The Role of Microglial Exosomes and miR-124-3p in Neuroinflammation and Neuronal Repair after Traumatic Brain Injury
Abstract
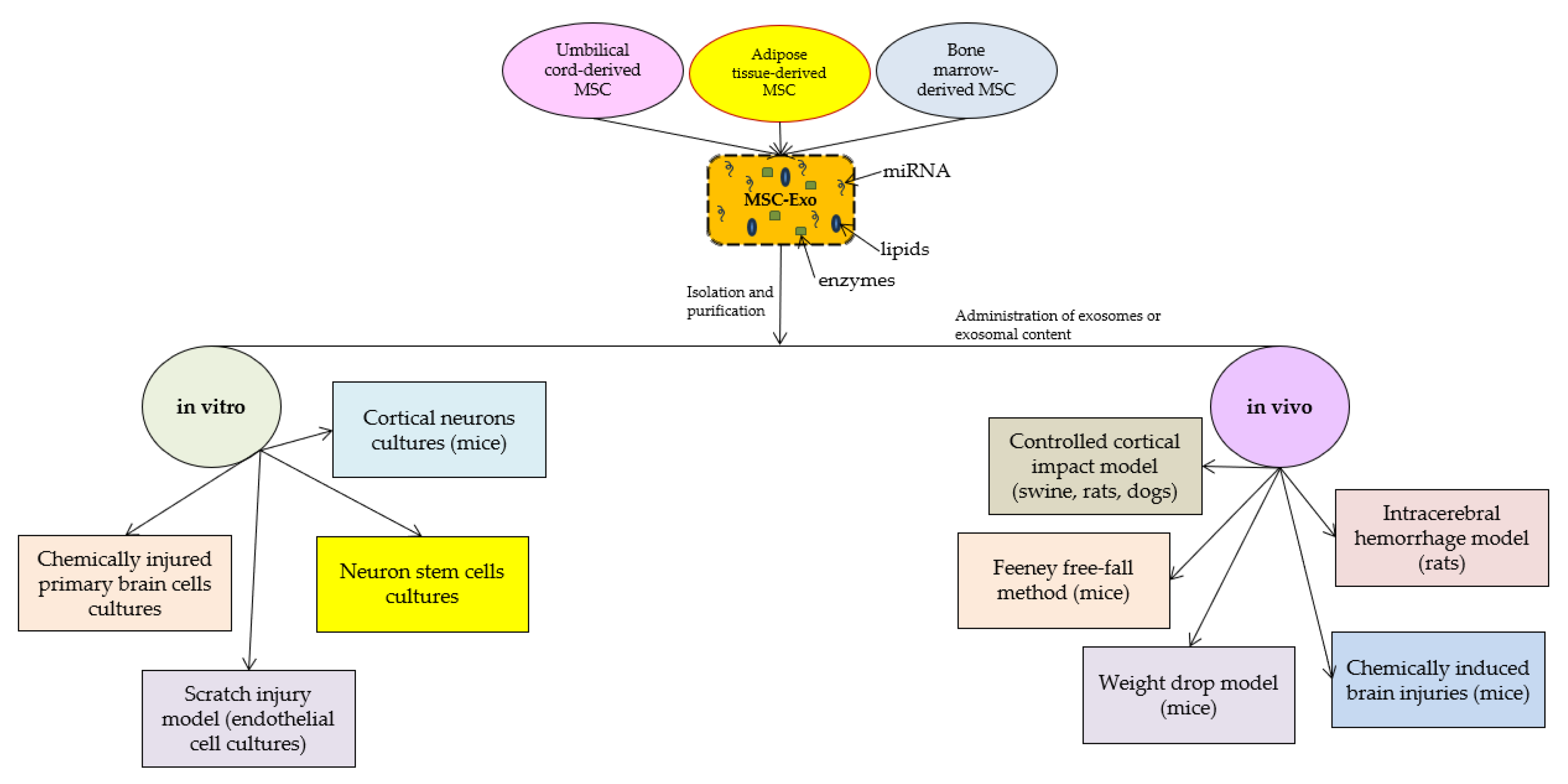
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Analysis
2.4. Data Synthesis
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Edgar, J.R. Q&A: What are exosomes, exactly? BMC Biol. 2016, 14, 46. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Zeng, X.; Schwarz, H.; Nanda, H.S.; Peng, X.; Zhou, Y. Exosomes, a New Star for Targeted Delivery. Front. Cell Dev. Biol. 2021, 9, 751079. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Kalluri, R. Mechanisms associated with biogenesis of exosomes in cancer. Mol. Cancer 2019, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Bio. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.; Sandsmark, D.K. Clinical Updates in Mild Traumatic Brain Injury (Concussion). Neuroimaging Clin. N. Am. 2023, 33, 271–278. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, S.; Ge, X.; Yin, Z.; Li, M.; Guo, M.; Hu, T.; Han, Z.; Kong, X.; Li, D.; et al. Mesenchymal stromal cell treatment attenuates repetitive mild traumatic brain injury-induced persistent cognitive deficits via suppressing ferroptosis. J. Neuroinflammation 2022, 19, 185. [Google Scholar] [CrossRef] [PubMed]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A web-based compendium of exosomal cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019, 47, D516–D519. [Google Scholar] [CrossRef] [PubMed]
- van Balkom, B.W.; Eisele, A.S.; Pegtel, D.M.; Bervoets, S.; Verhaar, M.C. Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting. J. Extracell. Vesicles 2015, 4, 26760. [Google Scholar] [CrossRef]
- Lasda, E.; Parker, R. Circular RNAs co-precipitate with extracellular vesicles: A possible mechanism for circRNA clearance. PLoS ONE 2016, 11, e0148407. [Google Scholar] [CrossRef]
- Chevillet, J.R.; Kang, Q.; Ruf, I.K.; Briggs, H.A.; Vojtech, L.N.; Hughes, S.M.; Cheng, H.H.; Arroyo, J.D.; Meredith, E.K.; Gallichotte, E.N.; et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. USA 2014, 111, 14888–14893. [Google Scholar] [CrossRef]
- Flavin, W.P.; Hosseini, H.; Ruberti, J.W.; Kavehpour, H.P.; Giza, C.C.; Prins, M.L. Traumatic brain injury and the pathways to cerebral tau accumulation. Front. Neurol. 2023, 14, 1239653. [Google Scholar] [CrossRef]
- Younger, D.S. Mild traumatic brain injury and sports-related concussion. Handb. Clin. Neurol. 2023, 196, 475–494. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders and Stroke. Traumatic Brain Injury. Available online: https://www.ninds.nih.gov/health-information/disorders/traumatic-brain-injury-tbi (accessed on 4 September 2023).
- Freire, M.A.M.; Rocha, G.S.; Bittencourt, L.O.; Falcao, D.; Lima, R.R.; Cavalcanti, J.R.L.P. Cellular and Molecular Pathophysiology of Traumatic Brain Injury: What Have We Learned So Far? Biology 2023, 12, 1139. [Google Scholar] [CrossRef] [PubMed]
- Center of Excellence for Medical Multimedia. Moderate to Severe TBI: Long-Term Effects. Retrieved 28 March 2019. Available online: https://tbi.cemmlibrary.org/Moderate-to-Severe-TBI/Long-Term-Effects (accessed on 4 September 2023).
- Matney, C.; Bowman, K.; Berwick, D. (Eds.) Traumatic Brain Injury: A Roadmap for Accelerating Progress. Chapter 6. Rehabilitation and Long-Term Care Needs After Traumatic Brain Injury; National Academies Press: Washington, DC, USA, 2022. [Google Scholar]
- Abio, A.; Bovet, P.; Valentin, B.; Bärnighausen, T.; Shaikh, M.A.; Posti, J.P.; Lowery Wilson, M. Changes in Mortality Related to Traumatic Brain Injuries in the Seychelles from 1989 to 2018. Front. Neurol. 2021, 12, 720434. [Google Scholar] [CrossRef] [PubMed]
- Purcarea, V. The evaluation of progress in the treatment of traumatic brain injury. J. Med. Life 2015, 8, 1–7. [Google Scholar] [PubMed]
- Hunea, I.; Bulgaru Iliescu, D.; Damian, S.-I.; Gîrlescu, N.; Diac, M.-M.; Afrăsânie, V.A.; Ciocoiu, M. Chemical Biomarkers of Diffusse Axonal Injury. Brain. Broad Res. Artif. Intell. Neurosci. 2020, 11, 18–32. [Google Scholar] [CrossRef]
- Lawrence, T.; Helmy, A.; Bouamra, O.; Woodford, M.; Lecky, F.; Hutchinson, P.J. Traumatic brain injury in England and Wales: Prospective audit of epidemiology, complications and standardised mortality. BMJ Open. 2016, 6, e012197. [Google Scholar] [CrossRef] [PubMed]
- Mayo Clinic Staff, 4 February 2021. Available online: https://www.mayoclinic.org/diseases-conditions/traumatic-brain-injury/diagnosis-treatment/drc-20378561 (accessed on 4 September 2023).
- Jha, S.; Ghewade, P. Management and Treatment of Traumatic Brain Injuries. Cureus. 2022, 14, e30617. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.W.; Lima, L.G.; Lobb, R.J.; Norris, E.L.; Hastie, M.L.; Krumeich, S.; Möller, A. Breast cancer-derived exosomes reflect the cell-of-origin phenotype. Proteomics 2019, 19, e1800180. [Google Scholar] [CrossRef] [PubMed]
- Atif, H.; Hicks, S.D. A Review of MicroRNA Biomarkers in Traumatic Brain Injury. J. Exp. Neurosci. 2019, 13, 1179069519832286. [Google Scholar] [CrossRef] [PubMed]
- Pinchi, E.; Frati, P.; Arcangeli, M.; Volonnino, G.; Tomassi, R.; Santoro, P.; Cipolloni, L. MicroRNAs: The New Challenge for Traumatic Brain Injury Diagnosis. Curr. Neuropharmacol. 2020, 18, 319–331. [Google Scholar] [CrossRef]
- Sun, P.; Liu, D.Z.; Jickling, G.C.; Sharp, F.R.; Yin, K.-J. MicroRNA-based therapeutics in central nervous system injuries. J. Cerebral Blood Flow Metab. 2018, 38, 1125–1148. [Google Scholar] [CrossRef]
- Vuokila, N.; Aronica, E.; Korotkov, A.; van Vliet, E.A.; Nuzhat, S.; Puhakka, N.; Pitkänen, A. Chronic Regulation of miR-124-3p in the Perilesional Cortex after Experimental and Human TBI. Int. J. Mol. Sci. 2020, 21, 2418. [Google Scholar] [CrossRef]
- Huang, S.; Ge, X.; Yu, J.; Han, Z.; Yin, Z.; Li, Y.; Chen, F.; Wang, H.; Zhang, J.; Lei, P. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018, 32, 512–528. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, X.; Du, M.; Wu, C.; Fu, J.; Tan, W.; Liao, Z.B. Recent advances in the role of miRNAs in post-traumatic stress disorder and traumatic brain injury. Mol. Psychiatry, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Xian, P.; Hei, Y.; Wang, R.; Wang, T.; Yang, J.; Li, J.; Di, Z.; Liu, Z.; Baskys, A.; Liu, W.; et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019, 9, 5956–5975. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Ma, B.; Li, N.; Wang, S.; Sun, Z.; Xue, C.; Han, Q.; Wei, J.; Zhao, R.C. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging 2020, 12, 18274–18296. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, J.; Wang, P.; Zhong, L.; Wang, S.; Feng, Q.; Wei, X.; Zhou, L. Hypoxia-pretreated mesenchymal stem cell-derived exosomes-loaded low-temperature extrusion 3D-printed implants for neural regeneration after traumatic brain injury in canines. Front. Bioeng. Biotechnol. 2022, 10, 1025138. [Google Scholar] [CrossRef]
- Li, D.; Huang, S.; Yin, Z.; Zhu, J.; Ge, X.; Han, Z.; Tan, J.; Zhang, S.; Zhao, J.; Chen, F.; et al. Increases in miR-124-3p in Microglial Exosomes Confer Neuroprotective Effects by Targeting FIP200-Mediated Neuronal Autophagy Following Traumatic Brain Injury. Neurochem. Res. 2019, 44, 1903–1923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Chopp, M.; Zhang, Z.G.; Mahmood, A.; Xiong, Y. Mesenchymal Stem Cell-Derived Exosomes Improve Functional Recovery in Rats after Traumatic Brain Injury: A Dose-Response and Therapeutic Window Study. Neurorehabil. Neural Repair 2020, 34, 616–626. [Google Scholar] [CrossRef]
- Ghosh, S.; Garg, S.; Ghosh, S. Cell-Derived Exosome Therapy: A Novel Approach to Treat Post-traumatic Brain Injury Mediated Neural Injury. ACS Chem. Neurosci. 2020, 11, 2045–2047. [Google Scholar] [CrossRef]
- Williams, A.M.; Dennahy, I.S.; Bhatti, U.F.; Halaweish, I.; Xiong, Y.; Chang, P.; Nikolian, V.C.; Chtraklin, K.; Brown, J.; Zhang, Y.; et al. Mesenchymal Stem Cell-Derived Exosomes Provide Neuroprotection and Improve Long-Term Neurologic Outcomes in a Swine Model of Traumatic Brain Injury and Hemorrhagic Shock. J. Neurotrauma. 2019, 36, 54–60. [Google Scholar] [CrossRef]
- Han, Y.; Seyfried, D.; Meng, Y.; Yang, D.; Schultz, L.; Chopp, M.; Seyfried, D. Multipotent mesenchymal stromal cell-derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. J. Neurosurg. 2018, 131, 290–300. [Google Scholar] [CrossRef]
- Liu, X.; Wu, C.; Zhang, Y.; Chen, S.; Ding, J.; Chen, Z.; Wu, K.; Wu, X.; Zhou, T.; Zeng, M.; et al. Hyaluronan-based hydrogel integrating exosomes for traumatic brain injury repair by promoting angiogenesis and neurogenesis. Carbohydr. Polym. 2023, 306, 120578. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, Y.; Zhang, R.; Wen, L.; Wu, K.; Li, Y.; Yao, Y.; Duan, R.; Jia, Y. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Recovery Following Spinal Cord Injury via Improvement of the Integrity of the Blood-Spinal Cord Barrier. Front. Neurosci. 2019, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, Y.; Bai, W.; Sun, L.; Tian, M. Human umbilical cord mesenchymal stem cell-derived exosome suppresses programmed cell death in traumatic brain injury via PINK1/Parkin-mediated mitophagy. CNS Neurosci. Ther. 2023, 29, 2236–2258. [Google Scholar] [CrossRef]
- Cui, L.; Luo, W.; Jiang, W.; Li, H.; Xu, J.; Liu, X.; Wang, B.; Wang, J.; Chen, G. Human umbilical cord mesenchymal stem cell-derived exosomes promote neurological function recovery in rat after traumatic brain injury by inhibiting the activation of microglia and astrocyte. Regen. Ther. 2022, 21, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Higgins, G.A.; Bhatti, U.F.; Biesterveld, B.E.; Dekker, S.E.; Kathawate, R.G.; Tian, Y.; Wu, Z.; Kemp, M.T.; Wakam, G.K.; et al. Early treatment with exosomes following traumatic brain injury and hemorrhagic shock in a swine model promotes transcriptional changes associated with neuroprotection. J. Trauma Acute Care Surg. 2020, 89, 536–543. [Google Scholar] [CrossRef]
- Williams, A.M.; Wu, Z.; Bhatti, U.F.; Biesterveld, B.E.; Kemp, M.T.; Wakam, G.K.; Vercruysse, C.A.; Chtraklin, K.; Siddiqui, A.Z.; Pickell, Z.; et al. Early single-dose exosome treatment improves neurologic outcomes in a 7-day swine model of traumatic brain injury and hemorrhagic shock. J. Trauma Acute Care Surg. 2020, 89, 388–396. [Google Scholar] [CrossRef]
- Heath, N.; Grant, L.; De Oliveira, T.M.; Rowlinson, R.; Osteikoetxea, X.; Dekker, N.; Overman, R. Rapid isolation and enrichment of extracellular vesicle preparations using anion exchange chromatography. Sci. Rep. 2018, 8, 5730. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Wang, C.; Lu, X.; Zhang, C.; Zhou, Z.; Chen, X.; Zhang, C.Y.; Zen, K.; Zhang, C. Comparison of commercial exosome isolation kits for circulating exosomal microRNA profiling. Anal. Bioanalical Chem. 2018, 410, 3805–3814. [Google Scholar] [CrossRef]
- Ge, X.; Guo, M.; Hu, T.; Li, W.; Huang, S.; Yin, Z.; Li, Y.; Chen, F.; Zhu, L.; Kang, C.; et al. Increased Microglial Exosomal miR-124-3p Alleviates Neurodegeneration and Improves Cognitive Outcome after rmTBI. Mol. Ther. 2020, 28, 503–522. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Wang, D.; Yan, W.; Zhang, S.; Li, D.; Han, Z.; Chen, F.; Lei, P. MiR-124-3p attenuates brain microvascular endothelial cell injury in vitro by promoting autophagy. Histol. Histopathol. 2022, 37, 159–168. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Huang, Y.; Zhang, H.; Lu, H.; Zheng, J.C. Exosomal miRNAs in central nervous system diseases: Biomarkers, pathological mediators, protective factors and therapeutic agents. Prog. Neurobiol. 2019, 183, 101694. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.G.; Wang, J.L.; Zhang, Y.X.; Li, L.; Reza, A.M.M.T.; Gurunathan, S. Biogenesis, Composition and Potential Therapeutic Applications of Mesenchymal Stem Cells Derived Exosomes in Various Diseases. Int. J. Nanomed. 2023, 18, 3177–3210. [Google Scholar] [CrossRef] [PubMed]
- Yari, H.; Mikhailova, M.V.; Mardasi, M.; Jafarzadehgharehziaaddin, M.; Shahrokh, S.; Thangavelu, L.; Ahmadi, H.; Shomali, N.; Yaghoubi, Y.; Zamani, M.; et al. Emerging role of mesenchymal stromal cells (MSCs)-derived exosome in neurodegeneration-associated conditions: A groundbreaking cell-free approach. Stem Cell Res. Ther. 2022, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomark. Res. 2019, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Reza-Zaldivar, E.E.; Hernández-Sapiéns, M.A.; Minjarez, B.; Gutiérrez-Mercado, Y.K.; Márquez-Aguirre, A.L.; Canales-Aguirre, A.A. Potential Effects of MSC-Derived Exosomes in Neuroplasticity in Alzheimer’s Disease. Front. Cell Neurosci. 2018, 12, 317. [Google Scholar] [CrossRef]
- Fayazi, N.; Sheykhhasan, M.; Soleimani Asl, S.; Najafi, R. Stem Cell-Derived Exosomes: A New Strategy of Neurodegenerative Disease Treatment. Mol. Neurobiol. 2021, 58, 3494–3514. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Rarinca, V.; Nicoara, M.; Ciobica, A.; Mavroudis, I. A short editorial view on the relevance of exosomes in some neuropsychiatric manifestations—Model studies. Acad. Rom. Sci. Ann. Ser. Biol. Sci. 2022, 11, 124–126. [Google Scholar] [CrossRef]
- Royo, N.C.; Conte, V.; Saatman, K.E.; Shimizu, S.; Belfield, C.M.; Soltesz, K.M.; Davis, J.E.; Fujimoto, S.T.; McIntosh, T.K. Hippocampal vulnerability following traumatic brain injury: A potential role for neurotrophin-4/5 in pyramidal cell neuroprotection. Eur. J. Neurosci. 2006, 23, 1089–1102. [Google Scholar] [CrossRef]
- Otani, N.; Nawashiro, H.; Shima, K. Pathophysiological Findings of Selective Vulnerability in the Hippocampus after Traumatic Brain Injury. J. Exp. Clin. Med. 2011, 3, 22–26. [Google Scholar] [CrossRef]
- Hu, Y.; Tao, W. Microenvironmental Variations after Blood-Brain Barrier Breakdown in Traumatic Brain Injury. Front. Mol. Neurosci. 2021, 14, 750810. [Google Scholar] [CrossRef]
- Cash, A.; Theus, M.H. Mechanisms of Blood-Brain Barrier Dysfunction in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef]
- Wang, W.-X.; Visavadiya, N.P.; Pandya, J.D.; Nelson, P.T.; Sullivan, P.G.; Springer, J.E. Mitochondria-Associated MicroRNAs in Rat Hippocampus Following Traumatic Brain Injury. Sanders-Brown Cent. Aging Fac. Publ. 2015, 265, 84–93. Available online: https://uknowledge.uky.edu/sbcoa_facpub/96 (accessed on 4 September 2023).
- Chen, W.; Guo, Y.; Yang, W.; Chen, L.; Ren, D.; Wu, C.; He, B.; Zheng, P.; Tong, W. Phosphorylation of connexin 43 induced by traumatic brain injury promotes exosome release. J. Neurophysiol. 2018, 119, 305–311. [Google Scholar] [PubMed]
- Mayorquin, L.C.; Rodriguez, A.V.; Sutachan, J.-J.; Albarracín, S.L. Connexin-Mediated Functional and Metabolic Coupling Between Astrocytes and Neurons. Front. Mol. Neurosci. 2018, 11, 118. [Google Scholar] [CrossRef]
- Dong, H.; Zhou, X.; Wang, X.; Yang, Y.; Luo, J.; Liu, Y.; Mao, Q. Complex role of connexin 43 in astrocytic tumors and possible promotion of glioma-associated epileptic discharge (Review). Mol. Med. Rep. 2017, 16, 7890–7900. [Google Scholar] [CrossRef] [PubMed]
- Vinken, M. Regulation of connexin signaling by the epigenetic machinery. Biochim. Biophys. Acta 2016, 1859, 262–268. [Google Scholar] [CrossRef]
- Zhou, K.Q.; Green, C.R.; Bennet, L.; Gunn, A.J.; Davidson, J.O. The Role of Connexin and Pannexin Channels in Perinatal Brain Injury and Inflammation. Front. Physiol. 2019, 10, 141. [Google Scholar] [CrossRef]
- Donat, C.K.; Scott, G.; Gentleman, S.M.; Sastre, M. Microglial Activation in Traumatic Brain Injury. Front. Aging Neurosci. 2017, 9, 208. [Google Scholar] [CrossRef]
- Li, Y.F.; Ren, X.; Zhang, L.; Wang, Y.H.; Chen, T. Microglial polarization in TBI: Signaling pathways and influencing pharmaceuticals. Front. Aging Neurosci. 2022, 14, 901117. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, H.; Yang, Y.; Ma, Y. Exosomal microRNAs have great potential in the neurorestorative therapy for traumatic brain injury. Exp. Neurol. 2022, 352, 114026. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Y.; Kong, C.; Su, X.; Zhang, X.; Bai, W.; He, X. MiR-124 Enriched Exosomes Promoted the M2 Polarization of Microglia and Enhanced Hippocampus Neurogenesis after Traumatic Brain Injury by Inhibiting TLR4 Pathway. Neurochem. Res. 2019, 44, 811–828. [Google Scholar] [CrossRef]
- Zhao, J.; He, Z.; Wang, J. MicroRNA-124: A Key Player in Microglia-Mediated Inflammation in Neurological Diseases. Front. Cell. Neurosci. 2021, 15, 771898. [Google Scholar] [CrossRef] [PubMed]
- Vuokila, N. MiR-124-3p as a Regulator for Post-TBI Recovery Process. Ph.D. Thesis, Molecular Medicine Publications of the University of Eastern Finland, Kuopio, Finland, 2020. [Google Scholar]
- Li, D.; Huang, S.; Zhu, J.; Hu, T.; Han, Z.; Zhang, S.; Zhao, J.; Chen, F.; Lei, P. Exosomes from MiR-21-5p-Increased Neurons Play a Role in Neuroprotection by Suppressing Rab11a-Mediated Neuronal Autophagy In Vitro after Traumatic Brain Injury. Med. Sci. Monit. 2019, 25, 1871–1885. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Xiong, Y.; Sun, Y.; Zeng, R.; Xue, H.; Hu, Y.; Liu, G. Circulating MiRNA-21-enriched extracellular vesicles promote bone remodeling in traumatic brain injury patients. Exp. Mol. Med. 2023, 55, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-P.; Li, C.; Ding, W.-C.; Peng, G.; Xiao, G.-L.; Chen, R.; Cheng, Q. Research Progress on the Inflammatory Effects of Long Non-coding RNA in Traumatic Brain Injury. Front. Mol. Neurosci. 2023, 15, 835012. [Google Scholar] [CrossRef]

| Study | Objective | Models and Administration | Key Findings |
|---|---|---|---|
| [36] | The effects of MSC-Exo on inflammation-induced astrocytic alterations |
|
|
| [37] | The effects of MSC-Exo on functional recovery, neuroinflammation, neuronal apoptosis, and neurogenesis |
|
|
| [38] | The regenerative potential of MSC-derived exosomes |
|
|
| [40] | The effects of MSC-Exo in relation to doses and times of administration |
|
|
| [42] | The effects of bone marrow-derived MSC-Exo on TBI and hemorrhagic shock in animal models |
|
|
| [43] | The effects of multipotent MSC-Exo on functional recovery, neurovascular remodeling, and neurogenesis |
|
|
| [44] | The efficiency of hyaluronan-collagen hydrogel incorporating bone marrow MSC-Exo in TBI treatment |
|
|
| [45] | The effects of MSC-Exo treatment on spinal cord injury |
|
|
| [46] | The neuroprotective effects of human umbilical cord MSC-Exo |
|
|
| [47] | The reparatory mechanism to which MSC-Exo contributes |
|
|
| [48] | The neuroreparatory molecular mechanisms that the MSC-Exo is contributing to |
|
|
| [49] | The impact of early single-dose exosome treatment in a 7-day survival model |
|
|
| [51] | The role of miRNAs in regulating post-traumatic neurodegeneration |
|
|
| [35] | To explore the regulatory mechanism of microglial exosomes on neuronal inflammation in TBI by investigating the impact of microglial exosomal miRNAs on injured neurons |
|
|
| [53] | The effects of miR-124-3p on brain microvascular endothelial cell function and their molecular mechanisms |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavroudis, I.; Balmus, I.-M.; Ciobica, A.; Nicoara, M.N.; Luca, A.C.; Palade, D.O. The Role of Microglial Exosomes and miR-124-3p in Neuroinflammation and Neuronal Repair after Traumatic Brain Injury. Life 2023, 13, 1924. https://doi.org/10.3390/life13091924
Mavroudis I, Balmus I-M, Ciobica A, Nicoara MN, Luca AC, Palade DO. The Role of Microglial Exosomes and miR-124-3p in Neuroinflammation and Neuronal Repair after Traumatic Brain Injury. Life. 2023; 13(9):1924. https://doi.org/10.3390/life13091924
Chicago/Turabian StyleMavroudis, Ioannis, Ioana-Miruna Balmus, Alin Ciobica, Mircea Nicusor Nicoara, Alina Costina Luca, and Dragos Octavian Palade. 2023. "The Role of Microglial Exosomes and miR-124-3p in Neuroinflammation and Neuronal Repair after Traumatic Brain Injury" Life 13, no. 9: 1924. https://doi.org/10.3390/life13091924
APA StyleMavroudis, I., Balmus, I.-M., Ciobica, A., Nicoara, M. N., Luca, A. C., & Palade, D. O. (2023). The Role of Microglial Exosomes and miR-124-3p in Neuroinflammation and Neuronal Repair after Traumatic Brain Injury. Life, 13(9), 1924. https://doi.org/10.3390/life13091924









