Allergic Proctocolitis: Literature Review and Proposal of a Diagnostic–Therapeutic Algorithm
Abstract
1. Introduction
2. Epidemiology
3. Pathogenesis
4. Clinical Manifestations
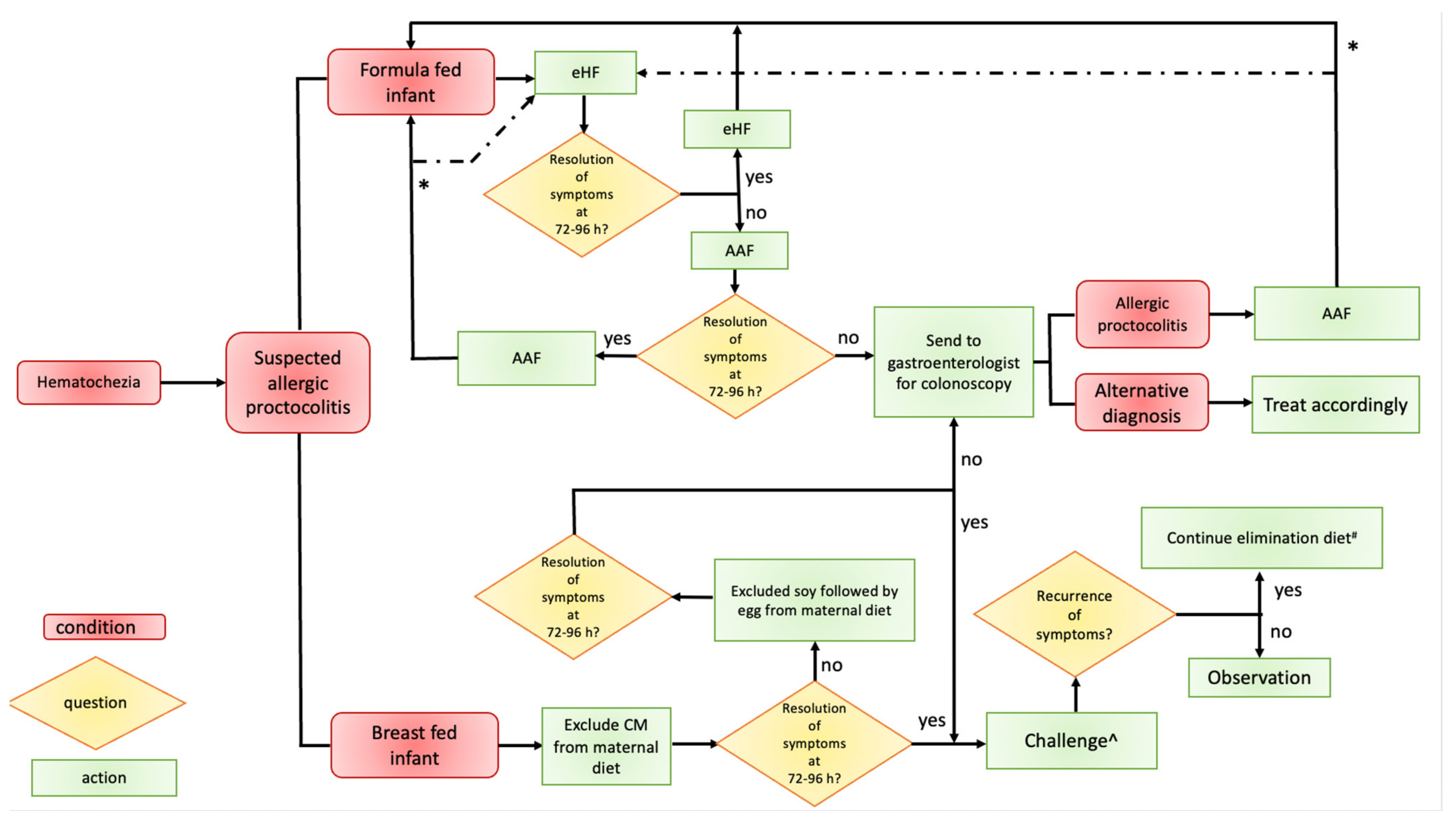
5. Diagnosis
- mild rectal bleeding in an otherwise healthy patient;
- resolution of clinical manifestations after elimination of trigger food(s) (if breastfed, resolution after maternal elimination diet);
- reappearance of symptoms on reintroduction of the trigger food(s);
- exclusion of other causes of rectal bleeding.
6. Non-Invasive Tests
7. Invasive Tests
8. Differential Diagnosis
9. Treatment
9.1. Exclusively Breastfed Infants
9.2. Infants Fed with Formula or Mixed Feeding
10. Reintroduction
10.1. Timing
10.2. Duration of the Elimination Diet
10.3. Setting
10.4. Mode of Introduction in a Hospital Setting
10.5. Method of Introduction at Home
- For formula-fed or no-longer-breastfed infants: 30 mL of cow’s milk is added to the amount of formula the infant is currently taking and increased by 30 mL every 2–3 days until the desired dose is reached [27]. This mode of gradual introduction is continued until an age-appropriate dose of cow’s milk is reached. The child is observed clinically to monitor for any recurrence of hematochezia, diarrhea, irritability, or other clinical manifestations. Any recurrence of the clinical manifestations generally occurs within 1–2 weeks of the introduction of the allergen. Reintroduction of cow’s milk is usually tolerated. If this fails, one option may be to introduce dairy-containing baked goods into the child’s diet before introducing pure cow’s milk [27].
10.6. Recurrence
11. Multidisciplinary Management of the AP
12. Parental Involvement
13. Prognosis
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Connors, L.; Keefe, A.O.; Rosenfield, L.; Kim, H. Non-IgE-mediated food hypersensitivity. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. S2), 56. [Google Scholar] [CrossRef]
- Mennini, M.; Fiocchi, A.G.; Cafarotti, A.; Montesano, M.; Mauro, A.; Villa, M.P.; Di Nardo, G. Food protein-induced allergic proctocolitis in infants: Literature review and proposal of a management protocol. World Allergy Organ. J. 2020, 13, 100471. [Google Scholar] [CrossRef]
- Rubin, M. Allergic intestinal bleeding in the newborn. Am. J. Med. Sci. 1940, 200, 385. [Google Scholar] [CrossRef]
- Gryboski, J.D.; Burkle, F.; Hillman, R. Milk induced colitis in an infant. Pediatrics 1966, 38, 299–302. [Google Scholar] [CrossRef]
- Gryboski, J.D. Gastrointestinal milk allergy in infants. Pediatrics 1967, 40, 354–362. [Google Scholar] [CrossRef]
- Elizur, A.; Cohen, M.; Goldberg, M.R.; Rajuan, N.; Cohen, A.; Leshno, M.; Katz, Y. Cow’s milk associated rectal bleeding: A population based prospective study. Pediatr. Allergy Immunol. 2012, 23, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Xanthakos, S.A.; Schwimmer, J.B.; Melin-Aldana, H.; Rothenberg, M.E.; Witte, D.P.; Cohen, M.B. Prevalence and Outcome of Allergic Colitis in Healthy Infants with Rectal Bleeding: A Prospective Cohort Study. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 16–22. [Google Scholar] [CrossRef]
- Arvola, T.; Ruuska, T.; Keränen, J.; Hyöty, H.; Salminen, S.; Isolauri, E. Rectal Bleeding in Infancy: Clinical, Allergological, and Microbiological Examination. Pediatrics 2006, 117, e760–e768. [Google Scholar] [CrossRef]
- Senocak, N.; Ertugrul, A.; Ozmen, S.; Bostanci, I. Clinical Features and Clinical Course of Food Protein—Induced Allergic Proctocolitis: 10-Year Experience of a Tertiary Medical Center. J. Allergy Clin. Immunol. Pract. 2022, 10, 1608–1613. [Google Scholar] [CrossRef]
- Martin, V.M.; Virkud, Y.V.; Seay, H.; Hickey, A.; Ndahayo, R.; Rosow, R.; Southwick, C.; Elkort, M.; Gupta, B.; Kramer, E.; et al. Prospective Assessment of Pediatrician-Diagnosed Food Protein–Induced Allergic Proctocolitis by Gross or Occult Blood. J. Allergy Clin. Immunol. Pract. 2020, 8, 1692–1699.e1. [Google Scholar] [CrossRef]
- Caubet, J.-C.; Szajewska, H.; Shamir, R.; Nowak-Węgrzyn, A. Non-IgE-mediated gastrointestinal food allergies in children. Pediatr. Allergy Immunol. 2017, 28, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Lozinsky, A.C.; Fleischer, D.M.; Vieira, M.C.; Du Toit, G.; Vandenplas, Y.; Dupont, C.; Knibb, R.; Uysal, P.; Cavkaytar, O.; et al. Diagnosis and management of Non-IgE gastrointestinal allergies in breastfed infants—An EAACI Position Paper. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Tsabouri, S.; Nicolaou, N.; Douros, K.; Papadopoulou, A.; Priftis, K.N. Food Protein Induced Proctocolitis: A Benign Condition with an Obscure Immunologic Mechanism. Endocr. Metab. Immune Disord. Drug Targets 2017, 17, 32–37. [Google Scholar] [CrossRef]
- Chehade, M.; Mayer, L. Oral tolerance and its relation to food hypersensitivities. J. Allergy Clin. Immunol. 2005, 115, 3–12. [Google Scholar] [CrossRef]
- Pérez-Machado, M.A.; Ashwood, P.; Thomson, M.A.; Latcham, F.; Sim, R.; Walker-Smith, J.A.; Murch, S.H. Reduced transforming growth factor-β1-producing T cells in the duodenal mucosa of children with food allergy. Eur. J. Immunol. 2003, 33, 2307–2315. [Google Scholar] [CrossRef]
- Van Wijk, F.; Nierkens, S.; de Jong, W.; Wehrens, E.J.M.; Boon, L.; van Kooten, P.; Knippels, L.M.J.; Pieters, R. The CD28/CTLA-4-B7 Signaling Pathway Is Involved in Both Allergic Sensitization and Tolerance Induction to Orally Administered Peanut Proteins. J. Immunol. 2007, 178, 6894–6900. [Google Scholar] [CrossRef]
- Beyer, K.; Castro, R.; Birnbaum, A.; Benkov, K.; Pittman, N.; Sampson, H.A. Human milk–specific mucosal lymphocytes of the gastrointestinal tract display a TH2 cytokine profile. J. Allergy Clin. Immunol. 2002, 109, 707–713. [Google Scholar] [CrossRef]
- Ozen, A.; Gulcan, E.M.; Ercan Saricoban, H.; Ozkan, F.; Cengizlier, R. Food Protein-Induced Non-Immunoglobulin E-Mediated Allergic Colitis in Infants and Older Children: What Cytokines Are Involved? Int. Arch. Allergy Immunol. 2015, 168, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-alfa -induced increase in intestinal epithelial tight junction permeability requires NF-kappa-B activation. Am. J. Physiol. Gastrointest Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef]
- Morita, H.; Nomura, I.; Orihara, K.; Yoshida, K.; Akasawa, A.; Tachimoto, H.; Ohtsuka, Y.; Namai, Y.; Futamura, M.; Shoda, T.; et al. Antigen-specific T-cell responses in patients with non–IgE-mediated gastrointestinal food allergy are predominantly skewed to TH2. J. Allergy Clin. Immunol. 2013, 131, 590–592.e6. [Google Scholar] [CrossRef]
- Kumagai, H.; Maisawa, S.-I.; Tanaka, M.; Takahashi, M.; Takasago, Y.; Nishijima, A.; Watanabe, S. Intestinal microbiota and secretory immunoglobulin A in feces of exclusively breast-fed infants with blood-streaked stools. Microbiol. Immunol. 2012, 56, 657–663. [Google Scholar] [CrossRef]
- Baldassarre, M.E.; Laforgia, N.; Fanelli, M.; Laneve, A.; Grosso, R.; Lifschitz, C. Lactobacillus GG Improves Recovery in Infants with Blood in the Stools and Presumptive Allergic Colitis Compared with Extensively Hydrolyzed Formula Alone. J. Pediatr. 2010, 156, 397–401. [Google Scholar] [CrossRef]
- Liu, S.-X.; Li, Y.-H.; Dai, W.-K.; Li, X.-S.; Qiu, C.-Z.; Ruan, M.-L.; Zou, B.; Dong, C.; Liu, Y.-H.; He, J.-Y.; et al. Fecal microbiota transplantation induces remission of infantile allergic colitis through gut microbiota re-establishment. World J. Gastroenterol. 2017, 23, 8570–8581. [Google Scholar] [CrossRef]
- Boné, J.; Claver, A.; Guallar, I.; Plaza, A. Allergic proctocolitis, food-induced enterocolitis: Immune mechanisms, diagnosis and treatment. Allergol. Immunopathol. 2009, 37, 36–42. [Google Scholar] [CrossRef]
- Zubeldia-Varela, E.; Barker-Tejeda, T.C.; Blanco-Pérez, F.; Infante, S.; Zubeldia, J.M.; Pérez-Gordo, M. Non-IgE-Mediated Gastrointestinal Food Protein-Induced Allergic Disorders. Clinical Perspectives and Analytical Approaches. Foods 2021, 10, 2662. [Google Scholar] [CrossRef]
- Nowak-Węgrzyn, A. Food protein-induced enterocolitis syndrome and allergic proctocolitis. Allergy Asthma Proc. 2015, 36, 172–184. [Google Scholar] [CrossRef]
- Liacouras, C.A. Food Protein-Induced Allergic Proctocolitis of Intancy-UpToDate. Available online: https://www.uptodate.com/contents/food-protein-induced-allergic-proctocolitis-of-infancy (accessed on 27 February 2023).
- Ravelli, A.; Villanacci, V.; Chiappa, S.; Bolognini, S.; Manenti, S.; Fuoti, M. Dietary Protein-Induced Proctocolitis in Childhood. Am. J. Gastroenterol. 2008, 103, 2605–2612. [Google Scholar] [CrossRef]
- Uncuoğlu, A.; Aydoğan, M.; Şimşek, I.E.; Çöğürlü, M.T.; Uçak, K.; Acar, H.C. A Prospective Assessment of Clinical Characteristics and Responses to Dietary Elimination in Food Protein–Induced Allergic Proctocolitis. J. Allergy Clin. Immunol. Pract. 2022, 10, 206–214.e1. [Google Scholar] [CrossRef]
- Lake, A.M. Food-Induced Eosinophilic Proctocolitis. J. Pediatr. Gastroenterol. Nutr. 2000, 30, S58–S60. [Google Scholar] [CrossRef]
- Kaya, A.; Toyran, M.; Civelek, E.; Misirlioglu, E.; Kirsaclioglu, C.; Kocabas, C.N. Characteristics and Prognosis of Allergic Proctocolitis in Infants. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 69–73. [Google Scholar] [CrossRef]
- Erdem, S.B.; Nacaroglu, H.T.; Karaman, S.; Erdur, C.B.; Karkıner, C.U.; Can, D. Tolerance development in food protein-induced allergic proctocolitis: Single centre experience. Allergol. Immunopathol. 2017, 45, 212–219. [Google Scholar] [CrossRef]
- Koksal, B.T.; Barıs, Z.; Ozcay, F.; Yilmaz Ozbek, O. Single and multiple food allergies in infants with proctocolitis. Allergol. Immunopathol. 2018, 46, 3–8. [Google Scholar] [CrossRef]
- Buyuktiryaki, B.; Celik, I.K.; Erdem, S.B.; Capanoglu, M.; Civelek, E.; Guc, B.U.; Guvenir, H.; Cakir, M.; Misirlioglu, E.D.; Akcal, O.; et al. Risk Factors Influencing Tolerance and Clinical Features of Food Protein–induced Allergic Proctocolitis. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 574–579. [Google Scholar] [CrossRef]
- Cetinkaya, P.G.; Kahveci, M.; Karaatmaca, B.; Esenboga, S.; Sahiner, U.M.; Sekerel, B.E.; Soyer, O. Predictors for late tolerance development in food protein-induced allergic proctocolitis. Allergy Asthma Proc. 2020, 41, E11–E18. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000, 106 Pt 1, 346–349. [Google Scholar] [CrossRef]
- Labrosse, R.; Graham, F.; Caubet, J.-C. Non-IgE-Mediated Gastrointestinal Food Allergies in Children: An Update. Nutrients 2020, 12, 2086. [Google Scholar] [CrossRef]
- Miceli Sopo, S.; Monaco, S.; Bersani, G.; Romano, A.; Fantacci, C. Proposal for management of the infant with suspected food protein-induced allergic proctocolitis. Pediatr. Allergy Immunol. 2018, 29, 215–218. [Google Scholar] [CrossRef]
- Venter, C.; Brown, T.; Shah, N.; Walsh, J.; Fox, A.T. Diagnosis and management of non-IgE-mediated cow’s milk allergy in infancy—A UK primary care practical guide. Clin. Transl. Allergy 2013, 3, 23. [Google Scholar] [CrossRef]
- Odze, R.D.; Bines, J.; Leichtner, A.M.; Goldman, H.; Antonioli, D.A. Allergic proctocolitis in infants: A prospective clinicopathologic biopsy study. Hum. Pathol. 1993, 24, 668–674. [Google Scholar] [CrossRef]
- Lozinsky, A.C.; de Morais, M.B. Eosinophilic colitis in infants. J. Pediatr. 2014, 90, 16–21. [Google Scholar] [CrossRef]
- Nowak-Węgrzyn, A.; Katz, Y.; Mehr, S.S.; Koletzko, S. Non-IgE-mediated gastrointestinal food allergy. J. Allergy Clin. Immunol. 2015, 135, 1114–1124. [Google Scholar] [CrossRef]
- Chang, J.; Wu, T.; Wang, K.; Huang, I.; Huang, B. Colon Mucosal Pathology in Infants Under Three Months of Age with Diarrhea Disorders. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Voganatsi, A.; Panyutich, A.; Miyasaki, K.T.; Murthy, R.K. Mechanism of extracellular release of human neutrophil calprotectin complex. J. Leukoc. Biol. 2001, 70, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Beşer, Ö.F.; Sancak, S.; Erkan, T.; Kutlu, T.; Çokuğraş, H.; Çokuğraş, F.Ç. Can Fecal Calprotectin Level Be Used as a Markers of Inflammation in the Diagnosis and Follow-Up of Cow’s Milk Protein Allergy? Allergy Asthma Immunol. Res. 2014, 6, 33. [Google Scholar] [CrossRef]
- Lee, Y.M.; Min, C.-Y.; Choi, Y.J.; Jeong, S.J. Delivery and feeding mode affects fecal calprotectin levels in infants < 7 months old. Early Hum. Dev. 2017, 108, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Castagno, E.; Viola, S. Fecal calprotectin in infants with presumptive allergic colitis. J. Pediatr. 2010, 157, 174. [Google Scholar] [CrossRef]
- Joshi, S.; Lewis, S.J.; Creanor, S.; Ayling, R.M. Age-related faecal calprotectin, lactoferrin and tumour M2-PK concentrations in healthy volunteers. Ann. Clin. Biochem. Int. J. Lab. Med. 2010, 47, 259–263. [Google Scholar] [CrossRef]
- Björkström, M.V.; Hall, L.; Söderlund, S.; Håkansson, E.G.; Håkansson, S.; Domellöf, M. Intestinal flora in very low-birth weight infants. Acta Paediatr. 2009, 98, 1762–1767. [Google Scholar] [CrossRef]
- Fagerberg, U.L.; Lööf, L.; Merzoug, R.D.; Hansson, L.-O.; Finkel, Y. Fecal Calprotectin Levels in Healthy Children Studied With an Improved Assay. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 468–472. [Google Scholar] [CrossRef]
- Campeotto, F.; Kalach, N.; Lapillonne, A.; Butel, M.J.; Dupont, C.; Kapel, N. Time course of faecal calprotectin in preterm newborns during the first month of life. Acta Paediatr. 2007, 96, 1531–1533. [Google Scholar] [CrossRef]
- Mennini, M.; Fierro, V.; Di Nardo, G.; Pecora, V.; Fiocchi, A. Microbiota in non-IgE-mediated food allergy. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 323–328. [Google Scholar] [CrossRef]
- Epifanio, M.; Spolidoro, J.V.; Missima, N.G.; Soder, R.B.; Garcia, P.C.R.; Baldisserotto, M. Cow’s milk allergy: Color Doppler ultrasound findings in infants with hematochezia. J. Pediatr. 2013, 89, 554–558. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Winter, H.S.; Antonioli, D.A.; Fukagawa, N.; Marcial, M.; Goldman, H. Allergy-related proctocolitis in infants: Diagnostic usefulness of rectal biopsy. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 1990, 3, 5–10. [Google Scholar]
- Sorea, S.; Dabadie, A.; Bridoux-Henno, L.; Balançon-Morival, M.; Jouan, H.; Le Gall, E. Hemorrhagic colitis in exclusively breast-fed infants. Arch Pediatr. 2003, 10, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Dargent, J.-L.; Souayah, H.; Hainaut, M. Granulomatous variant of allergic proctocolitis. Histopathology 2009, 55, 758–760. [Google Scholar] [CrossRef]
- Quitadamo, P.; Isoldi, S.; Mallardo, S.; Zenzeri, L.; Ceccanti, S.; Battagliere, I.; Del Bene, M.; Di Nardo, G. Rectal bleeding in infants: Diagnostic work-up and management. Curr. Pediatr. Rev. 2023, 19. [Google Scholar] [CrossRef]
- Patel, N. Lower Gastrointestinal Bleeding in Children. Available online: https://www.uptodate.com/contents/lower-gastrointestinal-bleeding-in-children-causes-and-diagnostic-approach (accessed on 27 February 2023).
- Leung, A.K.C.; Barankin, B.; Leong, K.F. Henoch-Schönlein Purpura in Children: An Updated Review. Curr. Pediatr. Rev. 2020, 16, 265–276. [Google Scholar] [CrossRef]
- Sangkhathat, S.; Patrapinyokul, S.; Wudhisuthimethawee, P.; Chedphaopan, J.; Mitamun, W. Massive gastrointestinal bleeding in infants with ascariasis. J. Pediatr. Surg. 2003, 38, 1696–1698. [Google Scholar] [CrossRef]
- Nowak-Węgrzyn, A.; Chehade, M.; Groetch, M.E.; Spergel, J.M.; Wood, R.A.; Allen, K.; Atkins, D.; Bahna, S.; Barad, A.V.; Berin, C.; et al. International consensus guidelines for the diagnosis and management of food protein–induced enterocolitis syndrome: Executive summary—Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2017, 139, 1111–1126.e4. [Google Scholar] [CrossRef]
- Lake, A.M.; Whitington, P.F.; Hamilton, S.R. Dietary protein-induced colitis in breast-fed infants. J. Pediatr. 1982, 101, 906–910. [Google Scholar] [CrossRef]
- Sampson, H.A.; Aceves, S.; Bock, S.A.; James, J.; Jones, S.; Lang, D.; Nadeau, K.; Nowak-Wegrzyn, A.; Oppenheimer, J.; Perry, T.T.; et al. Practice parameter Food allergy: A practice parameter update—2014. J. Allergy Clin. Immunol. 2014, 134, 1016–1025.e43. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y. Prevention and Management of Cow’s Milk Allergy in Non-Exclusively Breastfed Infants. Nutrients 2017, 9, 731. [Google Scholar] [CrossRef]
- Lucarelli, S.; Di Nardo, G.; Lastrucci, G.; D’Alfonso, Y.; Marcheggiano, A.; Federici, T.; Frediani, S.; Frediani, T.; Cucchiara, S. Allergic proctocolitis refractory to maternal hypoallergenic diet in exclusively breast-fed infants: A clinical observation. BMC Gastroenterol. 2011, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Qamer, S.; Deshmukh, M.; Patole, S. Probiotics for cow’s milk protein allergy: A systematic review of randomized controlled trials. Eur. J. Pediatr. 2019, 178, 1139–1149. [Google Scholar] [CrossRef]
- Anveden-Hertzberg, L.; Finkel, Y.; Sandstedt, B.; Karpe, B. Protocolitis in exclusively breast-fed infants. Eur. J. Pediatr. 1996, 155, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Sancakli, O.; Akın Aslan, A. Effects of elimination diets and clinical findings on mothers’ anxiety in infants with food allergy with non-life-threatening reactions. Eur. Ann. Allergy Clin. Immunol. 2022, 54, 108. [Google Scholar] [CrossRef]
- Lazare, F.B.; Brand, D.A.; Marciano, T.A.; Daum, F. Rapid resolution of milk protein intolerance in infancy. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.M.; Virkud, Y.V.; Phadke, N.A.; Su, K.-W.; Seay, H.; Atkins, M.R.; Keet, C.; Shreffler, W.G.; Yuan, Q. Increased IgE-Mediated Food Allergy With Food Protein-Induced Allergic Proctocolitis. Pediatrics 2020, 146, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, G.; Cremon, C.; Frediani, S.; Lucarelli, S.; Villa, M.P.; Stanghellini, V.; La Torre, G.; Martemucci, L.; Barbara, G. Allergic Proctocolitis Is a Risk Factor for Functional Gastrointestinal Disorders in Children. J. Pediatr. 2018, 195, 128–133.e1. [Google Scholar] [CrossRef]
- Hill, S.M.; Milla, P.J. Colitis caused by food allergy in infants. Arch. Dis. Child. 1990, 65, 132–133. [Google Scholar] [CrossRef]
| Age of onset of symptoms | First weeks to months of life (less than 6 months); it can occasionally occur in older children |
| Trigger foods | Most common: cow’s milk, soy Less common: egg, corn, wheat |
| Multiple food triggers | Occasional |
| Type of feeding at diagnosis | Breastfeeding (>60%) |
| Clinical manifestations | Presence of bright red blood with or without mucus in stool with or without diarrhea in otherwise healthy children Less common: flatulence, refusal to feed, abdominal colic |
| Atopic comorbidities | Eczema: 22–52% Atopic family history: 25–50% |
| Laboratory tests | Mild anemia Eosinophilia Increase in total IgE (occasional) Hypoalbuminemia (rare) |
| Stool exam | Eosinophils Visible or occult blood |
| Endoscopy/histology | Focal colitis, eosinophilic infiltrate, lymph node hyperplasia |
| Allergy test * (SPT and s-IgE) | Usually negative, positive in 10–35% of cases |
| Diagnosis | Clinical history and examination +/− OFC |
| Treatment | Avoidance of trigger food(s) (if breastfed, only consider exclusion of these foods in maternal diet) |
| Resolution of clinical symptoms with elimination diet | 72–96 h |
| Natural history | Resolution in the first year of life |
| Cow’s milk | In the breastfed infant: 1st choice: maternal elimination diet 2nd choice: eHF 3rd choice: AAF In the formula-fed infant: 1st choice: eHF 2nd choice: AAF if eHF fails |
| Soy | Elimination diet if there is no clinical response to cow’s milk elimination diet |
| Egg | Elimination diet in the case that there is no clinical response to the elimination of cow’s milk and soy |
| Pediatrician | - Manages the elimination diet in mild cases - Refers to allergist specialist |
| Allergologist | - Performs allergy tests to decide reintroduction settings and to assess prognosis - Confirm diagnosis with OFC - Considers prescribing eHF or AAF |
| Gastroenterologist | - Handles complex clinical cases - Performs endoscopic examination if necessary |
| Dietitian | - Supports patients with prolonged and/or multi-food diets |
| Psychologist | - Possible support to mothers in case of anxiety, especially if exclusion diets involve multiple foods |
| Therapeutic Options | Advantages | Disadvantages |
|---|---|---|
| Empiric diet | - Simple and effective - Can be managed by the pediatrician | - Risk of overdiagnosis - No definitive diagnosis - Often longer than necessary |
| Elimination diet after diagnostic OFC | - Excludes false positives - Diagnosis of certainty | - Recurrence of bleeding after a period of well-being |
| “Watchful waiting” (no change to diet) | - Avoids the elimination diet - More economical (avoids any eHF or AAF) | - Parental anxiety - Only 20% of patients overcome hematochezia without elimination diet - Possible occurrence of long-term anemia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barni, S.; Mori, F.; Giovannini, M.; Liotti, L.; Mastrorilli, C.; Pecoraro, L.; Saretta, F.; Castagnoli, R.; Arasi, S.; Caminiti, L.; et al. Allergic Proctocolitis: Literature Review and Proposal of a Diagnostic–Therapeutic Algorithm. Life 2023, 13, 1824. https://doi.org/10.3390/life13091824
Barni S, Mori F, Giovannini M, Liotti L, Mastrorilli C, Pecoraro L, Saretta F, Castagnoli R, Arasi S, Caminiti L, et al. Allergic Proctocolitis: Literature Review and Proposal of a Diagnostic–Therapeutic Algorithm. Life. 2023; 13(9):1824. https://doi.org/10.3390/life13091824
Chicago/Turabian StyleBarni, Simona, Francesca Mori, Mattia Giovannini, Lucia Liotti, Carla Mastrorilli, Luca Pecoraro, Francesca Saretta, Riccardo Castagnoli, Stefania Arasi, Lucia Caminiti, and et al. 2023. "Allergic Proctocolitis: Literature Review and Proposal of a Diagnostic–Therapeutic Algorithm" Life 13, no. 9: 1824. https://doi.org/10.3390/life13091824
APA StyleBarni, S., Mori, F., Giovannini, M., Liotti, L., Mastrorilli, C., Pecoraro, L., Saretta, F., Castagnoli, R., Arasi, S., Caminiti, L., Gelsomino, M., Klain, A., del Giudice, M. M., & Novembre, E. (2023). Allergic Proctocolitis: Literature Review and Proposal of a Diagnostic–Therapeutic Algorithm. Life, 13(9), 1824. https://doi.org/10.3390/life13091824









