Current Status of Vector-Borne Diseases in Croatia: Challenges and Future Prospects
Abstract
1. Introduction
2. Literature Search
3. Arthropod-Borne Viral Infections
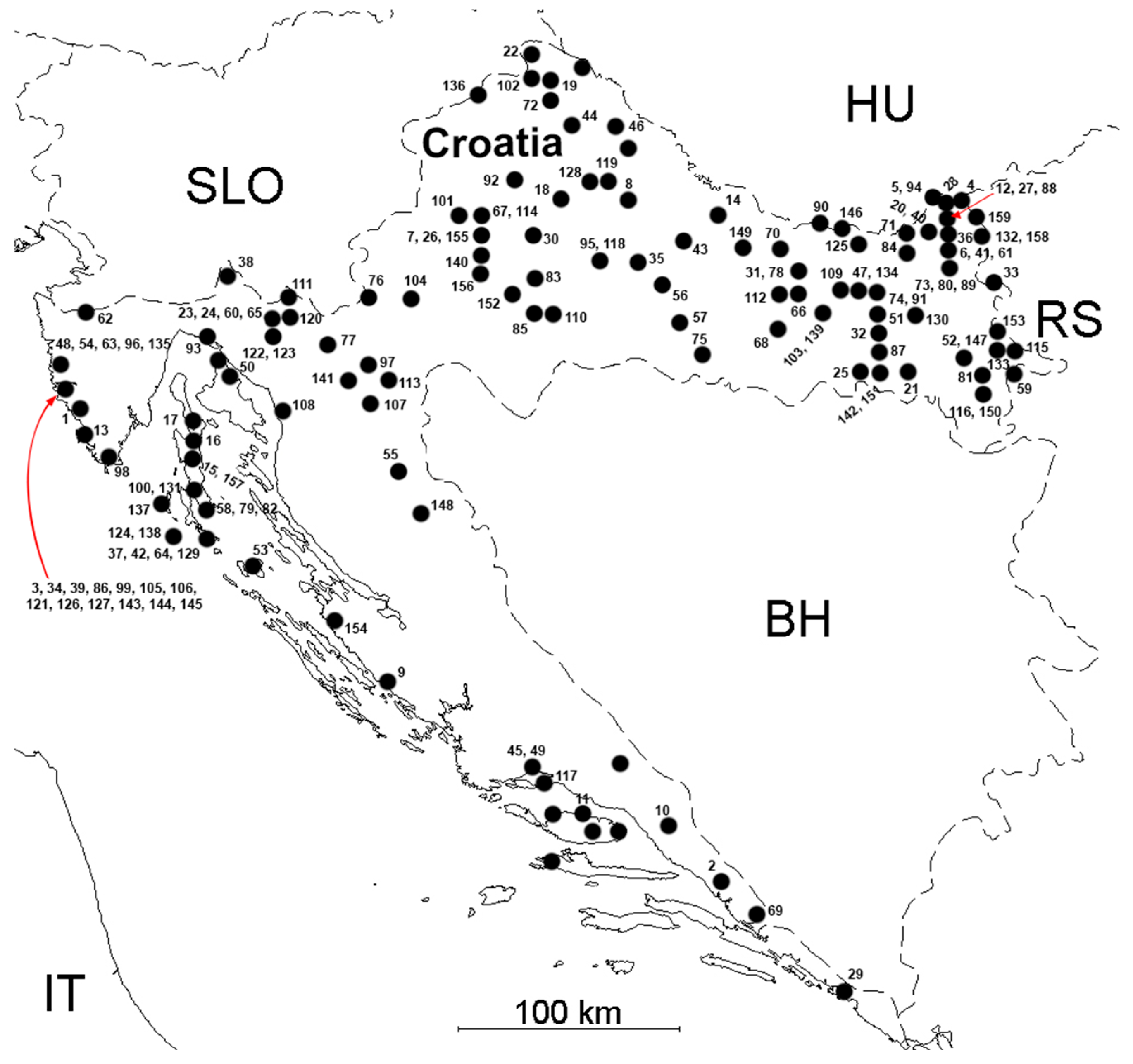
3.1. Tick-Borne Encephalitis
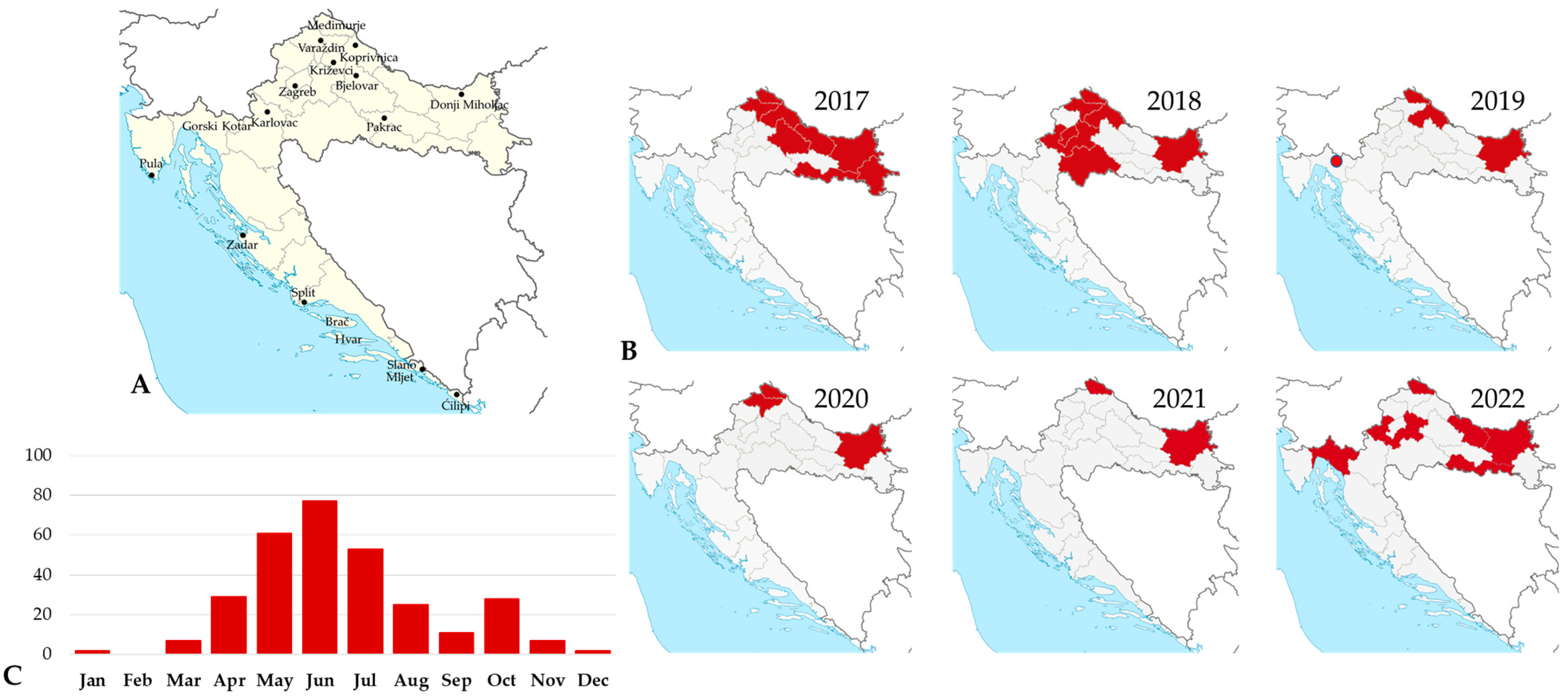
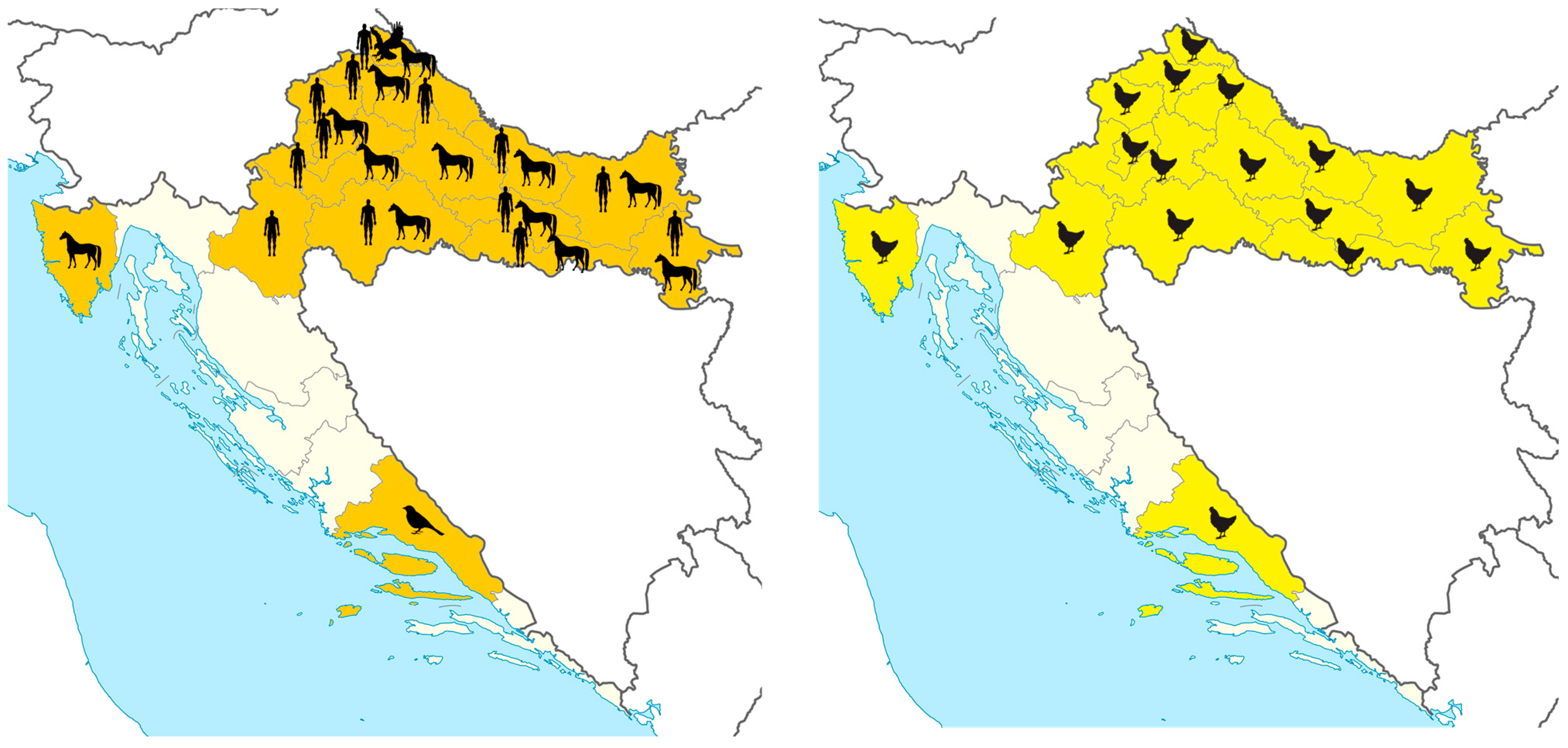
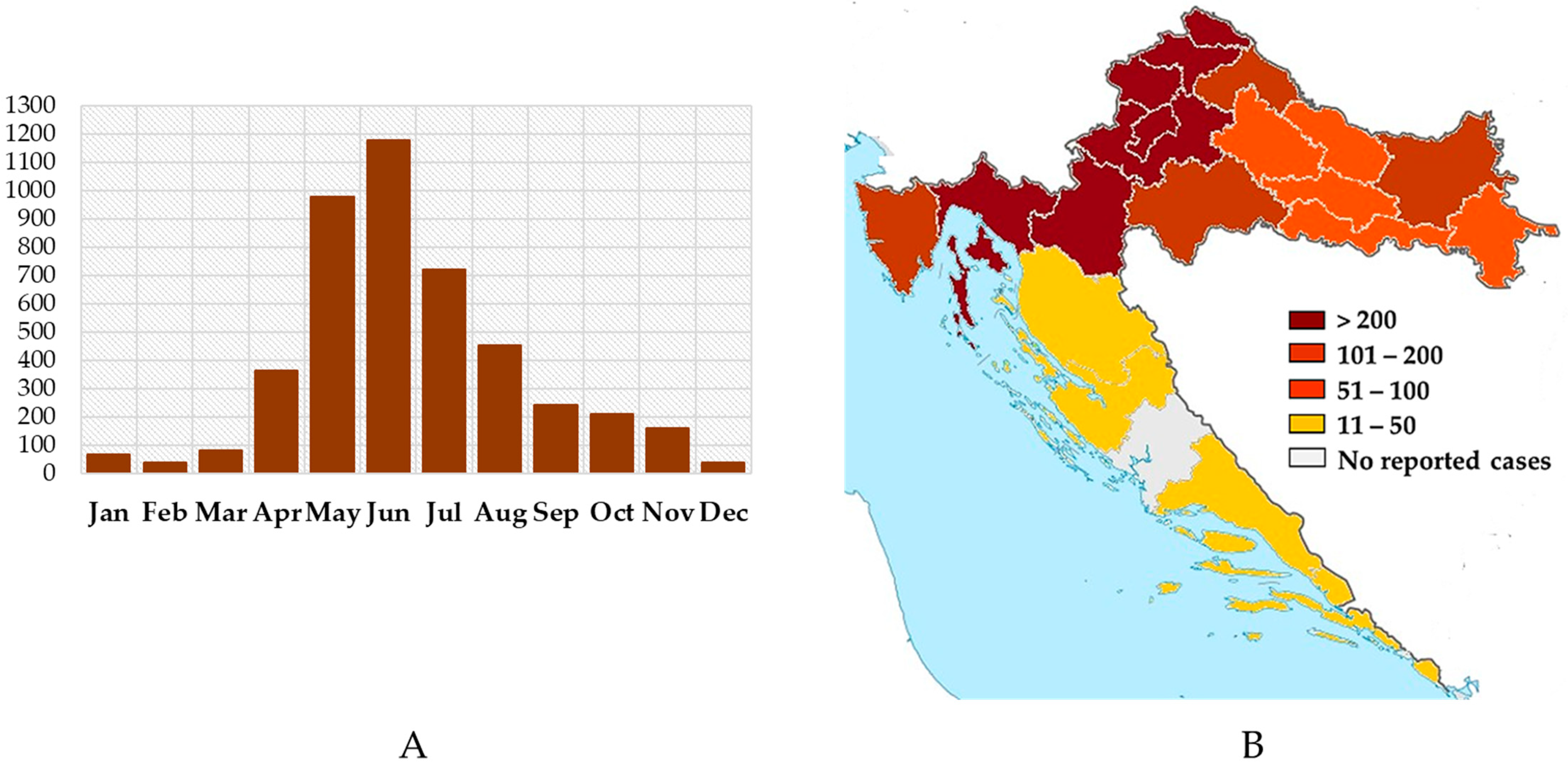
3.2. West Nile Virus Infections
3.3. Usutu Virus Infections
3.4. Toscana Virus Infections
3.5. Sandfly Fever
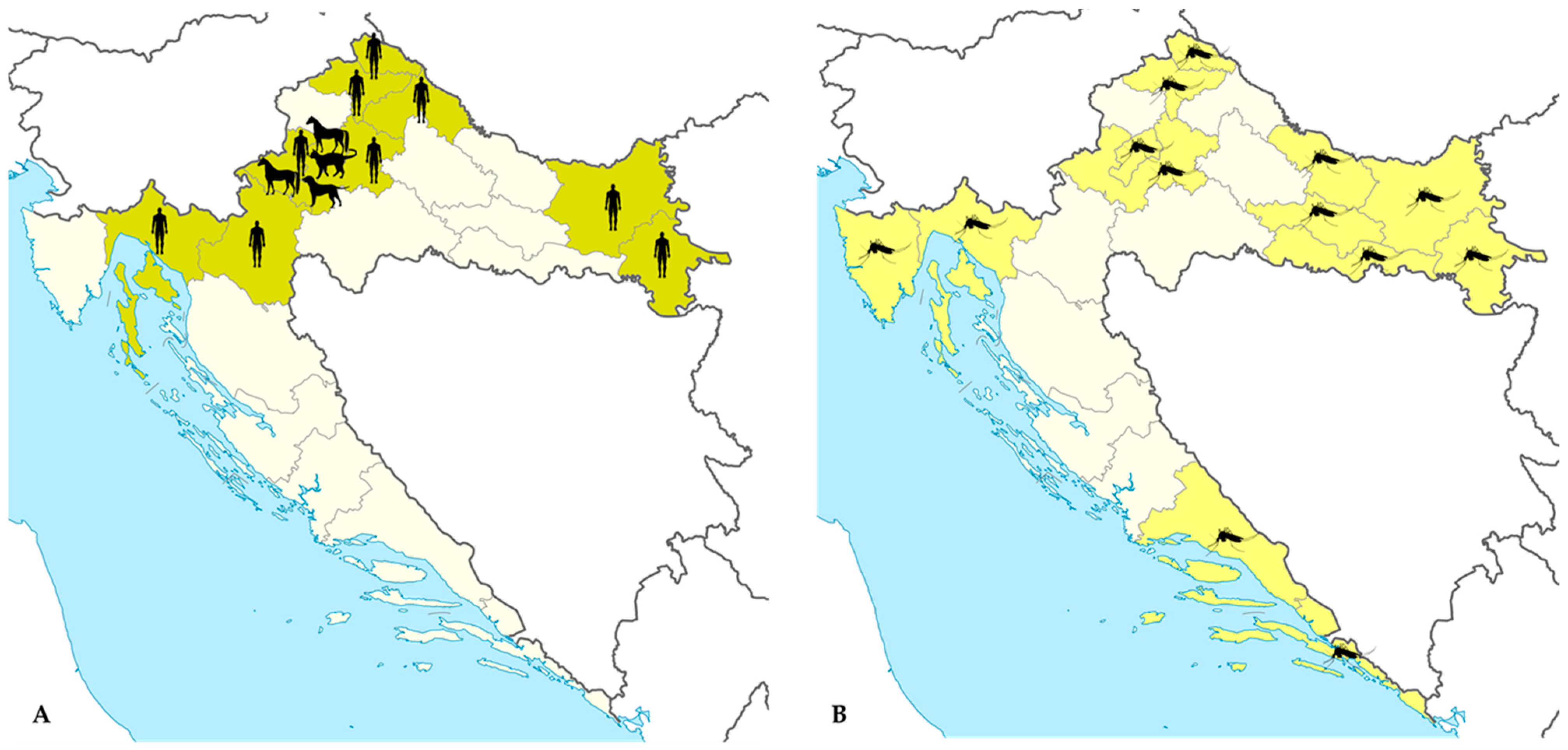
3.6. Tahyna Orthobunyavirus Infections
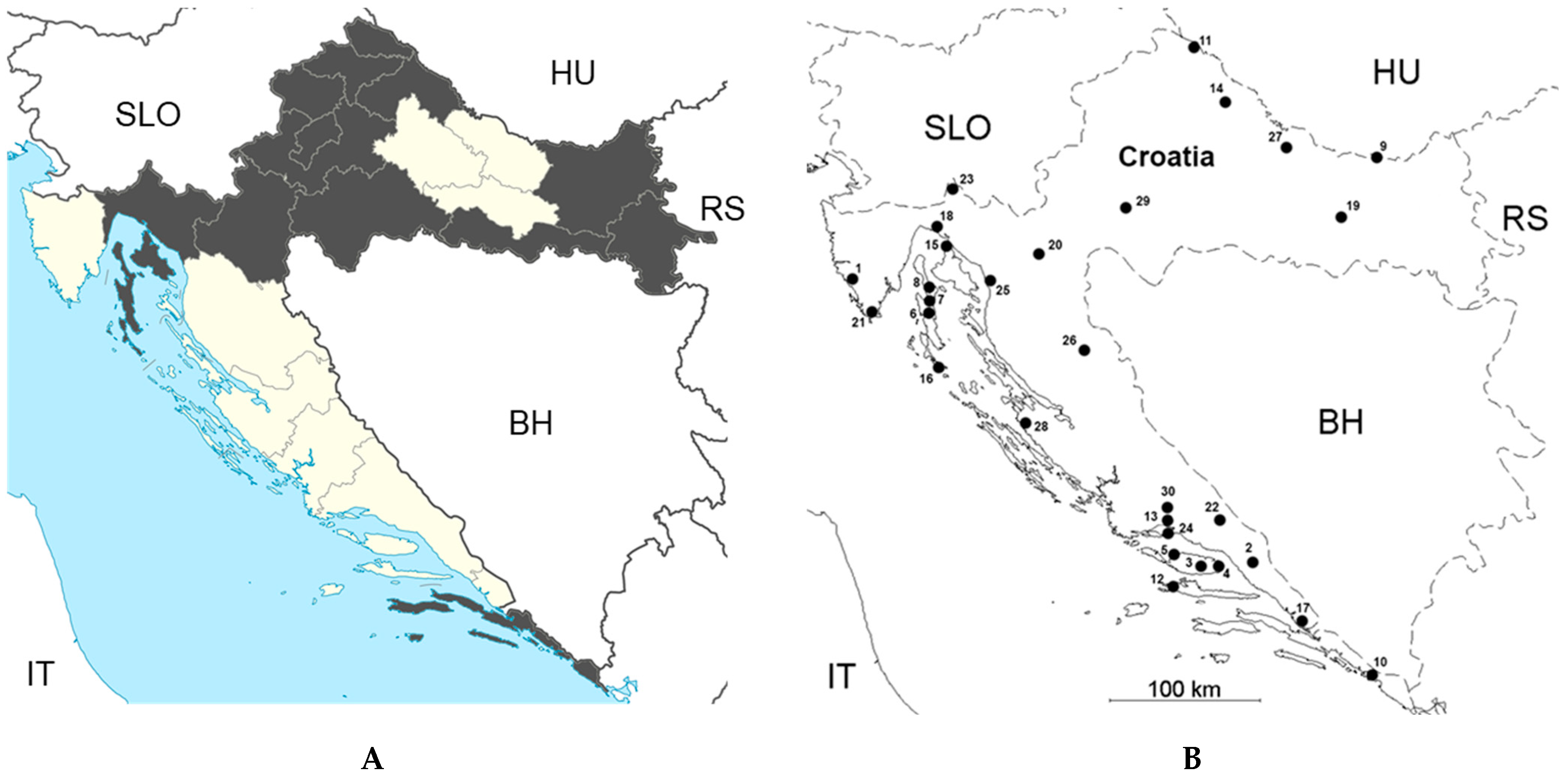
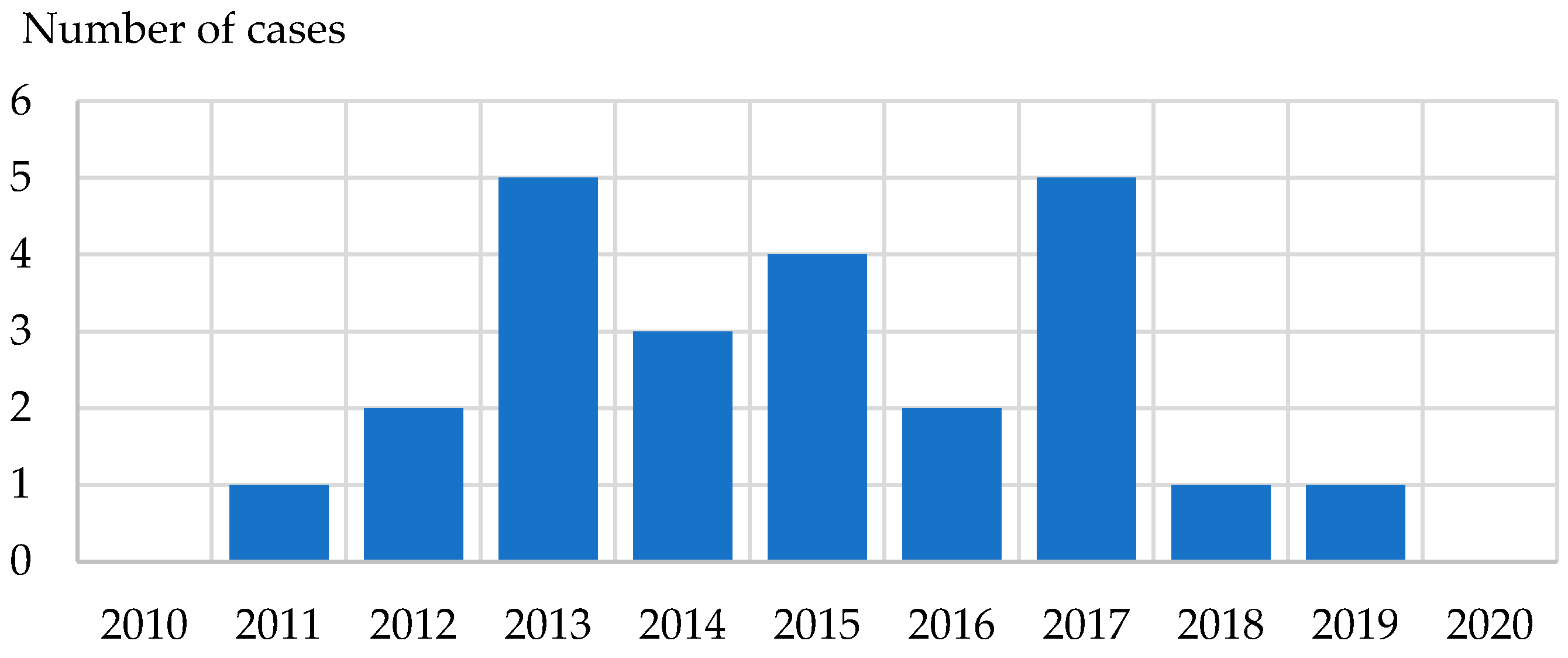
3.7. Bhanja Bandavirus Infections
3.8. Dengue, Chikungunya, and Zika Virus Infections
4. Vector-Borne Bacterial Infections
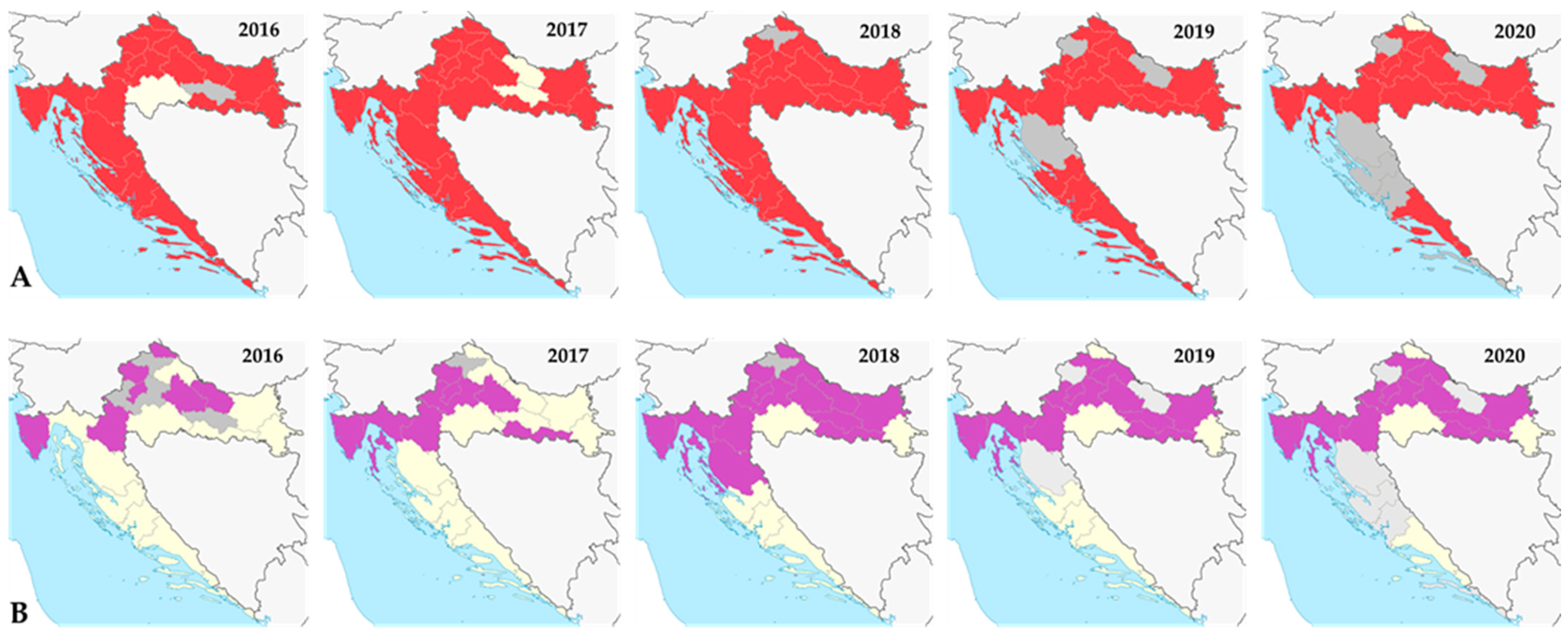
4.1. Lyme Disease (Borrelia burgdorferi s.l.)
4.2. Anaplasmosis (Anaplasma phagocytophilum)
4.3. Rickettsioses
5. Vector-Borne Parasitic Infections
5.1. Leishmaniais
5.2. Malaria
6. Monitoring of Invasive Mosquito Species in Croatia
7. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ilic, M.; Barbic, L.; Bogdanic, M.; Tabain, I.; Savic, V.; Kosanovic Licina, M.L.; Kaic, B.; Jungic, A.; Vucelja, M.; Angelov, V.; et al. Tick-borne encephalitis outbreak following raw goat milk consumption in a new micro-location, Croatia, June 2019. Ticks Tick-Borne Dis. 2020, 11, 101513. [Google Scholar] [CrossRef] [PubMed]
- Gjenero-Margan, I.; Aleraj, B.; Krajcar, D.; Lesnikar, V.; Klobučar, A.; Pem-Novosel, I.; Kurečić-Filipović, S.; Komparak, S.; Martić, R.; Duričić, S.; et al. Autochthonous dengue fever in Croatia, August–September 2010. Euro Surveill. 2011, 16, 19805. [Google Scholar] [CrossRef]
- Pem-Novosel, I.; Vilibic-Cavlek, T.; Gjenero-Margan, I.; Kaic, B.; Babic-Erceg, A.; Merdic, E.; Medic, A.; Ljubic, M.; Pahor, D.; Erceg, M. Dengue virus infection in Croatia: Seroprevalence and entomological study. New Microbiol. 2015, 38, 97–100. [Google Scholar] [PubMed]
- Vilibic-Cavlek, T.; Kaic, B.; Barbic, L.; Pem-Novosel, I.; Slavic-Vrzic, V.; Lesnikar, V.; Kurecic-Filipovic, S.; Babic-Erceg, A.; Listes, E.; Stevanovic, V.; et al. First evidence of simultaneous occurrence of West Nile virus and Usutu virus neuroinvasive disease in humans in Croatia during the 2013 outbreak. Infection 2014, 42, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Savic, V.; Klobucar, A.; Ferenc, T.; Ilic, M.; Bogdanic, M.; Tabain, I.; Stevanovic, V.; Santini, M.; Curman Posavec, M.; et al. Emerging Trends in the West Nile Virus Epidemiology in Croatia in the ‘One Health’ Context, 2011–2020. Trop. Med. Infect. Dis. 2021, 6, 140. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Savic, V.; Sabadi, D.; Peric, L.; Barbic, L.; Klobucar, A.; Miklausic, B.; Tabain, I.; Santini, M.; Vucelja, M.; et al. Prevalence and Molecular Epidemiology of West Nile and Usutu Virus Infections in Croatia in the “One Health” Context, 2018. Transbound. Emerg. Dis. 2019, 66, 1946–1957. [Google Scholar] [CrossRef]
- Punda-Polić, V.; Jerončić, A.; Mohar, B.; Šiško Kraljević, K. Prevalence of Toscana virus antibodies in residents of Croatia. Clin. Microbiol. Infect. 2012, 18, E200–E203. [Google Scholar] [CrossRef]
- Punda-Polić, V.; Mohar, B.; Duh, D.; Bradarić, N.; Korva, M.; Fajs, L.; Saksida, A.; Avšič-Županc, T. Evidence of an autochthonous Toscana virus strain in Croatia. J. Clin. Virol. 2012, 55, 4–7. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Zidovec-Lepej, S.; Ledina, D.; Knezevic, S.; Savic, V.; Tabain, I.; Ivic, I.; Slavuljica, I.; Bogdanic, M.; Grgic, I.; et al. Clinical, Virological, and Immunological Findings in Patients with Toscana Neuroinvasive Disease in Croatia: Report of Three Cases. Trop. Med. Infect. Dis. 2020, 5, 144. [Google Scholar] [CrossRef]
- Šalamun, M.; Kolarić, B.; Tabain, I.; Antolašić, L.; Milašinčić, L.; Artl, S.; Savić, V.; Barbić, L.; Stevanović, V.; Vilibić-Čavlek, T. Seroepidemiology of phlebovirus infections in Croatia, 2017–2018. In Proceedings of the 8th International Congress Veterinary Science and Profession, Zagreb, Croatia, 10–12 October 2019; p. 60. [Google Scholar]
- Vilibic-Cavlek, T.; Stevanovic, V.; Savic, V.; Markelic, D.; Sabadi, D.; Bogdanic, M.; Kovac, S.; Santini, M.; Tabain, I.; Potocnik-Hunjadi, T.; et al. Detection of Tahyna Orthobunyavirus-Neutralizing Antibodies in Patients with Neuroinvasive Disease in Croatia. Microorganisms 2022, 10, 1443. [Google Scholar] [CrossRef]
- Đaković Rode, O. Human granulocytic anaplasmosis in Croatia and new insights about anaplasma and ehrlichia species. Croat. J. Infect. 2015, 35, 5–15. (In Croatian) [Google Scholar]
- Punda-Polić, V.; Lukšić, B.; Čapkun, V. Epidemiological features of Mediterranean spotted fever, murine typhus, and Q fever in Split-Dalmatia County (Croatia), 1982–2002. Epidemiol. Infect. 2008, 136, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Šiško-Kraljević, K.; Jerončić, A.; Mohar, B.; Punda-Polić, V. Asymptomatic Leishmania Infantum Infections in Humans Living in Endemic and Non-Endemic Areas of Croatia, 2007 to 2009. Euro Surveill. 2013, 18, 20533. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Škrlec, I.; Alebić, T.; Jukić, M.; Včev, A. Malaria: The Past and the Present. Microorganisms 2019, 7, 179. [Google Scholar] [CrossRef]
- Vesenjak-Hirjan, J. Tick-Borne Encephalitis in Croatia; Rad JAZU, Book 372; Croatian Publisher RAD JAZU: Zagreb, Croatia, 1976; pp. 1–10. [Google Scholar]
- Vesenjak-Hirjan, J.; Rulnjević, J.; Šooš, E.; Galinović-Weissglas, M.; Beus, I. Appearance of Tick-Borne Encephalitis in the Mountain Range Medvednica; Rad JAZU, Book 372; Croatian Publisher RAD JAZU: Zagreb, Croatia, 1976; pp. 161–166. [Google Scholar]
- Borčić, B.; Kaić, B.; Gardašević-Morić, L.; Turković, B. Tick-borne meningoencephalitis in Gorski Kotar-new findings. Lijec. Vjesn. 2001, 123, 163–164. (In Croatian) [Google Scholar]
- Vesenjak-Hirjan, J.; Egri-Hećimović, E.; Brudnjak, Z.; Vince Šooš, E. Infections with Tick-Borne Encephalitis Virus in the Panonian Focus Stara Vas. Serological Studies 1961–1964; Rad JAZU, Book 372; Croatian Publisher RAD JAZU: Zagreb, Croatia, 1976; pp. 11–20. [Google Scholar]
- Vesenjak-Hirjan, J.; Galinović-Weisglass, M.; Brudnjak, Z. Infections with Tick-Borne Encephalitis Virus in Pannonian Focus Stara Vas. Serological Studies in 1972; Rad JAZU, Book 372; Croatian Publisher RAD JAZU: Zagreb, Croatia, 1976; pp. 29–36. [Google Scholar]
- Vesenjak-Hirjan, J.; Egri-Hećimović, E.; Šooš, E. Infections with Tick-Borne Encephalitis Virus in Mediterranean Focus Nadsela (Island of Brač); Rad JAZU, Book 372; Croatian Publisher RAD JAZU: Zagreb, Croatia, 1976; pp. 37–40. [Google Scholar]
- Vesenjak-Hirjan, J.; Galinović-Weisglass, M.; Urlić, V.; Bendiš, M.; Miović, P.; Vujošević, N.; Vuksanović, P. Occurrence of arboviruses in the Middle and the South Adriatic (Yugoslavia). In Arboviruses in the Mediterranean Countries; Vesenjak-Hirjan, J., Ed.; Gustav Fischer Verlag: Stuttgart, Germany; New York, NY, USA, 1980; pp. 303–310. [Google Scholar]
- Turković, B.; Brudnjak, Z. Natural foci of some viral zoonoses in Croatia. Acta Med. Croatica 1999, 53, 195–198. [Google Scholar]
- Golubić, D.; Dobler, G. Flaviviruses in the north-west Croatia. Croat. J. Infect. 2012, 32, 153–157. (In Croatian) [Google Scholar]
- Hađina, S.; Stevanović, V.; Kovač, S.; Vilibić Čavlek, T.; Perharić, M.; Starešina, V.; Maltar, L.; Kiš, T.; Madić, J.; Barbić, L. Serological evidence of tick-borne encephalitis virus infections in dogs in Croatia—Importance for the veterinary medicine and public health. In Proceedings of the 7th International Congress Veterinary Science and Profession, Zagreb, Croatia, 5–7 October 2017; Book of Abstracts. p. 59. [Google Scholar]
- Mašović, V. Sheep as Sentinels for West Nile Virus Infections. Master’s Thesis, Faculty of Veterinary Medicine, University of Zagreb, Zagreb, Croatia, 2023. [Google Scholar]
- Jemeršić, L.; Dežđek, D.; Brnić, D.; Prpić, J.; Janicki, Z.; Keros, T.; Roić, B.; Slavica, A.; Terzić, S.; Konjević, D.; et al. Detection and genetic characterization of tick-borne encephalitis virus (TBEV) derived from ticks removed from red foxes (Vulpes vulpes) and isolated from spleen samples of red deer (Cervus elaphus) in Croatia. Ticks Tick-Borne Dis. 2014, 5, 7–13. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Barbic, L.; Mrzljak, A.; Brnic, D.; Klobucar, A.; Ilic, M.; Janev-Holcer, N.; Bogdanic, M.; Jemersic, L.; Stevanovic, V.; et al. Emerging and Neglected Viruses of Zoonotic Importance in Croatia. Pathogens 2021, 10, 73. [Google Scholar] [CrossRef]
- Vesenjak-Hirjan, J.; Galinović-Weisglass, M.; Brudnjak, Z.; Calisher, C.H.; Tovornik, D.; Lazuick, J.S.; Rendić, Z. Island of Brač—Focus of arbovirus infections. In Arboviruses in the Mediterranean Countries; Vesenjak-Hirjan, J., Ed.; Gustav Fischer Verlag: Stuttgart, Germany; New York, NY, USA, 1980; pp. 311–317. [Google Scholar]
- Pem-Novosel, I.; Vilibic-Cavlek, T.; Gjenero-Margan, I.; Pandak, N.; Peric, L.; Barbic, L.; Listes, E.; Cvitkovic, A.; Stevanovic, V.; Savini, G. First outbreak of West Nile virus neuroinvasive disease in humans, Croatia, 2012. Vector Borne Zoonotic Dis. 2014, 14, 82–84. [Google Scholar] [CrossRef]
- Barbic, L.; Savic, V.; Bogdanic, M.; Madic, J.; Sabadi, D.; Al-Mufleh, M.; Stevanovic, V.; Hruskar, Z.; Lakoseljac, D.; Roncevic, D.; et al. Re-emergence of neuroinvasive flaviviruses in the 2022 transmission season in Croatia. In Proceedings of the 33rd ECCMID, Copenhagen, Denmark, 15–18 April 2023; p. 2914, Book of Abstracts. [Google Scholar]
- Madić, J.; Savini, G.; Di Gennaro, A.; Monaco, F.; Jukić, B.; Kovač, S.; Rudan, N.; Listeš, E. Serological Evidence for West Nile Virus Infection in Horses in Croatia. Vet. Rec. 2007, 160, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Barbić, L.; Listeš, E.; Katić, S.; Stevanović, V.; Madić, J.; Starešina, V.; Labrović, A.; Di Gennaro, A.; Savini, G. Spreading of West Nile virus infection in Croatia. Vet. Microbiol. 2012, 159, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Vuger, V. Dogs and Cats as Sentinels for West Nile Virus in Urban Areas. Master’s Thesis, Faculty of Veterinary Medicine University of Zagreb, Zagreb, Croatia, 2021. [Google Scholar]
- Klobucar, A.; Savic, V.; Curman Posavec, M.; Petrinic, S.; Kuhar, U.; Toplak, I.; Madic, J.; Vilibic-Cavlek, T. Screening of Mosquitoes for West Nile Virus and Usutu Virus in Croatia, 2015–2020. Trop. Med. Infect. Dis. 2021, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, A.; Jimenez-Clavero, M.A.; Barzon, L.; Cordioli, P.; Figuerola, J.; Koraka, P.; Martina, B.; Moreno, A.; Nowotny, N.; Pardigon, N.; et al. The challenge of West Nile virus in Europe: Knowledge gaps and research priorities. Euro Surveill. 2015, 20, 21135. [Google Scholar] [CrossRef]
- Barbic, L.; Vilibic-Cavlek, T.; Listes, E.; Stevanovic, V.; Gjenero-Margan, I.; Ljubin-Sternak, S.; Pem-Novosel, I.; Listes, I.; Mlinaric-Galinovic, G.; Di Gennaro, A.; et al. Demonstration of Usutu virus antibodies in horses, Croatia. Vector Borne Zoonotic Dis. 2013, 13, 772–774. [Google Scholar] [CrossRef]
- Santini, M.; Vilibic-Cavlek, T.; Barsic, B.; Barbic, L.; Savic, V.; Stevanovic, V.; Listes, E.; Di Gennaro, A.; Savini, G. First cases of human Usutu virus neuroinvasive infection in Croatia, August–September 2013: Clinical and laboratory features. J. Neurovirol. 2015, 21, 92–97. [Google Scholar] [CrossRef]
- Barbic, L.; Vuger, V.; Stevanovic, V.; Benvin, I.; Kovac, S.; Savic, V.; Vilibic-Cavlek, T.; Tabain, I.; Bogdanic, M.; Madic, J. First serological evidence of Usutu virus infection in cats—Possible role as sentinels for flavivirus infections. In Proceedings of the 32nd ECCMID, Lisbon, Portugal, 23–26 April 2022; p. 57, Book of Abstracts. [Google Scholar]
- Ayhan, N.; Prudhomme, J.; Laroche, L.; Bañuls, A.L.; Charrel, R.N. Broader Geographical Distribution of Toscana Virus in the Mediterranean Region Suggests the Existence of Larger Varieties of Sand Fly Vectors. Microorganisms 2020, 8, 114. [Google Scholar] [CrossRef]
- Ayhan, N.; Alten, B.; Ivovic, V.; Martinkovic, F.; Kasap, O.E.; Ozbel, Y.; de Lamballerie, X.; Charrel, R.N. Cocirculation of Two Lineages of Toscana Virus in Croatia. Front. Public Health 2017, 5, 336. [Google Scholar] [CrossRef]
- Vesenjak-Hirjan, J.; Calisher, C.H.; Beus, I.; Marton, E. First natural clinical human Bhanja virus infection. In Arboviruses in the Mediterranean Countries; Vesenjak-Hirjan, J., Ed.; Gustav Fischer Verlag: Stuttgart, Germany; New York, NY, USA, 1980; pp. 297–301. [Google Scholar]
- Borčić, B.; Punda-Polić, V. Sandfly fever epidemiology in Croatia. Acta. Medica. Iug. 1987, 41, 89–97. [Google Scholar]
- Madić, J.; Huber, D.; Lugović, B. Serologic survey for selected viral and rickettsial agents of brown bears (Ursus arctos) in Croatia. J. Wildl. Dis. 1993, 29, 572–576. [Google Scholar] [CrossRef]
- Stevanovic, V.; Vilibic-Cavlek, T.; Savic, V.; Klobucar, A.; Kovac, S.; Curman Posavec, M.; Petrinic, S.; Bogdanic, M.; Santini, M.; Tesic, V.; et al. Surveillance of Tahyna Orthobunyavirus in Urban Areas in Croatia-The “One Health” Approach. Trop. Med. Infect. Dis. 2022, 7, 320. [Google Scholar] [CrossRef] [PubMed]
- Vesenjak-Hirjan, J.; Calisher, C.H.; Brudnjak, Z.; Tovornik, D.; Skrtic, N.; Lazuick, J.S. Isolation of Bhanja virus from ticks in Yugoslavia. Am. J. Trop. Med. Hyg. 1977, 26, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Punda, V.; Beus, I.; Calisher, C.H.; Vesenjak-Hirjan, J. Laboratory infections with Bhanja virus. In Arboviruses in the Mediterranean Countries; Vesenjak-Hirjan, J., Ed.; Gustav Fischer Verlag: Stuttgart, Germany; New York, NY, USA, 1980; pp. 273–275. [Google Scholar]
- Vesenjak-Hirjan, J. Arboviruses in Yugoslavia. In Arboviruses in the Mediterranean Countries; Vesenjak-Hirjan, J., Ed.; Gustav Fischer Verlag: Stuttgart, Germany; New York, NY, USA, 1980; pp. 164–177. [Google Scholar]
- Vilibic-Cavlek, T.; Stevanovic, V.; Krcmar, S.; Savic, V.; Kovac, S.; Bogdanic, M.; Mauric Maljkovic, M.; Sabadi, D.; Santini, M.; Potocnik-Hunjadi, T.; et al. Detection of Bhanja Bandavirus in Patients with Neuroinvasive Disease of Unknown Etiology in Croatia. Microorganisms 2023, 11, 2155. [Google Scholar] [CrossRef]
- Pfäffle, M.P.; Santos-Silva, M.; Jaenson, T.G.T.; Vatansever, Z.; Petney, T.N. Haemaphysalis punctata Canestrini and Fanzago, 1878 (Figures 88–90). In Ticks of Europe and North Africa. A Guide to Species Identification; Estrada-Peňa, A., Mihalca, A.D., Petney, T.N., Eds.; Springer: Cham, Switzerland, 2017; pp. 237–242. [Google Scholar]
- Dumić, T.; Jurković, D.; Florijančič, T.; Ozimec, S.; Pintur, K.; Fabijanić, N.; Filipeti, B.; Beck, R. Tick infestation of Balkan chamois (Rupicapra rupicapra balcanica) from the area of Biokovo mountain in the Republic of Croatia. In Proceedings of the 3rd Inter-national Rupicapra Symposium, Makarska, Croatia, 16–18 June 2021. [Google Scholar]
- Dumić, T. The Ecological and Genetic Characteristics of Ectoparasites of Wild Ungulates from Different Habitats in Croatia. Ph.D. Thesis, Faculty of Agrobiotechnical Sciences Osijek, Osijek, Croatia, 2021. [Google Scholar]
- Hubálek, Z. Biogeography of tick-borne Bhanja virus (bunyaviridae) in Europe. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 372691. [Google Scholar] [CrossRef]
- Krčmar, S.; Klobučar, A.; Vucelja, M.; Boljfetić, M.; Kučinić, M.; Madić, J.; Cvek, M.; Bruvo Mađarić, B. DNA barcoding of hard ticks (Ixodidae), notes on distribution of vector species and new faunal record for Croatia. Ticks Tick-Borne Dis. 2022, 13, 101920. [Google Scholar] [CrossRef]
- Krčmar, S. Hard ticks (Acari, Ixodidae) of Croatia. ZooKeys 2012, 234, 19–57. [Google Scholar] [CrossRef]
- Konjević, D.; Janicki, Z.; Severin, K.; Stanko, M.; Živičnjak, T.; Slavica, A.; Starešina, V. An outbreak of tick paralysis in free-ranging mouflon (Ovis ammon musimon). J. Zoo Wildl. Med. 2007, 38, 585–587. [Google Scholar] [CrossRef]
- Perez, K. Hard Tick Prevalence in Karlovac County. Master’s Thesis, Faculty of Veterinary Medicine, University of Zagreb, Zagreb, Croatia, 2021. [Google Scholar]
- Mičetić, A. Fauna of Hard Ticks (Fam. Ixodidae) in Private Forests at Vicinity of Ponikve (Municipality Bakar). Master’s Thesis, Faculty of Forestry and Wood Technology, University of Zagreb, Zagreb, Croatia, 2021. [Google Scholar]
- Punda, V.; Ropac, D. The prevalence of Bhanja virus affected dogs in some regions of Croatia and Slovenia. Vet. Arh. 1985, 55, 157–164. [Google Scholar]
- Ropac, D.; Gould, E.; Punda, V.; Vesenjak-Hirjan, J. Dengue viruses in northeastern Croatia. Lijec. Vjesn. 1988, 110, 177–180. [Google Scholar]
- Vilibic-Cavlek, T.; Pem-Novosel, I.; Kaic, B.; Babić-Erceg, A.; Kucinar, J.; Klobucar, A.; Medic, A.; Pahor, D.; Barac-Juretic, K.; Gjenero-Margan, I. Seroprevalence and entomological study on Chikungunya virus at the Croatian littoral. Acta Microbiol. Immunol. Hung. 2015, 62, 199–206. [Google Scholar] [CrossRef][Green Version]
- Luksic, B.; Pandak, N.; Drazic-Maras, E.; Karabuva, S.; Radic, M.; Babic-Erceg, A.; Barbic, L.; Stevanovic, V.; Vilibic-Cavlek, T. First case of imported chikungunya infection in Croatia, 2016. Int. Med. Case Rep. J. 2017, 10, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Betica-Radic, L.; Venturi, G.; Fortuna, C.; Djuricic, S.; Salvia-Milos, A.; Tabain, I.; Barbic, L.; Stevanovic, V.; Listes, E.; et al. First detection of Zika virus infection in a Croatian traveler returning from Brazil, 2016. J. Infect. Dev. Ctries. 2017, 11, 662–667. [Google Scholar] [CrossRef]
- Burn, L.; Vyse, A.; Pilz, A.; Tran, T.M.P.; Fletcher, M.A.; Angulo, F.J.; Gessner, B.D.; Moïsi, J.C.; Stark, J.H. Incidence of Lyme Borreliosis in Europe: A Systematic Review (2005–2020). Vector Borne Zoonotic Dis. 2023, 23, 172–194. [Google Scholar] [CrossRef]
- Maretić, T.; Benić, B.; Đaković-Rode, O.; Beritić, D.; Ružić-Sabljić, E. First isolation of Borrelia sp. (Borrelia afzelii) from cerebrospinal fluid in a patient with neuroborreliosis in Croatia. Croat. J. Infect. 2009, 29, 65–70. (In Croatian) [Google Scholar]
- Burek, V.; Misić-Mayerus, L.; Maretić, T. Antibodies to Borrelia burgdorferi in various population groups in Croatia. Scand. J. Infect. Dis. 1992, 24, 683–684. [Google Scholar] [CrossRef] [PubMed]
- Poljak, I.; Troselj-Vukić, B.; Miletić, B.; Morović, M.; Ruzić-Sabljić, E.; Vucemilović, A.; Materljan, E. Low seroprevalence of Lyme borreliosis in the forested mountainous area of Gorski Kotar, Croatia. Croat. Med. J. 2000, 41, 433–436. [Google Scholar] [PubMed]
- Kovačević, J.; Bučanović, T.; Krčmar, S. Hard tick fauna (Acari: Ixodidae) in different types of habitats in the city of Osijek (Eastern Croatia). Nat. Croat. 2020, 29, 63–72. [Google Scholar] [CrossRef]
- Krčmar, S. Diversity, ecology, and seasonality of hard ticks (Acari: Ixodidae) in eastern Croatia. J. Vector Ecol. 2019, 44, 18–29. [Google Scholar] [CrossRef]
- Mikačić, D. Dynamics of tick occurrence (Ixodidae) in North Croatia. Vet Arh. 1969, 39, 183–186. (In Croatian) [Google Scholar]
- Sameroff, S.; Tokarz, R.; Vucelja, M.; Komal, J.; Oleynik, A.; Boljfetić, M.; Bjedov, L.; Yates, R.A.; Margaletić, J.; Oura, C.A.L.; et al. Virome of Ixodes ricinus, Dermacentor reticulatus, and Haemaphysalis concinna Ticks from Croatia. Viruses 2022, 14, 929. [Google Scholar] [CrossRef]
- Vucelja, M.; Krčmar, S.; Habuš, J.; Mojčec Perko, V.; Boljfetić, M.; Bjedov, L.; Margaletić, J. Altitudinal distribution, seasonal dynamics and Borrelia burgdorferi sensu lato infections in hard ticks (Acari: Ixodidae) in different forest communities in Inland Croatia. Sustainability 2023, 15, 4862. [Google Scholar] [CrossRef]
- Hillyard, P.D. Ticks of North-West Europe: Keys and Notes for Identification of the Species. In Synopses of the British Fauna (New Series) 52; Field Studies Council: Shrewsbury, UK, 1996; p. 178. [Google Scholar]
- Furnes, R.W.; Furness, E.N. Ixodes ricinus parasitism of birds increases at higher winter temperatures. J. Vector Ecol. 2018, 43, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Rijpkema, S.; Golubić, D.; Molkenboer, M.; Verbeek-De Kruif, N.; Schellekens, J. Identification of four genomic groups of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in a Lyme borreliosis endemic region of northern Croatia. Exp. Appl. Acarol. 1996, 20, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Jurković, D.; Beck, A.; Huber, D.; Mihaljević, Ž.; Polkinghorne, A.; Martinković, F.; Lukačević, D.; Pilat, M.; Brezak, R.; Bosnić, S.; et al. Seroprevalence of Vector-Borne Pathogens in Dogs from Croatia. Parasitol. Res. 2019, 118, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Štritof, Z. Seroprevalence of Lyme-borreliosis in horses in Croatia. In Proceedings of the Workshop with International Participation Emerging and Neglected Zoonoses in the “One Health” Context, Zagreb, Croatia, 18–19 October 2018. (In Croatian). [Google Scholar]
- Golubić, D.; Rijpkema, S.; Tkalec-Makovec, N.; Ruzić, E. Epidemiologic, ecologic and clinical characteristics of Lyme borrelliosis in northwest Croatia. Acta Med. Croatica 1998, 52, 7–13. [Google Scholar]
- Pandak, N.; Sprong, H.; Tijsse Klasen, E.; Trošelj-Vukić, B.; Golubić, D.; Šiško, M.; Miklaušić, B.; Čabraja, I.; Križanović, B. Borrelia and rickettsia in skin bioptates of erythema migrans patients. Croat. J. Infect. 2011, 31, 133–137. (In Croatian) [Google Scholar]
- Tadin, A.; Tokarz, R.; Markotić, A.; Margaletić, J.; Turk, N.; Habuš, J.; Svoboda, P.; Vucelja, M.; Desai, A.; Jain, K.; et al. Molecular Survey of Zoonotic Agents in Rodents and Other Small Mammals in Croatia. Am. J. Trop. Med. Hyg. 2016, 94, 466–473. [Google Scholar] [CrossRef]
- Huber, D.; Reil, I.; Duvnjak, S.; Jurković, D.; Lukačević, D.; Pilat, M.; Beck, A.; Mihaljević, Ž.; Vojta, L.; Polkinghorne, A.; et al. Molecular Detection of Anaplasma platys, Anaplasma phagocytophilum and Wolbachia Sp. but Not Ehrlichia canis in Croatian Dogs. Parasitol. Res. 2017, 116, 3019–3026. [Google Scholar] [CrossRef]
- Topolovec, J.; Puntarić, D.; Antolović-Pozgain, A.; Vuković, D.; Topolovec, Z.; Milas, J.; Drusko-Barisić, V.; Venus, M. Serologically Detected “New” Tick-Borne Zoonoses in Eastern Croatia. Croat. Med. J. 2003, 44, 626–629. [Google Scholar]
- Misić-Majerus, L.; Bujić, N.; Madarić, V.; Avsic-Zupanc, T.; Milinković, S. Human anaplasmosis (ehrlichiosis). Acta Med. Croatica 2006, 60, 411–419. (In Croatian) [Google Scholar]
- Bogdanić, M.; Savić, V.; Tabain, I.; Hunjak, B.; Milašinčić, L.; Antolašić, L.; Artl, S.; Barbić, L.; Jemeršić, L.; Prpić, J.; et al. Prevalence of tick-borne encephalitis, Lyme-borreliosis and human granulocytic anaplasmosis in patients with a history of a tick bite, Croatia (2017–2018). In Proceedings of the 8th International Congress Veterinary Science and Profession, Zagreb, Croatia, 10–12 October 2019. [Google Scholar]
- Trninić, K.; Cvitković, D.; Vlahović, K.; Ćurković, S.; Udiljak, Ž.; Kunštek, S.; Pavlak, M. Anaplasma phagocytophilum, the causative agent of vectorborne emergent zoonoses: A review of epidemiological studies. Vet. Stanica 2023, 54, 239–254. (In Croatian) [Google Scholar] [CrossRef]
- Jurković, D.; Mihaljević, Ž.; Duvnjak, S.; Silaghi, C.; Beck, R. First Reports of Indigenous Lethal Infection with Anaplasma Marginale, Anaplasma Bovis and Theileria Orientalis in Croatian Cattle. Ticks Tick-Borne Dis. 2020, 11, 101469. [Google Scholar] [CrossRef] [PubMed]
- Gotić, J.; Brkljača Bottegaro, N.; Kiš, I.; Crnogaj, M.; Mrljak, V.; Beck, R. A First Case of Equine Granulocytic Anaplasmosis in Croatia—A Case Report. Vet. Arh. 2017, 87, 113–120. [Google Scholar]
- Mrljak, V.; Kuleš, J.; Mihaljević, Ž.; Torti, M.; Gotić, J.; Crnogaj, M.; Živičnjak, T.; Mayer, I.; Šmit, I.; Bhide, M.; et al. Prevalence and Geographic Distribution of Vector-Borne Pathogens in Apparently Healthy Dogs in Croatia. Vector Borne Zoonotic Dis. 2017, 17, 398–408. [Google Scholar] [CrossRef]
- Mraović, J.; Jurić, B.; Krznarić, M.; Tus, Z.; Lončar, M.; Vrkić, V.; Marinculić, A.; Krivičić, K.; Pavlak, M. Epidemiological study of certain zoonoses in dogs and assessment of risk factors. Vet. Stanica 2019, 50, 423–434. (In Croatian) [Google Scholar]
- Dyachenko, V.; Pantchev, N.; Balzer, H.-J.; Meyersen, A.; Straubinger, R.K. First Case of Anaplasma platys Infection in a Dog from Croatia. Parasit. Vectors 2012, 5, 49. [Google Scholar] [CrossRef]
- Hornok, S.; Elek, V.; De La Fuente, J.; Naranjo, V.; Farkas, R.; Majoros, G.; Földvári, G. First Serological and Molecular Evidence on the Endemicity of Anaplasma ovis and A. marginale in Hungary. Vet. Microbiol. 2007, 122, 316–322. [Google Scholar] [CrossRef]
- Potgieter, F.T.; van Rensburg, L. Tick Transmission of Anaplasma centrale. Onderstepoort J. Vet. Res. 1987, 54, 5–7. [Google Scholar]
- Dzelalija, B.; Punda-Polic, V.; Medic, A.; Dobec, M. Rickettsiae and rickettsial diseases in Croatia: Implications for travel medicine. Travel Med. Infect. Dis. 2016, 14, 436–443. [Google Scholar] [CrossRef]
- Radulovic, S.; Feng, H.M.; Morovic, M.; Djelalija, B.; Popov, V.; Crocquet-Valdes, P.; Walker, D.H. Isolation of Rickettsia akari from a patient in a region where Mediterranean spotted fever is endemic. Clin. Infect. Dis. 1996, 22, 216–220. [Google Scholar] [CrossRef]
- Vilibić-Čavlek, T.; Ljubin-Sternak, S.; Kolarić, B.; Mlinarić-Galinović, G. Diagnosis of rickettsioses: Results of the Croatian Institute of Public Health. Croat. J. Infect. 2012, 32, 167–172. [Google Scholar]
- Dobec, M.; Golubic, D.; Punda-Polic, V.; Kaeppeli, F.; Sievers, M. Rickettsia helvetica in Dermacentor reticulatus ticks. Emerg. Infect. Dis. 2009, 15, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, V.; Kasap, O.E.; Ivovic, V.; Mikov, O.; Stefanovska, J.; Martinkovic, F.; Omeragic, J.; Pajovic, I.; Baymak, D.; Oguz, G.; et al. Sand Flies (Diptera: Psychodidae) in Eight Balkan Countries: Historical Review and Region-Wide Entomological Survey. Parasit. Vectors 2020, 13, 573. [Google Scholar] [CrossRef]
- Berriatua, E.; Maia, C.; Conceição, C.; Özbel, Y.; Töz, S.; Baneth, G.; Pérez-Cutillas, P.; Ortuño, M.; Muñoz, C.; Jumakanova, Z.; et al. Leishmaniases in the European Union and Neighboring Countries. Emerg. Infect. Dis. 2021, 27, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Vaselek, S. Systematic Review: Re-Emergence of Human Leishmaniasis in the Balkans. Trop. Med. Int. Health 2021, 26, 1189–1199. [Google Scholar] [CrossRef]
- Mulić, R.; Ropac, B.D.; Zorić, I.; Bradarić, N. Epidemiologic and Ecologic Characteristics of Some Diseases Transmitted by Arthropods on the Littoral of the Republic of Croatia. Mil. Med. 2002, 167, 321–325. [Google Scholar] [CrossRef]
- Bosnić, S.; Gradoni, L.; Khoury, C.; Maroli, M. A Review of Leishmaniasis in Dalmatia (Croatia) and Results from Recent Surveys on Phlebotomine Sandflies in Three Southern Counties. Acta Trop. 2006, 99, 42–49. [Google Scholar] [CrossRef]
- Gouzelou, E.; Haralambous, C.; Antoniou, M.; Christodoulou, V.; Martinković, F.; Živičnjak, T.; Smirlis, D.; Pratlong, F.; Dedet, J.-P.; Özbel, Y.; et al. Genetic Diversity and Structure in Leishmania Infantum Populations from Southeastern Europe Revealed by Microsatellite Analysis. Parasit. Vectors 2013, 6, 342. [Google Scholar] [CrossRef]
- Punda-Polić, V.; Sardelić, S.; Bradarić, N. Visceral Leishmaniasis in Southern Croatia. Lancet 1998, 351, 188. [Google Scholar] [CrossRef]
- Mulić, R.; Custović, A.; Ropac, D.; Tripković, I.; Stojanović, D.; Klismanić, Z. Occurrence of Visceral and Cutaneous Leishmaniasis in Croatia. Mil. Med. 2009, 174, 206–211. [Google Scholar] [CrossRef]
- Miščević, Z.; Milutinović, M.; Ivovic, V. Fauna and Distribution of Sandflies (Diptera, Phlebotomidae) in Yugoslavia, Croatia, Macedonia and Their Role in the Transmission of Parasitic and Viral Diseases. Acta Vet. 1998, 48, 163–172. [Google Scholar]
- Ivović, V.; Kalan, K.; Zupan, S.; Buzan, E. Illegal Waste Sites as A Potential Micro Foci of Mediterranean Leishmaniasis: First Records of Phlebotomine Sand Flies (Diptera: Psychodidae) From Slovenia. Acta Vet. 2015, 65, 348–357. [Google Scholar] [CrossRef]
- Vaselek, S. Canine Leishmaniasis in Balkan—A Review of Occurrence and Epidemiology. Acta Trop. 2021, 224, 106110. [Google Scholar] [CrossRef] [PubMed]
- Zivicnjak, T.; Martinković, F.; Marinculić, A.; Mrljak, V.; Kucer, N.; Matijatko, V.; Mihaljević, Z.; Barić-Rafaj, R. A Seroepidemiologic Survey of Canine Visceral Leishmaniosis among Apparently Healthy Dogs in Croatia. Vet. Parasitol. 2005, 131, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Živičnjak, T.; Martinković, F.; Khoury, C.; Bongiorno, G.; Bosnić, S.; Lukačević, D.; Maroli, M. Serological and Entomological Studies of Canine Leishmaniosis in Croatia. Vet. Arh. 2011, 81, 99–110. [Google Scholar]
- Žunić-Pedisić, F.; Knežević, B. Malaria among Croatian Seafarers between 2004 and 2014: Evaluation of Chemoprophylaxis Use and Occupational Disease Reporting. Arh. Hig. Rada Toksikol. 2021, 72, 295–298. [Google Scholar] [CrossRef]
- Caminade, C.; McIntyre, M.K.; Jones, A.E. Climate Change and Vector-Borne Diseases: Where Are We Next Heading? J. Infect. Dis. 2016, 214, 1300–1301. [Google Scholar] [CrossRef]
- World Health Organization (WHO)—Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 8 June 2023).
- Romanović, M.; Merdić, E. Investigation of Mosquito Larvae (Diptera, Culicidae) in the Coastal Area of Dalmatia, Croatia. Period. Biol. 2011, 113, 109–113. [Google Scholar]
- Merdić, E.; Boca, I. Seasonal Dynamics of the Anopheles maculipennis Complex in Osijek, Croatia. J. Vector. Ecol. 2004, 29, 257–263. [Google Scholar]
- Skrobonja, A.; Materljan, N.; Skrobonja, I. Beginnings and Success in Preventing Anophelism by Means of Gambusia Fish on the Island of Krk in Croatia from 1922 to 1927. Med. Glas. 2010, 7, 106–110. [Google Scholar]
- Gregurić Gracner, G.; Vucevac Bajt, V. History of Eradication of Malaria in Croatia. Orvostort. Kozl. 2002, 47, 145–155. [Google Scholar] [PubMed]
- Dugački, V., Dr. Rudolf Battara operation in Nin in 1902, the first systematic battle attempt against malaria in Croatia. Med. Jad. 2005, 35, 33–40. (In Croatian) [Google Scholar]
- Cigui, R. Malaria in Pula in the seventies of the 19th century and the epidemic in 1879. AMHA—Acta Med.-Hist. Adriat. 2012, 10, 69–81. (In Italian) [Google Scholar]
- Novak, M.; Martinčić, O.; Strinović, D.; Slaus, M. Skeletal and Dental Indicators of Health in the Late Mediaeval (12–15th Century) Population from Nin, Southern Croatia. Homo 2012, 63, 435–450. [Google Scholar] [CrossRef][Green Version]
- Fatović-Ferenčić, S. Brijuni Archipelago: Story of Kupelwieser, Koch, and Cultivation of 14 Islands. Croat. Med. J. 2006, 47, 369–371. [Google Scholar]
- Mulić, R.; Aljinović, L.; Gizdić, Z.; Petri, N.M. Malaria in Croatia: In the past, today and tomorrow. Lijec. Vjesn. 2000, 122, 51–55. [Google Scholar]
- Aleraj, B. Infectious diseases in Croatia in 2007. Croat. J. Infect. 2008, 28, 145–151. (In Croatian) [Google Scholar]
- Perić, D.; Škrobonja, I.; Škrobonja, A. Malaria in Croatia in the period between 1987 to 2006. Liječ. Vjesn. 2009, 131, 192–195. (In Croatian) [Google Scholar]
- Raju, N.; Poljak, I.; Troselj-Vukic, B. Malaria, a Travel Health Problem in the Maritime Community. J. Travel. Med. 2000, 7, 309–313. [Google Scholar]
- Klobucar, A.; Benic, N.; Krajcar, D.; Kosanovic-Licina, M.L.; Tesic, V.; Merdic, E.; Vrucina, I.; Savic, V.; Barbic, L.; Stevanovic, V.; et al. An overview of mosquitoes and emerging arboviral infections in the Zagreb area, Croatia. J. Infect. Dev. Ctries. 2016, 10, 1286–1293. [Google Scholar] [CrossRef]
- Klobucar, A.; Merdić, E.; Benić, N.; Baklaić, Z.; Krcmar, S. First record of Aedes albopictus in Croatia. J. Am. Mosq. Control. Assoc. 2006, 22, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Merdić, E.; Boca, I.; Sudarić Bogojević, M.; Landeka, N. Mosquitoes of Istria, a contribution to knowledge of Croatian mosquito fauna (Diptera, Culicidae). Period. Biol. 2008, 110, 351–360. [Google Scholar]
- Žitko, T.; Merdić, E. Seasonal and Spatial Oviposition Activity of Aedes albopictus (Diptera: Culicidae) in Adriatic Croatia. J. Med. Entomol. 2014, 51, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Gloria-Soria, A.; Payne, A.F.; Bialosuknia, S.M.; Stout, J.; Mathias, N.; Eastwood, G.; Ciota, A.T.; Kramer, L.D.; Armstrong, P.M. Vector Competence of Aedes albopictus Populations from the Northeastern United States for Chikungunya, Dengue, and Zika Viruses. Am. J. Trop. Med. Hyg. 2020, 104, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Martinet, J.P.; Bohers, C.; Vazeille, M.; Ferté, H.; Mousson, L.; Mathieu, B.; Depaquit, J.; Failloux, A.B. Assessing vector competence of mosquitoes from northeastern France to West Nile virus and Usutu virus. PLoS Negl. Trop. Dis. 2023, 17, e0011144. [Google Scholar] [CrossRef]
- Klobučar, A.; Lipovac, I.; Žagar, N.; Mitrović-Hamzić, S.; Tešić, V.; Vilibić-Čavlek, T.; Merdić, E. First record and spreading of the invasive mosquito Aedes japonicus japonicus (Theobald, 1901) in Croatia. Med. Vet. Entomol. 2019, 33, 171–176. [Google Scholar] [CrossRef]
- Ibáñez-Justicia, A.; van de Vossenberg, B.; van den Biggelaar, R.; Voogd, J.; Metz, E.; Jacobs, F.; Dik, M.; Stroo, A. Detection of Aedes flavopictus (Yamada, 1921), Netherlands, June 2019. Euro Surveill. 2019, 24, 1900433. [Google Scholar] [CrossRef]
- Chaves, L.F.; Friberg, M.D. Aedes albopictus and Aedes flavopictus (Diptera: Culicidae) pre-imaginal abundance patterns are associated with different environmental factors along an altitudinal gradient. Curr. Res. Insect. Sci. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Semenza, J.C.; Suk, J.E. Vector-borne diseases and climate change: A European perspective. FEMS Microbiol. Lett. 2018, 365, fnx244. [Google Scholar] [CrossRef]
- Caminade, C.; Medlock, J.M.; Ducheyne, E.; McIntyre, K.M.; Leach, S.; Baylis, M.; Morse, A.P. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: Recent trends and future scenarios. J. R. Soc. Interface 2012, 9, 2708–2717. [Google Scholar] [CrossRef]
- Haussig, J.M.; Young, J.J.; Gossner, C.M.; Mezei, E.; Bella, A.; Sirbu, A.; Pervanidou, D.; Drakulovic, M.B.; Sudre, B. Early start of the West Nile fever transmission season 2018 in Europe. Euro Surveill. 2018, 23, 1800428. [Google Scholar] [CrossRef] [PubMed]
- Janev Holcer, N.; Bucić, L.; Jeličić, P.; Vilibić-Čavlek, T.; Capak, K. The role of mosquito surveillance in the control of arboviral infections in Croatia. Croat. J. Infect. 2019, 39, 15–22. [Google Scholar] [CrossRef]
- Wallace, R.G.; Bergmann, L.; Kock, R.; Gilbert, M.; Hogerwerf, L.; Wallace, R.; Holmberg, M. The dawn of Structural One Health: A new science tracking disease emergence along circuits of capital. Soc. Sci. Med. 2015, 129, 68–77. [Google Scholar] [CrossRef] [PubMed]

| Locality | Altitude-Latitude (Degree/min/s) | UTM |
|---|---|---|
| 1. Bale | N 45°02′22″ E 13°47′09″ | VK 08 |
| 2. Biokovo Mt. | – | XH 79 |
| 3. Brač Island: | XH 49 | |
| Blato | N 43°17′02″ E 16°40′41″ | |
| Osridke | N 43°16′55″ E 16°49′24″ | |
| Sveti Toma | N 43°17′34″ E 16°47′00″ | |
| 4. Brač Island: | XH 59 | |
| Laščatna (North) | N 43°19′01″ E 16°53′25″ | |
| Laščatna (West) | N 43°19′02″ E 16°53′14″ | |
| 5. Brač Island (Supetar) | N 43°23′00″ E 16°33′23″ | XJ 20 |
| 6. Cres Island: | ||
| Belej | N 44°46′41″ E 14°25′46″ | VK 56 |
| Hrasta | N 44°48′50″ E 14°25′14″ | |
| 7. Cres Island: | VK 57 | |
| Cres | N 44°57′38″ E 14°24′33″ | |
| Vrana | N 44°50′40″ E 14°26′32″ | |
| 8. Cres Island (Vodice) | N 45°00′31″ E 14°23′58″ | VK 58 |
| 9. Donji Miholjac | N 45°45′44″ E 18°09′55″ | BR 87 |
| 10. Dubrovnik | N 42°40′06″ E 18°01′45″ | BN 62 |
| 11. Goričan | N 46°22′59″ E 16°40′45″ | XM 23 |
| 12. Hvar Island | – | XH 18 |
| 13. Kaštela | N 43°33′09″ E 16°20′36″ | XJ 12 |
| 14. Koprivnica | N 46°09′50″ E 16°50′00″ | XM 41 |
| 15. Krk Island (Njivice) | N 45°09′48″ E 14°32′32″ | VL 60 |
| 16. Lošinj Island: | ||
| Ćunski-Like | N 44°35′21″ E 14°24′35″ | VK 63 |
| Veli Lošinj | N 44°31′09″ E 14°30′09″ | |
| 17. Metković | N 43°03′12″ E 17°38′57″ | YH 06 |
| 18. Ponikve (Bakar) | N 45°10′22″ E 14°52′53″ | VL 61 |
| 19. Požega | N 45°19′53″ E 17°40′28″ | YL 12 |
| 20. Primišlje | N 45°10′55″ E 15°28′22″ | WL 30 |
| 21. Pula | N 44°51′59″ E 13°50′58″ | VK 16 |
| 22. Sinj | N 43°42′10″ E 16°38′15″ | XJ 34 |
| 23. Gumance (Smrekova Draga) | N 45°30′23″ E 14°25′02″ | VL 64 |
| 24. Split | N 43°30′29″ E 16°26′25″ | XJ 11 |
| 25. Sveti Juraj (Senj) | N 44°55′42″ E 14°55′13″ | VK 98 |
| 26. Kurjak (Udbina) | N 44°29′34″ E 15°39′24″ | WK 63 |
| 27. Virovitica | N 45°49′54″ E 17°23′08″ | XL 87 |
| 28. Zadar | N 44°07′11″ E 15°13′59″ | WJ 18 |
| 29. Zagreb–Karlovac–Sisak (Petrinja)–Bjelovar (Gornja Posavina) | – | WL 75 |
| 30. Muć | N 43°41′29″ E 16°32′29″ | XJ 13 |
| Location (Year) | N Positive /Tested Dogs | Seropositivity % (95% CI) | Reference |
|---|---|---|---|
| Continental, coastal (2017) | 27/435 | 6.21 (4.13–8.90) | [88] |
| Continental, coastal (2017) | 3/1080 | 0.28 (0.06–0.81) | [81] |
| Continental, coastal (2019) | 64/1433 | 4.47 (3.46–5.76) | [76] |
| Coastal; Istria (2019) | 5/134 | 3.73 (1.22–8.49) | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilibic-Cavlek, T.; Janev-Holcer, N.; Bogdanic, M.; Ferenc, T.; Vujica Ferenc, M.; Krcmar, S.; Savic, V.; Stevanovic, V.; Ilic, M.; Barbic, L. Current Status of Vector-Borne Diseases in Croatia: Challenges and Future Prospects. Life 2023, 13, 1856. https://doi.org/10.3390/life13091856
Vilibic-Cavlek T, Janev-Holcer N, Bogdanic M, Ferenc T, Vujica Ferenc M, Krcmar S, Savic V, Stevanovic V, Ilic M, Barbic L. Current Status of Vector-Borne Diseases in Croatia: Challenges and Future Prospects. Life. 2023; 13(9):1856. https://doi.org/10.3390/life13091856
Chicago/Turabian StyleVilibic-Cavlek, Tatjana, Natasa Janev-Holcer, Maja Bogdanic, Thomas Ferenc, Mateja Vujica Ferenc, Stjepan Krcmar, Vladimir Savic, Vladimir Stevanovic, Maja Ilic, and Ljubo Barbic. 2023. "Current Status of Vector-Borne Diseases in Croatia: Challenges and Future Prospects" Life 13, no. 9: 1856. https://doi.org/10.3390/life13091856
APA StyleVilibic-Cavlek, T., Janev-Holcer, N., Bogdanic, M., Ferenc, T., Vujica Ferenc, M., Krcmar, S., Savic, V., Stevanovic, V., Ilic, M., & Barbic, L. (2023). Current Status of Vector-Borne Diseases in Croatia: Challenges and Future Prospects. Life, 13(9), 1856. https://doi.org/10.3390/life13091856









