Synergism of Carbamoylated Erythropoietin and Insulin-like Growth Factor-1 in Immediate Early Gene Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Carbamoylation of EPO
2.2. Cell Culture
2.3. Trophic Factor Treatment
2.4. Inhibitor Studies
2.5. cDNA Preparation
2.6. Quantitative PCR
2.7. Western Blot Analysis
2.8. Digital Droplet PCR
2.9. Animal Studies
2.10. Immunohistochemistry
2.11. Statistical Analysis
3. Results
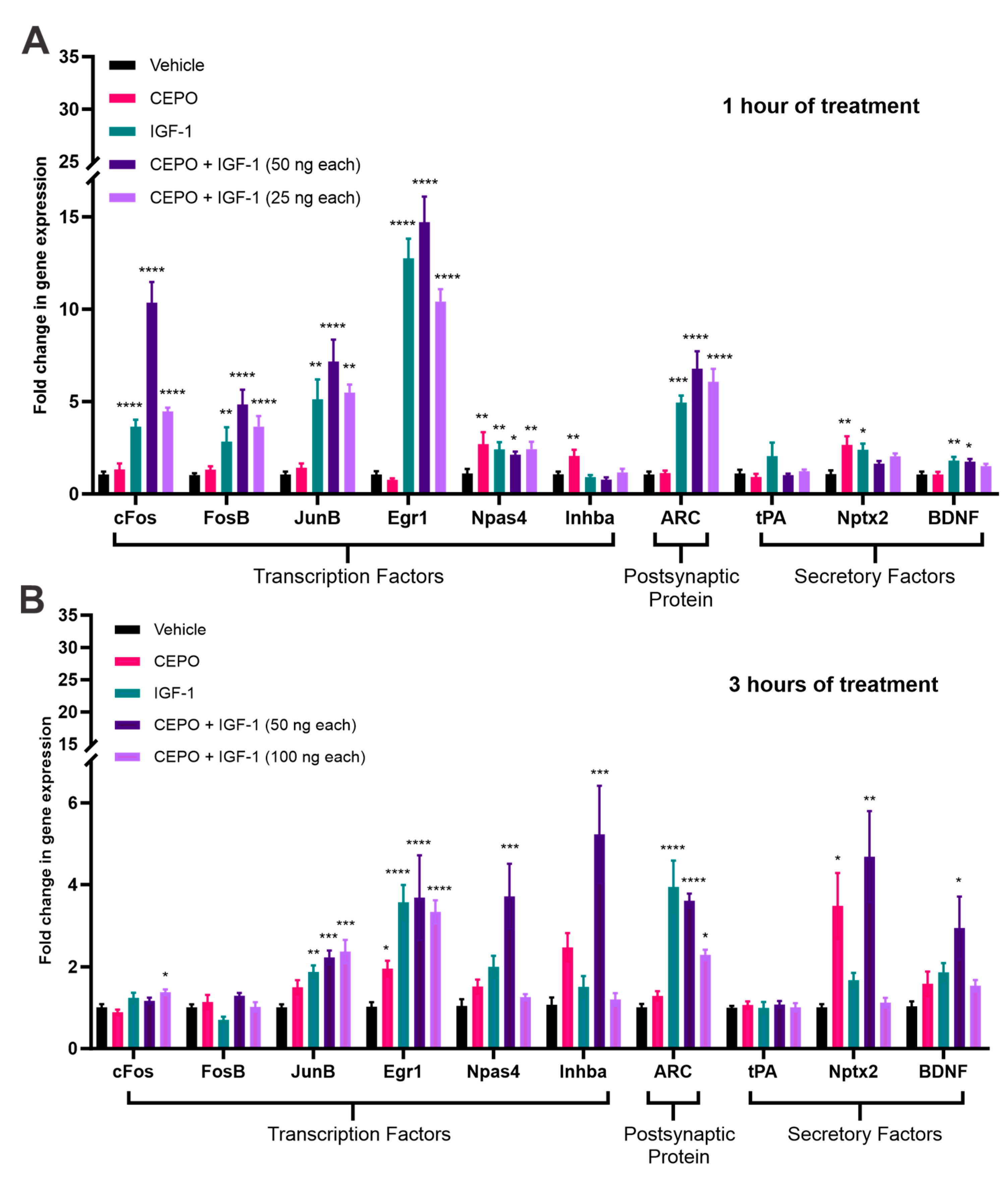
3.1. CEPO + IGF-1 Combination Therapy Robustly Activates IEG Expression
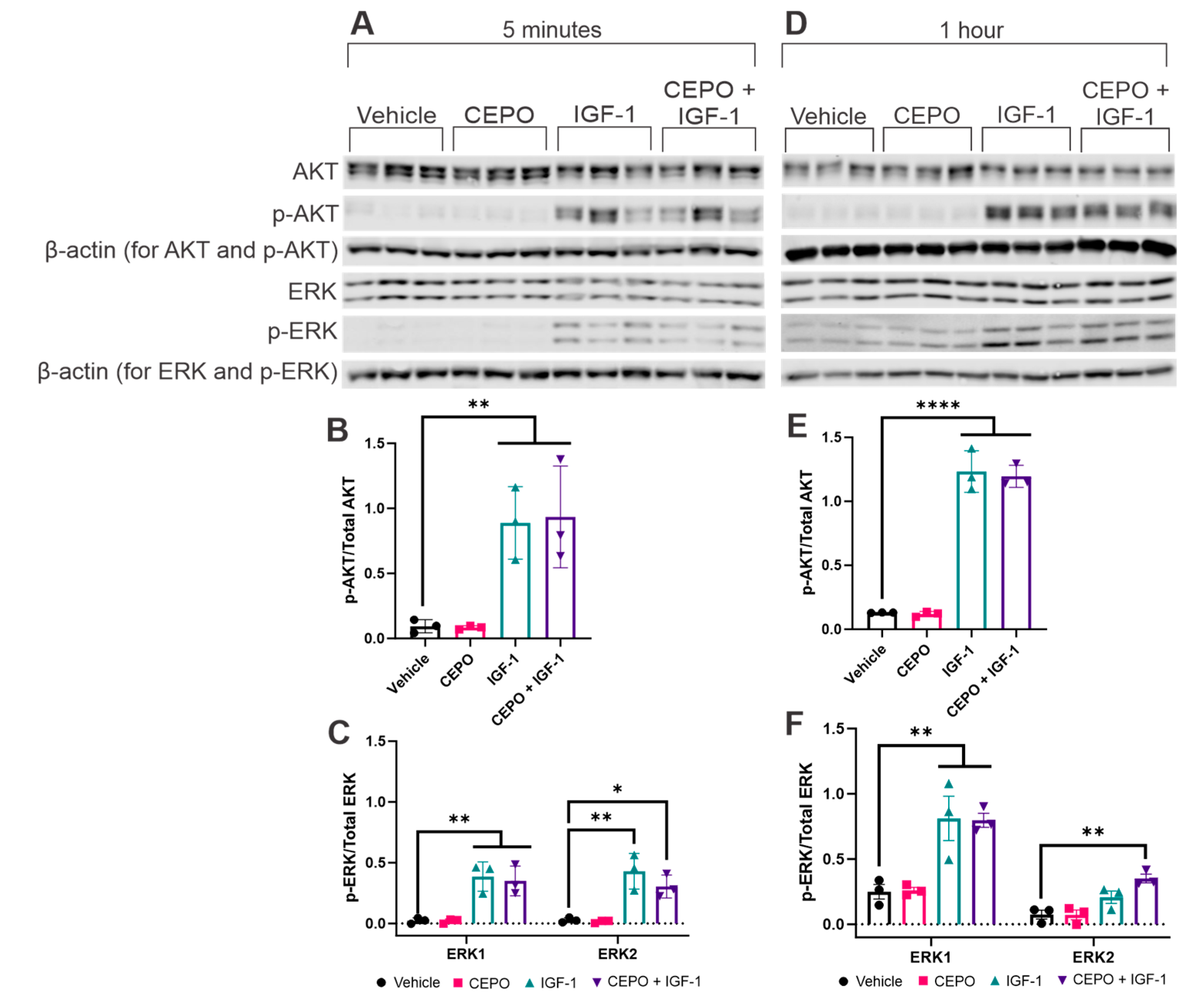
3.2. IGF-1, but Not CEPO, Upregultes AKT and ERK Phosphorylation
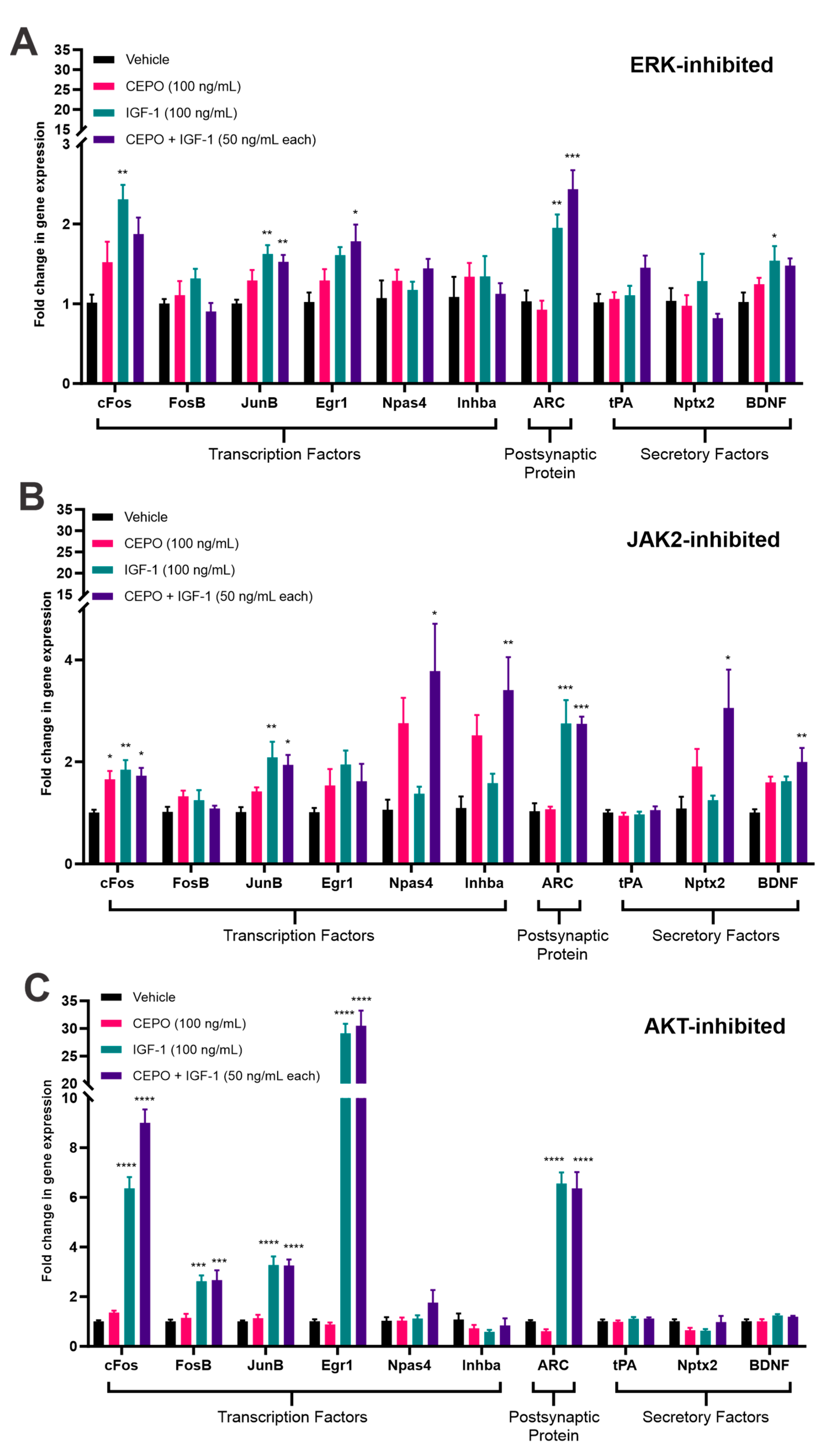
3.3. ERK and JAK2, but Not AKT, Inhibition Cause a Decrease in IEG Expression
3.4. IGF1R Levels Are More Than 50-Fold Higher Than EPOR, CD131, or IR in PC-12 Cells
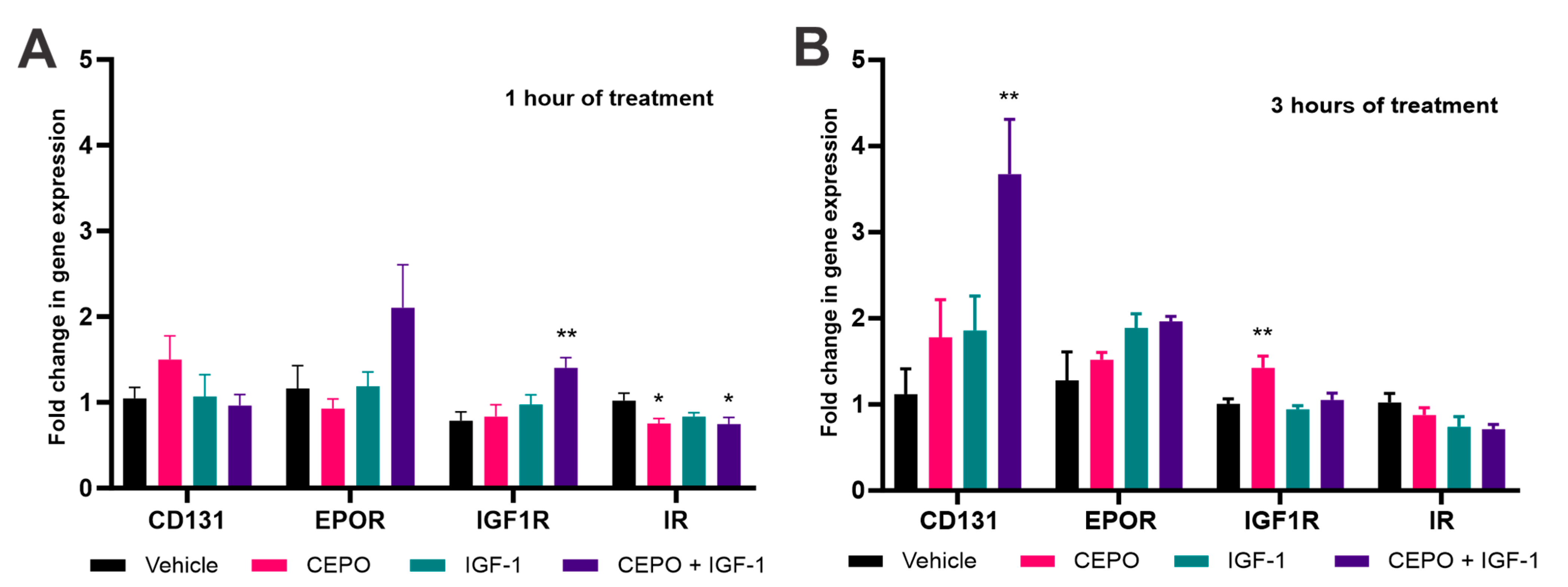
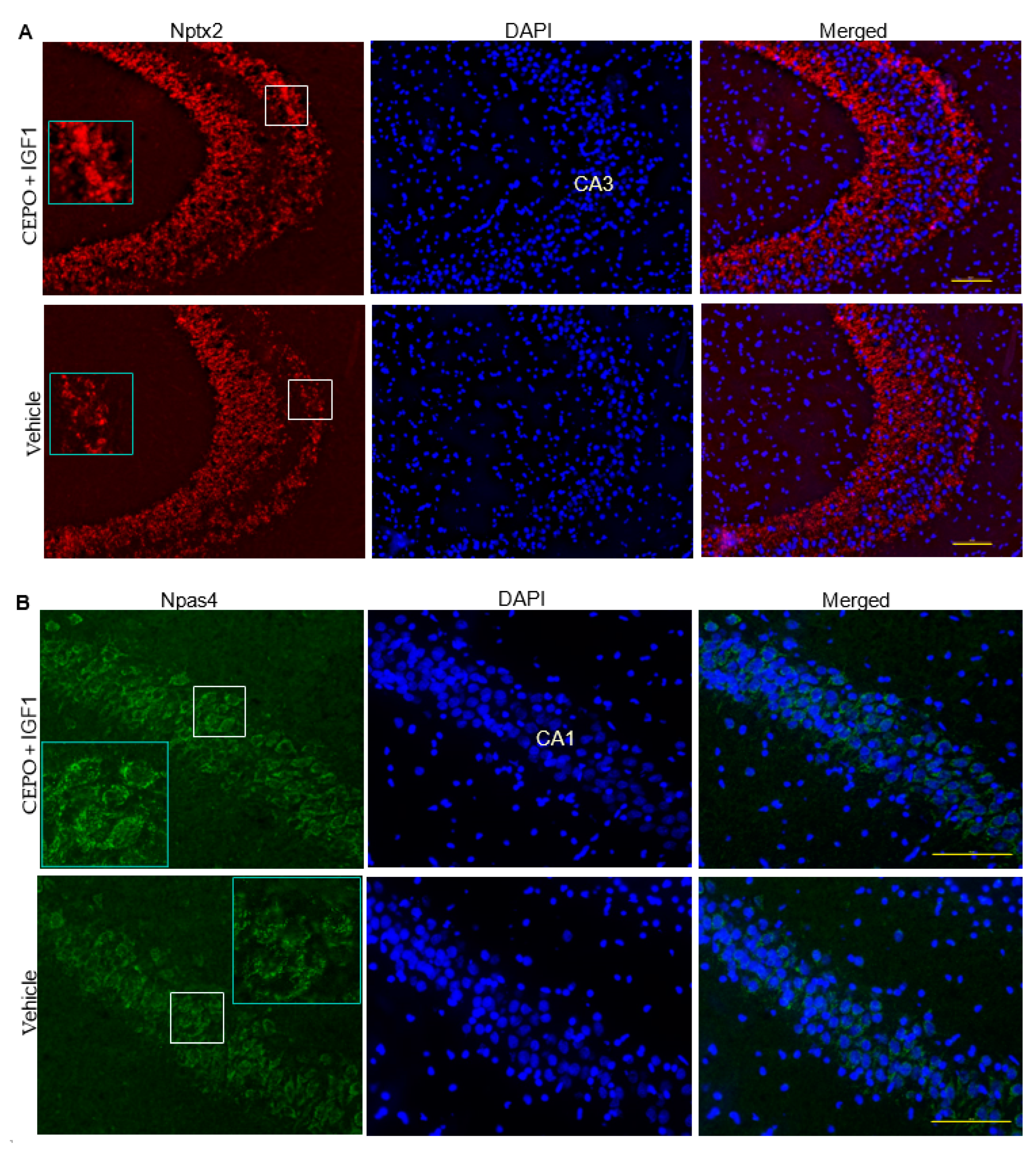
3.5. Npas4 and Nptx2 Are Regulated by CEPO + IGF-1 in the Rat Hippocampus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skaper, S.D. Neurotrophic Factors: An Overview. In Neurotrophic Factors: Methods and Protocols; Skaper, S.D., Ed.; Springer: New York, NY, USA, 2018; pp. 1–17. [Google Scholar]
- Segal, R.A.; Greenberg, M.E. Intracellular Signaling Pathways Activated by Neurotrophic Factors. Annu. Rev. Neurosci. 1996, 19, 463–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Luo, S.; Guo, X.; Luo, X.; Zhang, Y. Associations between brain-derived neurotrophic factor and cognitive impairment in panic disorder. Brain Behav. 2020, 10, e01885. [Google Scholar] [CrossRef]
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C.-M. Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Garcia, R.I.; Kwok, S.; Banerjee, A.; Petravicz, J.; Woodson, J.; Mellios, N.; Tropea, D.; Sur, M. Functional recovery with recombinant human IGF1 treatment in a mouse model of Rett Syndrome. Proc. Natl. Acad. Sci. USA 2014, 111, 9941–9946. [Google Scholar] [CrossRef] [PubMed]
- Costales, J.; Kolevzon, A. The therapeutic potential of insulin-like growth factor-1 in central nervous system disorders. Neurosci. Biobehav. Rev. 2016, 63, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.L.; Mooney, M.; Sonntag, W.E. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience 1998, 87, 559–569. [Google Scholar] [CrossRef]
- Miskowiak, K.W.; Vinberg, M.; Christensen, E.M.; Bukh, J.D.; Harmer, C.J.; Ehrenreich, H.; Kessing, L.V. Recombinant human erythropoietin for treating treatment-resistant depression: A double-blind, randomized, placebo-controlled phase 2 trial. Neuropsychopharmacology 2014, 39, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.W.; Vinberg, M.; Macoveanu, J.; Ehrenreich, H.; Køster, N.; Inkster, B.; Paulson, O.B.; Kessing, L.V.; Skimminge, A.; Siebner, H.R. Effects of Erythropoietin on Hippocampal Volume and Memory in Mood Disorders. Biol. Psychiatry 2015, 78, 270–277. [Google Scholar] [CrossRef]
- Kolevzon, A.; Breen, M.S.; Siper, P.M.; Halpern, D.; Frank, Y.; Rieger, H.; Weismann, J.; Trelles, M.P.; Lerman, B.; Rapaport, R.; et al. Clinical trial of insulin-like growth factor-1 in Phelan-McDermid syndrome. Mol. Autism 2022, 13, 17. [Google Scholar] [CrossRef]
- Miskowiak, K.; Inkster, B.; O’Sullivan, U.; Selvaraj, S.; Goodwin, G.M.; Harmer, C.J. Differential effects of erythropoietin on neural and cognitive measures of executive function 3 and 7 days post-administration. Exp. Brain Res. 2008, 184, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.; O’Sullivan, U.; Harmer, C.J. Erythropoietin enhances hippocampal response during memory retrieval in humans. J. Neurosci. 2007, 27, 2788–2792. [Google Scholar] [CrossRef] [PubMed]
- Digicaylioglu, M.; Garden, G.; Timberlake, S.; Fletcher, L.; Lipton, S.A. Acute neuroprotective synergy of erythropoietin and insulin-like growth factor I. Proc. Natl. Acad. Sci. USA 2004, 101, 9855–9860. [Google Scholar] [CrossRef]
- Fletcher, L.; Kohli, S.; Sprague, S.M.; Scranton, R.A.; Lipton, S.A.; Parra, A.; Jimenez, D.F.; Digicaylioglu, M. Intranasal delivery of erythropoietin plus insulin-like growth factor-I for acute neuroprotection in stroke. Laboratory investigation. J. Neurosurg. 2009, 111, 164–170. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Digicaylioglu, M.; Russo, R.; Kaul, M.; Achim, C.L.; Fletcher, L.; Masliah, E.; Lipton, S.A. Erythropoietin plus insulin-like growth factor-I protects against neuronal damage in a murine model of human immunodeficiency virus-associated neurocognitive disorders. Ann. Neurol. 2010, 68, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Leist, M.; Ghezzi, P.; Grasso, G.; Bianchi, R.; Villa, P.; Fratelli, M.; Savino, C.; Bianchi, M.; Nielsen, J.; Gerwien, J.; et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science 2004, 305, 239–242. [Google Scholar] [CrossRef]
- Tiwari, N.K.; Sathyanesan, M.; Kumar, V.; Newton, S.S. A Comparative Analysis of Erythropoietin and Carbamoylated Erythropoietin Proteome Profiles. Life 2021, 11, 359. [Google Scholar] [CrossRef]
- Sathyanesan, M.; Watt, M.J.; Haiar, J.M.; Scholl, J.L.; Davies, S.R.; Paulsen, R.T.; Wiederin, J.; Ciborowski, P.; Newton, S.S. Carbamoylated erythropoietin modulates cognitive outcomes of social defeat and differentially regulates gene expression in the dorsal and ventral hippocampus. Transl. Psychiatry 2018, 8, 113. [Google Scholar] [CrossRef]
- Adamcio, B.; Sargin, D.; Stradomska, A.; Medrihan, L.; Gertler, C.; Theis, F.; Zhang, M.; Müller, M.; Hassouna, I.; Hannke, K.; et al. Erythropoietin enhances hippocampal long-term potentiation and memory. BMC Biol. 2008, 6, 37. [Google Scholar] [CrossRef]
- Esmaeili Tazangi, P.; Moosavi, S.M.; Shabani, M.; Haghani, M. Erythropoietin improves synaptic plasticity and memory deficits by decrease of the neurotransmitter release probability in the rat model of Alzheimer’s disease. Pharmacol. Biochem. Behav. 2015, 130, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lykissas, G.M.; Batistatou, K.A.; Charalabopoulos, A.K.; Beris, E.A. The Role of Neurotrophins in Axonal Growth, Guidance, and Regeneration. Curr. Neurovasc. Res. 2007, 4, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Okuno, H. Regulation and function of immediate-early genes in the brain: Beyond neuronal activity markers. Neurosci. Res. 2011, 69, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Lanahan, A.; Worley, P. Immediate-early genes and synaptic function. Neurobiol. Learn. Mem. 1998, 70, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lin, Y. Npas4: Linking Neuronal Activity to Memory. Trends Neurosci. 2016, 39, 264–275. [Google Scholar] [CrossRef]
- Tiwari, N.K.; Sathyanesan, M.; Schweinle, W.; Newton, S.S. Carbamoylated erythropoietin induces a neurotrophic gene profile in neuronal cells. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 132–141. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Duman, C.H.; Schlesinger, L.; Terwilliger, R.; Russell, D.S.; Newton, S.S.; Duman, R.S. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav. Brain Res. 2009, 198, 366–371. [Google Scholar] [CrossRef]
- Sathyanesan, M.; Girgenti, M.J.; Warner-Schmidt, J.; Newton, S.S. Indomethacin induced gene regulation in the rat hippocampus. Mol. Brain 2015, 8, 59. [Google Scholar] [CrossRef]
- Atanasova, M.; Whitty, A. Understanding cytokine and growth factor receptor activation mechanisms. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 502–530. [Google Scholar] [CrossRef]
- Schneider, H.; Chaovapong, W.; Matthews, D.J.; Karkaria, C.; Cass, R.T.; Zhan, H.; Boyle, M.; Lorenzini, T.; Elliott, S.G.; Giebel, L.B. Homodimerization of erythropoietin receptor by a bivalent monoclonal antibody triggers cell proliferation and differentiation of erythroid precursors. Blood 1997, 89, 473–482. [Google Scholar] [CrossRef]
- De Meyts, P.; Wallach, B.; Christoffersen, C.T.; Ursø, B.; Grønskov, K.; Latus, L.J.; Yakushiji, F.; Ilondo, M.M.; Shymko, R.M. The insulin-like growth factor-I receptor. Structure, ligand-binding mechanism and signal transduction. Horm. Res. 1994, 42, 152–169. [Google Scholar] [CrossRef]
- Elliott, S.; Lorenzini, T.; Yanagihara, D.; Chang, D.; Elliott, G. Activation of the erythropoietin (EPO) receptor by bivalent anti-EPO receptor antibodies. J. Biol. Chem. 1996, 271, 24691–24697. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, Y.-J.; Li, D.; Gu, R.; Wang, W.-H. Dual effect of insulin-like growth factor on the apical 70-pS K channel in the thick ascending limb of rat kidney. Am. J. Physiol.-Cell Physiol. 2004, 286, C1258–C1263. [Google Scholar] [CrossRef]
- Chang, M.C.; Park, J.M.; Pelkey, K.A.; Grabenstatter, H.L.; Xu, D.; Linden, D.J.; Sutula, T.P.; McBain, C.J.; Worley, P.F. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat. Neurosci. 2010, 13, 1090–1097. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, S.; Chang, M.C.; Worley, P.; Kirkwood, A.; Quinlan, E.M. Obligatory role for the immediate early gene NARP in critical period plasticity. Neuron 2013, 79, 335–346. [Google Scholar] [CrossRef]
- Xu, D.; Hopf, C.; Reddy, R.; Cho, R.W.; Guo, L.; Lanahan, A.; Petralia, R.S.; Wenthold, R.J.; O’Brien, R.J.; Worley, P. Narp and NP1 Form Heterocomplexes that Function in Developmental and Activity-Dependent Synaptic Plasticity. Neuron 2003, 39, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wade, S.D.; Graykowski, D.; Xiao, M.-F.; Zhao, B.; Giannini, L.A.A.; Hanson, J.E.; van Swieten, J.C.; Sheng, M.; Worley, P.F.; et al. The neuronal pentraxin Nptx2 regulates complement activity and restrains microglia-mediated synapse loss in neurodegeneration. Sci. Transl. Med. 2023, 15, eadf0141. [Google Scholar] [CrossRef]
- Xiao, M.F.; Xu, D.; Craig, M.T.; Pelkey, K.A.; Chien, C.C.; Shi, Y.; Zhang, J.; Resnick, S.; Pletnikova, O.; Salmon, D.; et al. NPTX2 and cognitive dysfunction in Alzheimer’s Disease. Elife 2017, 6, e23798. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.-F.; Roh, S.-E.; Zhou, J.; Chien, C.-C.; Lucey, B.P.; Craig, M.T.; Hayes, L.N.; Coughlin, J.M.; Leweke, F.M.; Jia, M.; et al. A biomarker-authenticated model of schizophrenia implicating NPTX2 loss of function. Sci. Adv. 2021, 7, eabf6935. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Bloodgood, B.L.; Hauser, J.L.; Lapan, A.D.; Koon, A.C.; Kim, T.-K.; Hu, L.S.; Malik, A.N.; Greenberg, M.E. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature 2008, 455, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Pollina, E.A.; Gilliam, D.T.; Landau, A.T.; Lin, C.; Pajarillo, N.; Davis, C.P.; Harmin, D.A.; Yap, E.-L.; Vogel, I.R.; Griffith, E.C.; et al. A NPAS4–NuA4 complex couples synaptic activity to DNA repair. Nature 2023, 614, 732–741. [Google Scholar] [CrossRef]
- Ramamoorthi, K.; Fropf, R.; Belfort, G.M.; Fitzmaurice, H.L.; McKinney, R.M.; Neve, R.L.; Otto, T.; Lin, Y. Npas4 Regulates a Transcriptional Program in CA3 Required for Contextual Memory Formation. Science 2011, 334, 1669–1675. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Yin, J.; Zhang, J.; Jiang, Y. Insulin-like growth factor receptor signaling in tumorigenesis and drug resistance: A challenge for cancer therapy. J. Hematol. Oncol. 2020, 13, 64. [Google Scholar] [CrossRef]
- Bahrami, S.; Drabløs, F. Gene regulation in the immediate-early response process. Adv. Biol. Regul. 2016, 62, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Dahlke, S.P.; Sung, M.; Samal, B.; Emery, A.C.; Elkahloun, A.; Eiden, L.E. ERK-dependent induction of the immediate-early gene Egr1 and the late gene Gpr50 contribute to two distinct phases of PACAP Gs-GPCR signaling for neuritogenesis. J. Neuroendocr. 2022, 34, e13182. [Google Scholar] [CrossRef]
- Sgambato, V.; Pagès, C.; Rogard, M.; Besson, M.J.; Caboche, J. Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. J. Neurosci. 1998, 18, 8814–8825. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.; Hernández-Losa, J.; Lyons, R.J.; Castellone, M.D.; Gutkind, J.S. Regulation of the Transcriptional Activity of c-Fos by ERK: A NOVEL ROLE FOR THE PROLYL ISOMERASE PIN1*. J. Biol. Chem. 2005, 280, 35081–35084. [Google Scholar] [CrossRef]
- Siddhartha, G.J.; Catherine, Y.; Jared, E.T. Dynamics and heterogeneity of Erk-induced immediate-early gene expression. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ohe, S.; Kubota, Y.; Yamaguchi, K.; Takagi, Y.; Nashimoto, J.; Kozuka-Hata, H.; Oyama, M.; Furukawa, Y.; Takekawa, M. ERK-mediated NELF-A phosphorylation promotes transcription elongation of immediate-early genes by releasing promoter-proximal pausing of RNA polymerase II. Nat. Commun. 2022, 13, 7476. [Google Scholar] [CrossRef]
| EPOR | CD131 | IGF1R | IR |
|---|---|---|---|
| 7.0 | 0.9 | 584.8 | 5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rothschadl, M.J.; Sathyanesan, M.; Newton, S.S. Synergism of Carbamoylated Erythropoietin and Insulin-like Growth Factor-1 in Immediate Early Gene Expression. Life 2023, 13, 1826. https://doi.org/10.3390/life13091826
Rothschadl MJ, Sathyanesan M, Newton SS. Synergism of Carbamoylated Erythropoietin and Insulin-like Growth Factor-1 in Immediate Early Gene Expression. Life. 2023; 13(9):1826. https://doi.org/10.3390/life13091826
Chicago/Turabian StyleRothschadl, Morgan J., Monica Sathyanesan, and Samuel S. Newton. 2023. "Synergism of Carbamoylated Erythropoietin and Insulin-like Growth Factor-1 in Immediate Early Gene Expression" Life 13, no. 9: 1826. https://doi.org/10.3390/life13091826
APA StyleRothschadl, M. J., Sathyanesan, M., & Newton, S. S. (2023). Synergism of Carbamoylated Erythropoietin and Insulin-like Growth Factor-1 in Immediate Early Gene Expression. Life, 13(9), 1826. https://doi.org/10.3390/life13091826






