Dermatophyte Infections Worldwide: Increase in Incidence and Associated Antifungal Resistance
Abstract
:1. Introduction
2. Epidemiology of Dermatophyte Infections
3. Clinical Perspectives of Tinea
4. Standard Treatment of Tinea Infections and Current Limitations
5. Treatment Failure and Diagnostic Challenges
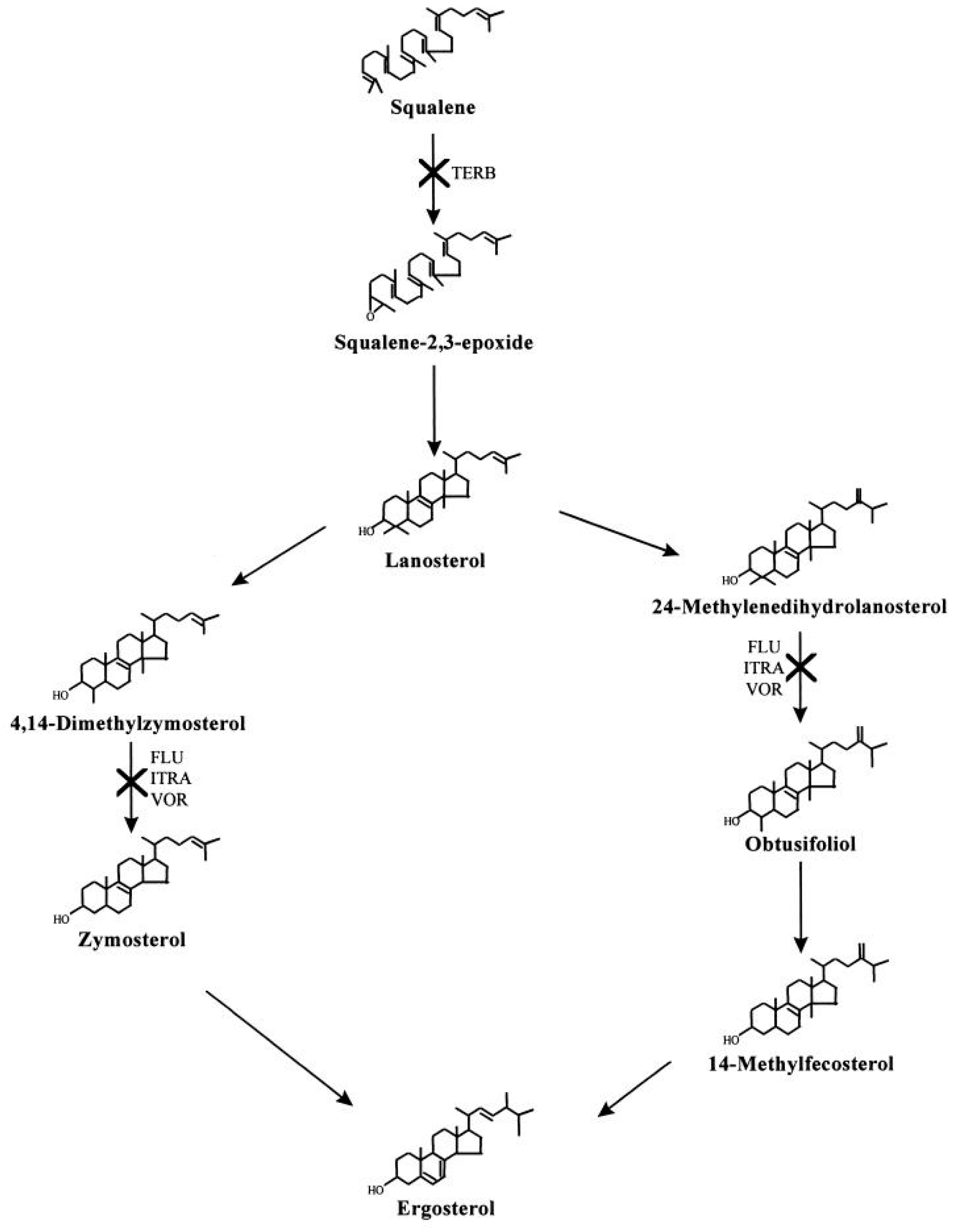
6. Emergence of Drug Resistant Organisms
7. The Impact of Increasing Trends of Fungal Infections and Growing Antifungal Resistance
8. Management Prospective and Alternative Treatments
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51 (Suppl. 4), 2–15. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, I.; Summerbell, R.C. The dermatophytes. Clin. Microbiol. Rev. 1995, 8, 240–259. [Google Scholar] [CrossRef] [PubMed]
- Panackal, A.A.; Halpern, E.F.; Watson, A.J. Cutaneous fungal infections in the United States: Analysis of the National Ambulatory Medical Care Survey (NAMCS) and National Hospital Ambulatory Medical Care Survey (NHAMCS), 1995–2004. Int. J. Dermatol. 2009, 48, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.R.; Lim, H.W.; Margolis, D.; Weinstock, M.A.; Goodman, C.; Faulkner, E.; Gould, C.; Gemmen, E.; Dall, T. The burden of skin diseases: 2004: A joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J. Am. Acad. Dermatol. 2006, 55, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Vena, G.A.; Chieco, P.; Posa, F.; Garofalo, A.; Bosco, A.; Cassano, N. Epidemiology of dermatophytoses: Retrospective analysis from 2005 to 2010 and comparison with previous data from 1975. New Microbiol. 2012, 35, 207–213. [Google Scholar] [PubMed]
- Ely, J.W.; Rosenfeld, S.; Seabury Stone, M. Diagnosis and management of tinea infections. Am. Fam. Physician 2014, 90, 702–710. [Google Scholar] [PubMed]
- Kemna, M.E.; Elewski, B.E. A U.S. epidemiologic survey of superficial fungal diseases. J. Am. Acad. Dermatol. 1996, 35, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Boddy, L. Chapter 9—Interactions with Humans and Other Animals. In The Fungi, 3rd ed.; Watkinson, S.C., Boddy, L., Money, N.P., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 293–336. [Google Scholar] [CrossRef]
- Abd Elmegeed, A.S.; Ouf, S.A.; Moussa, T.A.; Eltahlawi, S.M. Dermatophytes and other associated fungi in patients attending to some hospitals in Egypt. Braz. J. Microbiol. 2015, 46, 799–805. [Google Scholar] [CrossRef]
- de Hoog, G.S.; Dukik, K.; Monod, M.; Packeu, A.; Stubbe, D.; Hendrickx, M.; Kupsch, C.; Stielow, J.B.; Freeke, J.; Goker, M.; et al. Toward a Novel Multilocus Phylogenetic Taxonomy for the Dermatophytes. Mycopathologia 2017, 182, 5–31. [Google Scholar] [CrossRef]
- White, T.C.; Findley, K.; Dawson, T.L., Jr.; Scheynius, A.; Boekhout, T.; Cuomo, C.A.; Xu, J.; Saunders, C.W. Fungi on the skin: Dermatophytes and Malassezia. Cold Spring Harb. Perspect. Med. 2014, 4, a019802. [Google Scholar] [CrossRef]
- Brasch, J. Current knowledge of host response in human tinea. Mycoses 2009, 52, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Uprety, S. The menace of chronic and recurrent dermatophytosis in India: Is the problem deeper than we perceive? Indian. Dermatol. Online J. 2016, 7, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Pathania, S.; Rudramurthy, S.M.; Narang, T.; Saikia, U.N.; Dogra, S. A prospective study of the epidemiological and clinical patterns of recurrent dermatophytosis at a tertiary care hospital in India. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Verma, P.; Chandra, U.; Tiwary, N.K. Risk factors for chronic and chronic-relapsing tinea corporis, tinea cruris and tinea faciei: Results of a case-control study. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Canavan, T.N.; Elewski, B.E. Identifying Signs of Tinea Pedis: A Key to Understanding Clinical Variables. J. Drugs Dermatol. 2015, 14, s42–s47. [Google Scholar] [PubMed]
- Appelt, L.; Nenoff, P.; Uhrlass, S.; Kruger, C.; Kuhn, P.; Eichhorn, K.; Buder, S.; Beissert, S.; Abraham, S.; Aschoff, R.; et al. Terbinafine-resistant dermatophytoses and onychomycosis due to Trichophyton rubrum. Hautarzt 2021, 72, 868–877. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Mda, G.; Santana, G.B.; Criado, P.R.; Benard, G. Chronic widespread dermatophytosis due to Trichophyton rubrum: A syndrome associated with a Trichophyton-specific functional defect of phagocytes. Front. Microbiol. 2015, 6, 801. [Google Scholar] [CrossRef]
- Hryncewicz-Gwozdz, A.; Kalinowska, K.; Plomer-Niezgoda, E.; Bielecki, J.; Jagielski, T. Increase in resistance to fluconazole and itraconazole in Trichophyton rubrum clinical isolates by sequential passages in vitro under drug pressure. Mycopathologia 2013, 176, 49–55. [Google Scholar] [CrossRef]
- Muller, V.L.; Kappa-Markovi, K.; Hyun, J.; Georgas, D.; Silberfarb, G.; Paasch, U.; Uhrlass, S.; Nenoff, P.; Schaller, J. Tinea capitis et barbae caused by Trichophyton tonsurans: A retrospective cohort study of an infection chain after shavings in barber shops. Mycoses 2021, 64, 428–436. [Google Scholar] [CrossRef]
- Ghannoum, M.; Isham, N.; Hajjeh, R.; Cano, M.; Al-Hasawi, F.; Yearick, D.; Warner, J.; Long, L.; Jessup, C.; Elewski, B. Tinea capitis in Cleveland: Survey of elementary school students. J. Am. Acad. Dermatol. 2003, 48, 189–193. [Google Scholar] [CrossRef]
- Pilz, J.F.; Koberle, M.; Kain, A.; Seidl, P.; Zink, A.; Biedermann, T.; Pilz, A.C. Increasing incidence of Trichophyton tonsurans in Munich-A single-centre observation. Mycoses 2023, 66, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Alkeswani, A.; Cantrell, W.; Elewski, B. Treatment of Tinea Capitis. Skin. Appendage Disord. 2019, 5, 201–210. [Google Scholar] [CrossRef]
- Al Aboud, A.M.; Crane, J.S. Tinea Capitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; Available online: https://www.ncbi.nlm.nih.gov/books/NBK536909/ (accessed on 14 August 2023).
- Takenaka, M.; Murota, H.; Nishimoto, K. Epidemiological survey of 42 403 dermatophytosis cases examined at Nagasaki University Hospital from 1966 to 2015. J. Dermatol. 2020, 47, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Chaudhry, M.; Elewski, B. Tinea corporis, tinea cruris, tinea nigra, and piedra. Dermatol. Clin. 2003, 21, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Kim, S.L.; Jang, Y.H.; Lee, S.J.; Kim, D.W.; Bang, Y.J.; Jun, J.B. Increasing Prevalence of Trichophyton rubrum Identified through an Analysis of 115,846 Cases over the Last 37 Years. J. Korean Med. Sci. 2015, 30, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.K.; Mahajan, R. Management of tinea corporis, tinea cruris, and tinea pedis: A comprehensive review. Indian Dermatol. Online J. 2016, 7, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Moskaluk, A.E.; VandeWoude, S. Current Topics in Dermatophyte Classification and Clinical Diagnosis. Pathogens 2022, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Zhang, L.L.; Liu, Z.H. Tinea Faciei. J. Pediatr. 2022, 250, 108–109. [Google Scholar] [CrossRef]
- Zhan, P.; Geng, C.; Li, Z.; Jiang, Q.; Jin, Y.; Li, C.; Liu, W. The epidemiology of tinea manuum in Nanchang area, South China. Mycopathologia 2013, 176, 83–88. [Google Scholar] [CrossRef]
- Kuruvella, T.; Pandey, S. Tinea Barbae. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chamorro, M.J.; House, S.A. Tinea Manuum. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hawkins, D.M.; Smidt, A.C. Superficial fungal infections in children. Pediatr. Clin. N. Am. 2014, 61, 443–455. [Google Scholar] [CrossRef]
- Kaushik, N.; Pujalte, G.G.; Reese, S.T. Superficial Fungal Infections. Prim. Care 2015, 42, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Ameen, M. Epidemiology of superficial fungal infections. Clin. Dermatol. 2010, 28, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, G.; Jain, H.C.; Lynde, C.W.; Foley, K.A.; Daigle, D.; Cooper, E.A.; Summerbell, R.C. The prevalence of unsuspected onychomycosis and its causative organisms in a multicentre Canadian sample of 30,000 patients visiting physicians’ offices. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, M.; Makimura, K.; Sato, K.; Abe, S.; Tsuboi, R. Molecular detection of dermatophytes and nondermatophytes in onychomycosis by nested polymerase chain reaction based on 28S ribosomal RNA gene sequences. Br. J. Dermatol. 2009, 161, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Chadeganipour, M.; Mohammadi, R. Causative Agents of Onychomycosis: A 7-Year Study. J. Clin. Lab. Anal. 2016, 30, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, C.; Bouchara, J.P.; Mignon, B. Updates on the epidemiology of dermatophyte infections. Mycopathologia 2008, 166, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Shemer, A.; Grunwald, M.H.; Davidovici, B.; Nathansohn, N.; Amichai, B. A novel two-step kit for topical treatment of tinea pedis—An open study. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1099–1101. [Google Scholar] [CrossRef]
- Ozturk, P.; Arican, O.; Kurutas, E.B.; Karakas, T.; Gungor, M. Local oxidative stress in interdigital tinea pedis. J. Dermatol. 2013, 40, 114–117. [Google Scholar] [CrossRef]
- Mirmirani, P.; Tucker, L.Y. Epidemiologic trends in pediatric tinea capitis: A population-based study from Kaiser Permanente Northern California. J. Am. Acad. Dermatol. 2013, 69, 916–921. [Google Scholar] [CrossRef]
- Fuller, L.C.; Child, F.C.; Midgley, G.; Higgins, E.M. Scalp ringworm in south-east London and an analysis of a cohort of patients from a paediatric dermatology department. Br. J. Dermatol. 2003, 148, 985–988. [Google Scholar] [CrossRef]
- Hay, R.J.; Ashbee, H.R. Mycology. In Rook’s Textbook of Dermatology; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 1–93. [Google Scholar] [CrossRef]
- Yee, G.; Al Aboud, A.M. Tinea Corporis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Pippin, M.M.; Madden, M.L.; Das, M. Tinea Cruris. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Julapalli, M.R.; Levy, M.L. Chapter 67—Viral and Fungal Skin Infections. In Feigin and Cherry’s Textbook of Pediatric Infectious Diseases, 6th ed.; Feigin, R.D., Cherry, J.D., Demmler-Harrison, G.J., Kaplan, S.L., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2009; pp. 794–809. [Google Scholar] [CrossRef]
- Schuller, J.; Remme, J.J.; Rampen, F.H.; Van Neer, F.C. Itraconazole in the treatment of tinea pedis and tinea manuum: Comparison of two treatment schedules. Mycoses 1998, 41, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.A.; Dinehart, S.M.; Farmer, E.R.; Goltz, R.W.; Graham, G.F.; Hardinsky, M.K.; Lewis, C.W.; Pariser, D.M.; Skouge, J.W.; Webster, S.B.; et al. Guidelines of care for superficial mycotic infections of the skin: Tinea corporis, tinea cruris, tinea faciei, tinea manuum, and tinea pedis. Guidelines/Outcomes Committee. American Academy of Dermatology. J. Am. Acad. Dermatol. 1996, 34, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Rand, S. Overview: The treatment of dermatophytosis. J. Am. Acad. Dermatol. 2000, 43, S104–S112. [Google Scholar] [CrossRef] [PubMed]
- Korting, H.C.; Schollmann, C. The significance of itraconazole for treatment of fungal infections of skin, nails and mucous membranes. J. Dtsch. Dermatol. Ges. 2009, 7, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zeichner, J.A. New Topical Therapeutic Options in the Management of Superficial Fungal Infections. J. Drugs Dermatol. 2015, 14, s35–s41. [Google Scholar]
- Gupta, A.K.; Cooper, E.A. Update in antifungal therapy of dermatophytosis. Mycopathologia 2008, 166, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Leelavathi, M.; Noorlaily, M. Onychomycosis nailed. Malays Fam. Physician 2014, 9, 2–7. [Google Scholar]
- Gupta, A.K.; Simpson, F.C. Efinaconazole: A new topical treatment for onychomycosis. Ski. Ther. Lett. 2014, 19, 1–4. [Google Scholar]
- Rodriguez, D.A. Efinaconazole Topical Solution, 10%, for the Treatment of Mild and Moderate Toenail Onychomycosis. J. Clin. Aesthet. Dermatol. 2015, 8, 24–29. [Google Scholar]
- Poulakos, M.; Grace, Y.; Machin, J.D.; Dorval, E. Efinaconazole and Tavaborole. J. Pharm. Pract. 2017, 30, 245–255. [Google Scholar] [CrossRef]
- Gamal, A.; Elshaer, M.; Long, L.; McCormick, T.S.; Elewski, B.; Ghannoum, M.A. Antifungal Activity of Efinaconazole Compared to Fluconazole, Itraconazole, and Terbinafine against Terbinafine- and Itraconazole-Resistant and -Susceptible Clinical Isolates of Dermatophytes, Candida, and Mold. J. Am. Podiatr. Med. Assoc. 2023, 113, e1–e30. [Google Scholar] [CrossRef] [PubMed]
- Ghelardi, E.; Bulgheroni, A.; Mailland, F.; Celandroni, F.; Gueye, S.A. In vitro evaluation of the potential of ciclopirox to induce resistance in Trichophyton rubrum, in comparison to terbinafine, amorolfine, and itraconazole. J. Am. Acad. Dermatol. 2013, 68, 106. [Google Scholar] [CrossRef]
- Ryder, N.S.; Wagner, S.; Leitner, I. In vitro activities of terbinafine against cutaneous isolates of Candida albicans and other pathogenic yeasts. Antimicrob. Agents Chemother. 1998, 42, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Kovitwanichkanont, T.; Chong, A.H. Superficial fungal infections. Aust. J. Gen. Pract. 2019, 48, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Kreijkamp-Kaspers, S.; Hawke, K.; Guo, L.; Kerin, G.; Bell-Syer, S.E.; Magin, P.; Bell-Syer, S.V.; van Driel, M.L. Oral antifungal medication for toenail onychomycosis. Cochrane Database Syst. Rev. 2017, 7, CD010031. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.E.; Prouty, M. Terbinafine used safely in autoimmune hepatitis for treatment of tinea corporis. BMJ Case Rep. 2021, 14, e243143. [Google Scholar] [CrossRef] [PubMed]
- Itraconazole. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Puttick, M.P.; Phillips, P. Itraconazole: Precautions regarding drug interactions and bioavailability. Can. J. Infect. Dis. 1994, 5, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Lipner, S.R.; Scher, R.K. Onychomycosis: Treatment and prevention of recurrence. J. Am. Acad. Dermatol. 2019, 80, 853–867. [Google Scholar] [CrossRef]
- Bishnoi, A.; Vinay, K.; Dogra, S. Emergence of recalcitrant dermatophytosis in India. Lancet Infect. Dis. 2018, 18, 250–251. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Mukherjee, P.K.; Warshaw, E.M.; Evans, S.; Korman, N.J.; Tavakkol, A. Molecular analysis of dermatophytes suggests spread of infection among household members. Cutis 2013, 91, 237–245. [Google Scholar]
- Rex, J.H.; Walsh, T.J.; Sobel, J.D.; Filler, S.G.; Pappas, P.G.; Dismukes, W.E.; Edwards, J.E. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin. Infect. Dis. 2000, 30, 662–678. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Weyers, W.; Diaz-Cascajo, C.; Weyers, I. Erythema annulare centrifugum: Results of a clinicopathologic study of 73 patients. Am. J. Dermatopathol. 2003, 25, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Al Hasan, M.; Fitzgerald, S.M.; Saoudian, M.; Krishnaswamy, G. Dermatology for the practicing allergist: Tinea pedis and its complications. Clin. Mol. Allergy 2004, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E. Onychomycosis: Pathogenesis, diagnosis, and management. Clin. Microbiol. Rev. 1998, 11, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Masih, A.; Khurana, A.; Singh, P.K.; Gupta, M.; Hagen, F.; Meis, J.F.; Chowdhary, A. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses 2018, 61, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Gamage, H.; Sivanesan, P.; Hipler, U.C.; Elsner, P.; Wiegand, C. Superficial fungal infections in the department of dermatology, University Hospital Jena: A 7-year retrospective study on 4556 samples from 2007 to 2013. Mycoses 2020, 63, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, J.; Noguchi, H.; Hase, M.; Tokuhisa, Y.; Shimizu, T.; Ogawa, T.; Hiruma, M.; Harada, K.; Kano, R. Epidemiological study of terbinafine-resistant dermatophytes isolated from Japanese patients. J. Dermatol. 2021, 48, 564–567. [Google Scholar] [CrossRef]
- Jia, S.; Long, X.; Hu, W.; Zhu, J.; Jiang, Y.; Ahmed, S.; de Hoog, G.S.; Liu, W.; Jiang, Y. The epidemic of the multiresistant dermatophyte Trichophyton indotineae has reached China. Front. Immunol. 2022, 13, 1113065. [Google Scholar] [CrossRef]
- Caplan, A.S.; Chaturvedi, S.; Zhu, Y.; Todd, G.C.; Yin, L.; Lopez, A.; Travis, L.; Smith, D.J.; Chiller, T.; Lockhart, S.R.; et al. Notes from the Field: First Reported U.S. Cases of Tinea Caused by Trichophyton indotineae—New York City, December 2021–March 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 536–537. [Google Scholar] [CrossRef]
- Chen, E.; Ghannoum, M.; Elewski, B.E. Treatment-resistant tinea corporis, a potential public health issue. Br. J. Dermatol. 2021, 184, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.J.; Arendrup, M.C.; Verma, S.; Saunte, D.M.L. The Emerging Terbinafine-Resistant Trichophyton Epidemic: What Is the Role of Antifungal Susceptibility Testing? Dermatology 2022, 238, 60–79. [Google Scholar] [CrossRef] [PubMed]
- Saunte, D.M.L.; Hare, R.K.; Jorgensen, K.M.; Jorgensen, R.; Deleuran, M.; Zachariae, C.O.; Thomsen, S.F.; Bjornskov-Halkier, L.; Kofoed, K.; Arendrup, M.C. Emerging Terbinafine Resistance in Trichophyton: Clinical Characteristics, Squalene Epoxidase Gene Mutations, and a Reliable EUCAST Method for Detection. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Maeda, M.; Alshahni, M.M.; Tanaka, R.; Yaguchi, T.; Bontems, O.; Salamin, K.; Fratti, M.; Monod, M. Terbinafine Resistance of Trichophyton Clinical Isolates Caused by Specific Point Mutations in the Squalene Epoxidase Gene. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Yaguchi, T.; Maeda, M.; Alshahni, M.M.; Salamin, K.; Guenova, E.; Feuermann, M.; Monod, M. Gene Amplification of CYP51B: A New Mechanism of Resistance to Azole Compounds in Trichophyton indotineae. Antimicrob. Agents Chemother. 2022, 66, e0005922. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, P.; Dingemans, G.; Meis, J.F.; Chowdhary, A. Evaluation of DermaGenius® resistance real-time polymerase chain reaction for rapid detection of terbinafine-resistant Trichophyton species. Mycoses 2021, 64, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Das, S.; Tigga, R.; Pandey, R.; Bhattacharya, S.N.; Taneja, B. Whole genome sequences of two Trichophyton indotineae clinical isolates from India emerging as threats during therapeutic treatment of dermatophytosis. 3 Biotech 2021, 11, 402. [Google Scholar] [CrossRef]
- Ghannoum, M. Azole Resistance in Dermatophytes: Prevalence and Mechanism of Action. J. Am. Podiatr. Med. Assoc. 2016, 106, 79–86. [Google Scholar] [CrossRef]
- Rayens, E.; Norris, K.A. Prevalence and Healthcare Burden of Fungal Infections in the United States, 2018. Open Forum Infect. Dis. 2022, 9, ofab593. [Google Scholar] [CrossRef]
- Marconi, V.C.; Kradin, R.; Marty, F.M.; Hospenthal, D.R.; Kotton, C.N. Disseminated dermatophytosis in a patient with hereditary hemochromatosis and hepatic cirrhosis: Case report and review of the literature. Med. Mycol. 2010, 48, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Pathan, S.; Vincent, Q.B.; Liu, L.; Cypowyj, S.; Prando, C.; Migaud, M.; Taibi, L.; Ammar-Khodja, A.; Stambouli, O.B.; et al. Deep dermatophytosis and inherited CARD9 deficiency. N. Engl. J. Med. 2013, 369, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Huang, C.; Zhang, Y.; Li, R. Invasive dermatophyte infection: A systematic review. Mycoses 2021, 64, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Tack, D.A.; Fleishcer, A., Jr.; McMichael, A.; Feldman, S. The epidemic of tinea capitis disproportionately affects school-aged African Americans. Pediatr. Dermatol. 1999, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Coloe, J.R.; Diab, M.; Moennich, J.; Diab, D.; Pawaskar, M.; Balkrishnan, R.; Bechtel, M.A. Tinea capitis among children in the Columbus area, Ohio, USA. Mycoses 2010, 53, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Hall, D.C.; Cooper, E.A.; Ghannoum, M.A. Diagnosing Onychomycosis: What’s New? J. Fungi 2022, 8, 464. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Venkataraman, M.; Hall, D.C.; Cooper, E.A.; Summerbell, R.C. The emergence of Trichophyton indotineae: Implications for clinical practice. Int. J. Dermatol. 2023, 62, 857–861. [Google Scholar] [CrossRef]
- Bieber, K.; Harder, M.; Stander, S.; Boch, K.; Kridin, K.; Kohler, B.; Anemuller, W.; Ernst, A.L.; Zillikens, D.; Cavalar, M.; et al. DNA chip-based diagnosis of onychomycosis and tinea pedis. J. Dtsch. Dermatol. Ges. 2022, 20, 1112–1121. [Google Scholar] [CrossRef]
- Kidd, S.E.; Weldhagen, G.F. Diagnosis of dermatophytes: From microscopy to direct PCR. J. Microbiol. Aust. 2022, 43, 9–13. [Google Scholar] [CrossRef]
- FDA. LUZU (Luliconazole) Cream, 1%. 2013. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204153s000lbl.pdf (accessed on 5 October 2023).
- FDA. JUBLIA® (Efinaconazole) Topical Solution, 10%. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/203567s000lbl.pdf (accessed on 7 November 2022).
- Wiederhold, N.P.; Fothergill, A.W.; McCarthy, D.I.; Tavakkol, A. Luliconazole demonstrates potent in vitro activity against dermatophytes recovered from patients with onychomycosis. Antimicrob. Agents Chemother. 2014, 58, 3553–3555. [Google Scholar] [CrossRef]
- Jerajani, H.; Janaki, C.; Kumar, S.; Phiske, M. Comparative assessment of the efficacy and safety of sertaconazole (2%) cream versus terbinafine cream (1%) versus luliconazole (1%) cream in patients with dermatophytoses: A pilot study. Indian J. Dermatol. 2013, 58, 34–38. [Google Scholar] [CrossRef] [PubMed]
- FDA. KERYDIN® (Tavaborole) Topical Solution, 5%. 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/204427s006lbl.pdf (accessed on 28 August 2023).
- Sharma, N.; Sharma, D. An upcoming drug for onychomycosis: Tavaborole. J. Pharmacol. Pharmacother. 2015, 6, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Ciaravino, V.; Coronado, D.; Lanphear, C.; Shaikh, I.; Ruddock, W.; Chanda, S. Tavaborole, a novel boron-containing small molecule for the topical treatment of onychomycosis, is noncarcinogenic in 2-year carcinogenicity studies. Int. J. Toxicol. 2014, 33, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Scher, R.K.; Nakamura, N.; Tavakkol, A. Luliconazole: A review of a new antifungal agent for the topical treatment of onychomycosis. Mycoses 2014, 57, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kishida, H.; Okubo, A. Efficacy and safety of luliconazole 5% nail solution for the treatment of onychomycosis: A multicenter, double-blind, randomized phase III study. J. Dermatol. 2017, 44, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Rich, P.; Spellman, M.; Purohit, V.; Zang, C.; Crook, T.J. Tavaborole 5% Topical Solution for the Treatment of Toenail Onychomycosis in Pediatric Patients: Results from a Phase 4 Open-Label Study. J. Drugs Dermatol. 2019, 18, 190–195. [Google Scholar] [PubMed]
- Elewski, B.E.; Aly, R.; Baldwin, S.L.; Gonzalez Soto, R.F.; Rich, P.; Weisfeld, M.; Wiltz, H.; Zane, L.T.; Pollak, R. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: Results from 2 randomized phase-III studies. J. Am. Acad. Dermatol. 2015, 73, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Francuzik, W.; Fritz, K.; Salavastru, C. Laser therapies for onychomycosis—Critical evaluation of methods and effectiveness. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 936–942. [Google Scholar] [CrossRef]
- Nijenhuis-Rosien, L.; Kleefstra, N.; Wolfhagen, M.J.; Groenier, K.H.; Bilo, H.J.; Landman, G.W. Laser therapy for onychomycosis in patients with diabetes at risk for foot complications: Study protocol for a randomized, double-blind, controlled trial (LASER-1). Trials 2015, 16, 108. [Google Scholar] [CrossRef]
- Gupta, A.K.; Versteeg, S.G. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1111–1118. [Google Scholar] [CrossRef]
| Tinea Infection | Body Area Affected | Most Common Causative Pathogens |
|---|---|---|
| Tinea Capitis | Head and scalp | T. tonsurans, Microsporum canis [11] |
| Tinea Corporis | Trunk and extremities | T. rubrum, T. mentagrophytes, T. tonsurans [25] |
| Tinea Cruris | Groin, pubic region, intertriginous anogenital region | T. rubrum, T. mentagrophytes [26,27,28] |
| Tinea Faciei | Face | T. rubrum, T. mentagrophytes [30,31] |
| Tinea Barbae | Beard and mustache area | T. verrucosum, T. rubrum, T. mentagrophytes [32] |
| Tinea Manuum | Hands | T. rubrum [33] |
| Tinea Pedis | Feet | T. rubrum, T. mentagrophytes, Epidermophyton floccosum [18,34,35,36] |
| Onychomycosis (Tinea Unguium) | Nails | T. rubrum, T. mentagrophytes [37,38] |
| Tinea Infection | Systemic Therapy | Local Therapy |
|---|---|---|
| Tinea Capitis [24] | Terbinafine or Griseofulvin. If kerion is present, add steroids. | Not recommended. Itraconazole or Fluconazole may be used in some cases. |
| Tinea Corporis [45,46] | Indicated for severe infection caused by T. rubrum. Terbinafine, Itraconazole, Fluconazole, or Griseofulvin. Terbinafine is indicated for Majocchi Granuloma. | Azoles or Allylamines |
| Tinea Cruris [27,47] | Indicated for chronic or recurrent infection. Terbinafine, Itraconazole, or Fluconazole. | Azoles or Allylamines |
| Tinea Faciei [30,48] | Indicated for severe or refractory infection or involvement of vellus hair | Azoles or Allylamines |
| Tinea Barbae [32] | Terbinafine, Itraconazole, Fluconazole, or Ketoconazole | Azoles or Allylamines as adjunct therapy |
| Tinea Manuum [33,49,50] | Indicated for co-infection of the nail, two feet-one hand syndrome, and chronic or recurrent infection. Terbinafine or Itraconazole may be effective. | Azoles or Allylamines |
| Tinea Pedis [6,50,51,52,53] | Indicated for treatment-resistant infection. Terbinafine, Itraconazole, Fluconazole, Ketoconazole, or Griseofulvin. | Indicated for uncomplicated or mild interdigital infection. Azoles or Allylamines. Luliconazole or Naftifine may be used for interdigital infection. Initial treatment with topical corticosteroids may be beneficial. |
| Onychomycosis (Tinea Unguium) [54,55,56,57,58,59] | Indicated for moderate to severe infection. Terbinafine or Itraconazole. Avoid Griseofulvin (lower efficacy) and Ketoconazole (hepatotoxicity). | Indicated for mild to moderate infection. Efinaconazole, Ciclopirox, or Amorolfine. |
| Dermatophyte Pathogen | Resistance Mechanisms | Primary Associated Antifungal(s) |
|---|---|---|
| T. indotineae | F397L, L393F, F415S, or H440Y squalene epoxidase gene point mutations [84,86], Ala448Thr amino acid substitution in erg1 [87], overexpression of TinCYP51B gene [85] | Terbinafine, Itraconazole, Voriconazole |
| T. interdigitale | F397L, L393F, F415S, H440Y F484Y or I121M V237I squalene epoxidase gene point mutations [81] | Terbinafine |
| T. mentagrophytes | F397L or L393F squalene epoxidase gene point mutations [81] | Terbinafine |
| T. rubrum | F397L, L393F, F415S, H440Y F484Y or I121M V237I squalene epoxidase gene point mutations [81], azole efflux pump (i.e., overexpression of TruMDR2 and TruMDR3 genes) [85] | Terbinafine, Itraconazole, Voriconazole |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruithoff, C.; Gamal, A.; McCormick, T.S.; Ghannoum, M.A. Dermatophyte Infections Worldwide: Increase in Incidence and Associated Antifungal Resistance. Life 2024, 14, 1. https://doi.org/10.3390/life14010001
Kruithoff C, Gamal A, McCormick TS, Ghannoum MA. Dermatophyte Infections Worldwide: Increase in Incidence and Associated Antifungal Resistance. Life. 2024; 14(1):1. https://doi.org/10.3390/life14010001
Chicago/Turabian StyleKruithoff, Caroline, Ahmed Gamal, Thomas S. McCormick, and Mahmoud A. Ghannoum. 2024. "Dermatophyte Infections Worldwide: Increase in Incidence and Associated Antifungal Resistance" Life 14, no. 1: 1. https://doi.org/10.3390/life14010001
APA StyleKruithoff, C., Gamal, A., McCormick, T. S., & Ghannoum, M. A. (2024). Dermatophyte Infections Worldwide: Increase in Incidence and Associated Antifungal Resistance. Life, 14(1), 1. https://doi.org/10.3390/life14010001







