.-I.; Boț, M.; Barabás, E.; Chibelean, C.B. Spectrum and antibiotic resistance of uropathogens in Romanian females. Antibiotics 2020, 9, 472. [Google Scholar] [CrossRef]
- Rusu, A.; Petca, A.; Mares, C.; Petca, R.C.; Popescu, R.I.; Negoita, S.; Danau, R.A.; Chibelean, C.B.; Jinga, V. Urinary infections in a Romanian population: Antimicrobial resistance of uropathogens—A multiregional study. Farmacia 2023, 71, 165–173. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Collection of Clinical Specimens during Field Investigation of Outbreaks; World Health Organization: Geneva, Switzerland, 2000; pp. 1–51. [Google Scholar]
- Available online: https://www.eucast.org/ast_of_bacteria (accessed on 25 October 2023).
- Petca, R.C.; Popescu, R.I.; Mares, C.; Petca, A.; Mehedintu, C.; Sandu, I.; Maru, N. Antibiotic resistance profile of common uropathogens implicated in urinary tract infections in Romania. Farmacia 2019, 67, 994–1004. [Google Scholar] [CrossRef]
- Chibelean, C.B.; Petca, R.C.; Mareș, C.; Popescu, R.I.; Enikő, B.; Mehedințu, C.; Petca, A. A clinical perspective on the antimicrobial resistance spectrum of uropathogens in a Romanian male population. Microorganisms 2020, 8, 848. [Google Scholar] [CrossRef] [PubMed]
- Ejrnæs, K. Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli. Dan. Med. Bull. 2011, 58, B4187. [Google Scholar] [PubMed]
- Muhammad, A.; Khan, S.N.; Ali, N.; Rehman, M.U.; Ali, I. Prevalence and antibiotic susceptibility pattern of uropathogens in outpatients at a tertiary care hospital. New Microbes New Infect. 2020, 36, 100716. [Google Scholar] [CrossRef] [PubMed]
- Jalil, M.B.; Al Atbee, M.Y.N. The prevalence of multiple drug resistance Escherichia coli and Klebsiella pneumoniae isolated from patients with urinary tract infections. J. Clin. Lab. Anal. 2022, 36, e24619. [Google Scholar] [CrossRef]
- Maione, A.; Galdiero, E.; Cirillo, L.; Gambino, E.; Gallo, M.A.; Sasso, F.P.; Petrillo, A.; Guida, M.; Galdiero, M. Prevalence, resistance patterns and biofilm production ability of bacterial uropathogens from cases of community-acquired urinary tract infections in South Italy. Pathogens 2023, 12, 537. [Google Scholar] [CrossRef] [PubMed]
- Odoki, M.; Almustapha Aliero, A.; Tibyangye, J.; Nyabayo Maniga, J.; Wampande, E.; Drago Kato, C.; Agwu, E.; Bazira, J. Prevalence of bacterial urinary tract infections and associated factors among patients attending hospitals in Bushenyi District, Uganda. Int. J. Microbiol. 2019, 2019, 4246780. [Google Scholar] [CrossRef]
- Silago, V.; Moremi, N.; Mtebe, M.; Komba, E.; Masoud, S.; Mgaya, F.X.; Mirambo, M.M.; Nyawale, H.A.; Mshana, S.E.; Matee, M.I. Multidrug-resistant uropathogens causing community acquired urinary tract infections among patients attending health facilities in Mwanza and Dar es Salaam, Tanzania. Antibiotics 2022, 11, 1718. [Google Scholar] [CrossRef]
- Luty, R.S.; Fadil, A.G.; Najm, J.M.; Abduljabbar, H.H.; Kashmar, S.A.A. Uropathogens antibiotic susceptibility as an indicator for the empirical therapy used for urinary tract infections: A retrospective observational study. Iran J Microbiol. 2020, 12, 395–403. [Google Scholar] [CrossRef]
- Gomila, A.; Carratalà, J.; Eliakim-Raz, N.; Shaw, E.; Wiegand, I.; Vallejo-Torres, L.; Gorostiza, A.; Vigo, J.M.; Morris, S.; Stoddart, M.; et al. Risk factors and prognosis of complicated urinary tract infections caused by Pseudomonas aeruginosa in hospitalized patients: A retrospective multicenter cohort study. Infect. Drug Resist. 2018, 11, 2571–2581. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary tract infections: The current scenario and future prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Alshomrani, M.K.; Alharbi, A.A.; Alshehri, A.A.; Arshad, M.; Dolgum, S. Isolation of Staphylococcus aureus urinary tract infections at a community-based healthcare center in Riyadh. Cureus 2023, 15, e35140. [Google Scholar] [CrossRef] [PubMed]
- Al-Badr, A.; Al-Shaikh, G. Recurrent urinary tract infections management in women: A review. Sultan Qaboos Univ. Med. J. 2013, 13, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Mody, L.; Juthani-Mehta, M. Urinary tract infections in older women: A clinical review. JAMA 2014, 311, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.P.; Flores, J.T.; Barros Junior Ode, O.; Rodrigues, G.B.; Mourão Cde, M.; Moreira, R.M. Epidemiological and clinical aspects of urinary tract infection in community-dwelling elderly women. Braz. J. Infect. Dis. 2012, 16, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Magliano, E.; Grazioli, V.; Deflorio, L.; Leuci, A.I.; Mattina, R.; Romano, P.; Cocuzza, C.E. Gender and age-dependent etiology of community-acquired urinary tract infections. Sci. World J. 2012, 2012, 349597. [Google Scholar] [CrossRef]
- Akhtar, A.; Ahmad Hassali, M.A.; Zainal, H.; Ali, I.; Khan, A.H. A cross-sectional assessment of urinary tract infections among geriatric patients: Prevalence, medication regimen complexity, and factors associated with treatment outcomes. Front. Public Health 2021, 9, 657199. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Shi, Y.; Li, M.; Hu, X.; Bai, R. Trends in incidence of urinary tract infection in Mainland China from 1990 to 2019. Int. J. Gen. Med. 2021, 14, 1413–1420. [Google Scholar] [CrossRef]
- Almukhtar, S.H. Urinary tract infection among women aged (18–40) years old in Kirkuk City, Iraq. Open Nurs. J. 2018, 12, 248–254. [Google Scholar] [CrossRef]
- Saber, S.; Yasmin, N.; Alam, M.T.; Hossain, M.M.; Alam, R.F. Study on urinary tract infection among females of reproductive age group in tertiary care teaching hospital, Dhaka, Bangladesh. EJMED 2021, 3, 85–89. [Google Scholar] [CrossRef]
- Ku, J.H.; Bruxvoort, K.J.; Salas, S.B.; Varley, C.D.; Casey, J.A.; Raphael, E.; Robinson, S.C.; Nachman, K.E.; Lewin, B.J.; Contreras, R.; et al. Multidrug resistance of Escherichia coli from outpatient uncomplicated urinary tract infections in a large United States integrated healthcare organization. Open Forum Infect. Dis. 2023, 10, ofad287. [Google Scholar] [CrossRef] [PubMed]
- Naber, K.G.; Wagenlehner, F.; Kresken, M.; Cheng, W.Y.; Catillon, M.; Duh, M.S.; Yu, L.; Khanal, A.; Mulgirigama, A.; Joshi, A.V.; et al. Escherichia coli resistance, treatment patterns and clinical outcomes among females with uUTI in Germany: A retrospective physician-based chart review study. Sci. Rep. 2023, 13, 12077. [Google Scholar] [CrossRef] [PubMed]
- Dehbanipour, R.; Rastaghi, S.; Sedighi, M.; Maleki, N.; Faghri, J. High prevalence of multidrug-resistance uropathogenic Escherichia coli strains, Isfahan, Iran. J. Nat. Sci. Biol. Med. 2016, 7, 22–26. [Google Scholar] [PubMed]
- Miftode, I.L.; Nastase, E.V.; Miftode, R.Ș.; Miftode, E.G.; Iancu, L.S.; Luncă, C.; Anton Păduraru, D.T.; Costache, I.I.; Stafie, C.S.; Dorneanu, O.S. Insights into multidrug-resistant K. pneumoniae urinary tract infections: From susceptibility to mortality. Exp. Ther. Med. 2021, 22, 1086. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, H.; Pu, S.; Huang, S.; Niu, S. Resistance trends of Klebsiella pneumoniae causing urinary tract infections in Chongqing, 2011–2019. Infect. Drug Resist. 2021, 14, 475–481. [Google Scholar] [CrossRef]
- Ballén, V.; Gabasa, Y.; Ratia, C.; Ortega, R.; Tejero, M.; Soto, S. Antibiotic resistance and virulence profiles of Klebsiella pneumoniae strains isolated from different clinical sources. Front. Cell. Infect. Microbiol. 2021, 11, 738223. [Google Scholar] [CrossRef]
- Mo, L.; Wang, J.; Qian, J.; Peng, M. Antibiotic sensitivity of Proteus mirabilis urinary tract infection in patients with urinary calculi. Int. J. Clin. Pract. 2022, 2022, 7273627. [Google Scholar] [CrossRef]
- Khan, S.; Khan, S.-U.-H.; Wazir, W.; Khattak, M.; Khan, A.A.; Rasool, F.; Ali, S. Characterization of antibiotic resistance in Proteus species isolated from urinary tract infections in a tertiary care hospital. Int. J. Infect. Dis. 2021, 101, 353. [Google Scholar] [CrossRef]
- de Sousa, T.; Hébraud, M.; Alves, O.; Costa, E.; Maltez, L.; Pereira, J.E.; Martins, Â.; Igrejas, G.; Poeta, P. Study of antimicrobial resistance, biofilm formation, and motility of Pseudomonas aeruginosa derived from urine samples. Microorganisms 2023, 11, 1345. [Google Scholar] [CrossRef]
- Adejobi, A.; Ojo, O.; Alaka, O.; Odetoyin, B.; Onipede, A. Antibiotic resistance pattern of Pseudomonas spp. from patients in a tertiary hospital in South-West Nigeria. Germs 2021, 11, 238–245. [Google Scholar] [CrossRef]
- Shrestha, S.; Amatya, R.; Adhikari, R.P. Prevalence and antibiogram of Pseudomonas aeruginosa isolated from clinical specimens in a Teaching Hospital, Kathmandu. Int. J. Infect. Dis. 2016, 45, 115–116. [Google Scholar] [CrossRef]
- Kraszewska, Z.; Skowron, K.; Kwiecińska-Piróg, J.; Grudlewska-Buda, K.; Przekwas, J.; Wiktorczyk-Kapischke, N.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Antibiotic resistance of Enterococcus spp. isolated from the urine of patients hospitalized in the University Hospital in North-Central Poland, 2016–2021. Antibiotics 2022, 11, 1749. [Google Scholar] [CrossRef] [PubMed]
- Codelia-Anjum, A.; Lerner, L.B.; Elterman, D.; Zorn, K.C.; Bhojani, N.; Chughtai, B. Enterococcal urinary tract infections: A review of the pathogenicity, epidemiology, and treatment. Antibiotics 2023, 12, 778. [Google Scholar] [CrossRef] [PubMed]
- Salm, J.; Salm, F.; Arendarski, P.; Kramer, T.S. High antimicrobial resistance in urinary tract infections in male outpatients in routine laboratory data, Germany, 2015 to 2020. Euro Surveill. 2022, 27, 2101012. [Google Scholar] [CrossRef]
- Lin, E.; Bhusal, Y.; Horwitz, D.; Shelburne, S.A.; Trautner, B.W. Overtreatment of Enterococcal bacteriuria. Arch. Intern. Med. 2012, 172, 33–38. [Google Scholar] [CrossRef]
- Ezeh, C.K.; Eze, C.N.; Dibua, M.E.U.; Emencheta, S.C. A meta-analysis on the prevalence of resistance of Staphylococcus aureus to different antibiotics in Nigeria. Antimicrob. Resist. Infect Control. 2023, 12, 40. [Google Scholar] [CrossRef]
- Rafiee, M.; Tabarraei, A.; Yazdi, M.; Mohebbi, A.; Ghaemi, E.A. Antimicrobial resistance patterns of Staphylococcus saprophyticus isolates causing urinary tract infections in Gorgan, North of Iran. Med. Lab. J. 2023, 17, 33–38. [Google Scholar]
- Mareș, C.; Petca, R.-C.; Petca, A.; Popescu, R.-I.; Jinga, V. Does the COVID pandemic modify the antibiotic resistance of uropathogens in female patients? A new storm? Antibiotics 2022, 11, 376. [Google Scholar] [CrossRef]
| 18–29 Years | 30–45 Years | 46–59 Years | >60 Years | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Gram-negative | 21 | 3.6 | 48 | 9.1 | 92 | 17.5 | 258 | 49.2 | 417 | 79.5 |
| Escherichia coli | 14 | 2.6 | 31 | 5.9 | 60 | 11.4 | 185 | 35. | 290 | 55.3 |
| Klebsiella spp. | 3 | 0.5 | 12 | 2.2 | 22 | 4.1 | 41 | 7.8 | 78 | 14.8 |
| Proteus spp. | 2 | 0.3 | 5 | 0.9 | 6 | 1.1 | 18 | 3.4 | 31 | 5.9 |
| Pseudomonas spp. | - | - | - | - | 4 | 0.7 | 14 | 2.6 | 18 | 3.4 |
| Gram-positive | 9 | 1.7 | 9 | 1.7 | 25 | 4.7 | 64 | 12.2 | 107 | 20.4 |
| Enterococcus spp. | 6 | 1.1 | 4 | 0.7 | 19 | 3.6 | 53 | 10.1 | 82 | 15.6 |
| Staphylococcus spp. | 3 | 0.5 | 5 | 0.9 | 6 | 1.1 | 11 | 2.1 | 25 | 4.7 |
| Total | 28 | 5.3 | 57 | 10.8 | 117 | 22.3 | 322 | 61.4 | 524 | |
| Antibiotics | Gram-Negative Organism Isolated | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Escherichia coli | Klebsiella spp. | Proteus spp. | Pseudomonas spp. | |||||||||||||||||||||
| S | R | NA | S | R | NA | S | R | NA | S | R | NA | |||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |||||||||
| Amikacin | 229 | 78.9 | 54 | 18.6 | 7 | 2.4 | 71 | 91.0 | 7 | 8.9 | - | - | 31 | 100.0 | - | - | - | - | 12 | 66.6 | 5 | 27.7 | 1 | 5.5 |
| Amoxicillin-Clavulanic ac. | 192 | 66.2 | 96 | 33.1 | 2 | 0.6 | 47 | 60.2 | 28 | 35.8 | 3 | 3.8 | 22 | 70.9 | 7 | 22.5 | 2 | 6.4 | - | - | - | - | - | - |
| Trimethoprim-Sulfamethoxazole | 190 | 65.5 | 94 | 32.4 | 6 | 2.0 | 49 | 62.8 | 19 | 24.3 | 10 | 12.8 | 13 | 41.9 | 17 | 54.8 | 1 | 3.2 | - | - | - | - | - | - |
| Ceftazidime | 251 | 86.5 | 39 | 13.4 | - | - | 62 | 79.4 | 15 | 19.2 | 1 | 1.2 | 29 | 93.5 | 2 | 6.4 | - | - | 10 | 55.5 | 7 | 38.8 | 1 | 5.5 |
| Fosfomycin | 280 | 96.5 | - | - | 10 | 3.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Imipenem | 270 | 93.1 | 2 | 0.6 | 18 | 6.2 | 69 | 88.4 | 1 | 1.2 | 8 | 10.2 | 27 | 87.0 | 1 | 3.2 | 3 | 9.6 | 12 | 66.6 | 4 | 22.2 | 1 | 5.5 |
| Levofloxacin | 194 | 66.8 | 93 | 32.0 | 3 | 1.0 | 58 | 74.3 | 20 | 25.6 | - | - | 16 | 51.6 | 15 | 48.3 | - | - | 11 | 61.1 | 7 | 38.8 | - | - |
| Meropenem | 236 | 81.3 | 1 | 0.3 | 53 | 18.2 | 67 | 85.8 | 3 | 3.8 | 8 | 10.2 | 23 | 74.1 | - | - | 8 | 25.8 | 14 | 77.7 | 4 | 22.2 | - | - |
| Nitrofurantoin | 228 | 78.6 | 4 | 1.3 | 58 | 20.0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Antibiotics | Gram-Positive Organism Isolated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enterococcus spp. | Staphylococcus spp. | |||||||||||
| S | R | NA | S | R | NA | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Amikacin | - | - | - | - | - | - | 16 | 64.0 | 6 | 24.0 | 3 | 12.0 |
| Ampicillin | 70 | 85.3 | 11 | 13.4 | 1 | 1.2 | - | - | - | - | - | - |
| Trimethoprim-Sulfamethoxazole | - | - | - | - | - | - | 17 | 68.0 | 7 | 28.0 | 1 | 4.0 |
| Fosfomycin | 74 | 90.2 | 4 | 4.8 | 4 | 4.8 | - | - | - | - | - | - |
| Levofloxacin | 40 | 48.7 | 41 | 50.0 | 1 | 1.2 | 19 | 76.0 | 5 | 20.0 | 1 | 4.0 |
| Linezolid | 73 | 89.0 | 7 | 8.5 | 2 | 2.4 | 21 | 84.0 | 2 | 8.0 | 2 | 8.0 |
| Nitrofurantoin | 71 | 86.5 | 8 | 9.7 | 3 | 3.6 | 16 | 64.0 | 1 | 4.0 | 8 | 32.0 |
| Penicillin | 48 | 58.5 | 32 | 39.0 | 2 | 2.4 | 10 | 40.0 | 14 | 56.0 | 1 | 4.0 |
| Vancomycin | 66 | 80.4 | 11 | 13.4 | 5 | 6.0 | - | - | - | - | - | - |
| Antibiotics | Gram-Negative | Gram-Positive | Total | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | R | NA | S | R | NA | S | R | NA | ||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Amikacin | 343 | 82.2 | 66 | 15.8 | 1 | 0.2 | 16 | 64.0 | 6 | 24.0 | 3 | 12.0 | 359 | 81.2 | 72 | 16.2 | 4 | 0.9 |
| Amoxicillin-Clavulanic ac. | 261 | 65.4 | 131 | 32.8 | 7 | 1.7 | - | - | - | - | - | - | 261 | 65.4 | 131 | 32.8 | 7 | 1.7 |
| Ampicillin | - | - | - | - | - | - | 70 | 85.3 | 11 | 13.4 | 1 | 1.2 | 70 | 85.3 | 11 | 13.4 | 1 | 1.2 |
| Trimethoprim-Sulfamethoxazole | 252 | 63.1 | 130 | 32.5 | 17 | 4.2 | 17 | 68.0 | 7 | 28.0 | 1 | 4.0 | 269 | 63.4 | 137 | 32.3 | 18 | 4.2 |
| Ceftazidime | 352 | 84.4 | 63 | 15.1 | 2 | 0.4 | - | - | - | - | - | - | 352 | 84.4 | 63 | 15.1 | 2 | 0.4 |
| Fosfomycin | 280 | 96.5 | - | - | 10 | 3.4 | 74 | 90.2 | 4 | 4.8 | 4 | 4.8 | 354 | 95.1 | 4 | 1.07 | 14 | 3.7 |
| Imipenem | 378 | 90.6 | 8 | 1.91 | 30 | 7.1 | - | - | - | - | - | - | 378 | 90.6 | 8 | 1.91 | 30 | 7.1 |
| Levofloxacin | 279 | 66.9 | 135 | 32.3 | 3 | 0.7 | 59 | 55.1 | 46 | 42.9 | 2 | 1.8 | 338 | 64.5 | 181 | 34.5 | 5 | 0.9 |
| Linezolid | - | - | - | - | - | - | 94 | 87.8 | 9 | 8.4 | 4 | 3.7 | 94 | 87.8 | 9 | 8.4 | 4 | 3.7 |
| Meropenem | 340 | 81.5 | 8 | 1.9 | 69 | 16.5 | - | - | - | - | - | - | 340 | 81.5 | 8 | 1.9 | 69 | 16.5 |
| Nitrofurantoin | 228 | 78.6 | 4 | 1.3 | 58 | 20.0 | 87 | 81.3 | 9 | 8.4 | 11 | 10.2 | 315 | 79.3 | 13 | 3.2 | 69 | 17.3 |
| Penicillin | - | - | - | - | - | - | 58 | 54.2 | 46 | 42.9 | 3 | 2.8 | 58 | 54.2 | 46 | 42.9 | 3 | 2.8 |
| Vancomycin | - | - | - | - | - | - | 66 | 80.4 | 11 | 13.4 | 5 | 6.0 | 66 | 80.4 | 11 | 13.4 | 5 | 6.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mareș, C.; Petca, R.-C.; Popescu, R.-I.; Petca, A.; Mulțescu, R.; Bulai, C.A.; Ene, C.V.; Geavlete, P.A.; Geavlete, B.F.; Jinga, V. Update on Urinary Tract Infection Antibiotic Resistance—A Retrospective Study in Females in Conjunction with Clinical Data. Life 2024, 14, 106. https://doi.org/10.3390/life14010106
Mareș C, Petca R-C, Popescu R-I, Petca A, Mulțescu R, Bulai CA, Ene CV, Geavlete PA, Geavlete BF, Jinga V. Update on Urinary Tract Infection Antibiotic Resistance—A Retrospective Study in Females in Conjunction with Clinical Data. Life. 2024; 14(1):106. https://doi.org/10.3390/life14010106
Chicago/Turabian StyleMareș, Cristian, Răzvan-Cosmin Petca, Răzvan-Ionuț Popescu, Aida Petca, Răzvan Mulțescu, Cătălin Andrei Bulai, Cosmin Victor Ene, Petrișor Aurelian Geavlete, Bogdan Florin Geavlete, and Viorel Jinga. 2024. "Update on Urinary Tract Infection Antibiotic Resistance—A Retrospective Study in Females in Conjunction with Clinical Data" Life 14, no. 1: 106. https://doi.org/10.3390/life14010106
APA StyleMareș, C., Petca, R.-C., Popescu, R.-I., Petca, A., Mulțescu, R., Bulai, C. A., Ene, C. V., Geavlete, P. A., Geavlete, B. F., & Jinga, V. (2024). Update on Urinary Tract Infection Antibiotic Resistance—A Retrospective Study in Females in Conjunction with Clinical Data. Life, 14(1), 106. https://doi.org/10.3390/life14010106











