Fighting Cardiac Thromboembolism during Transcatheter Procedures: An Update on the Use of Cerebral Protection Devices in Cath Labs and EP Labs
Abstract
1. Introduction
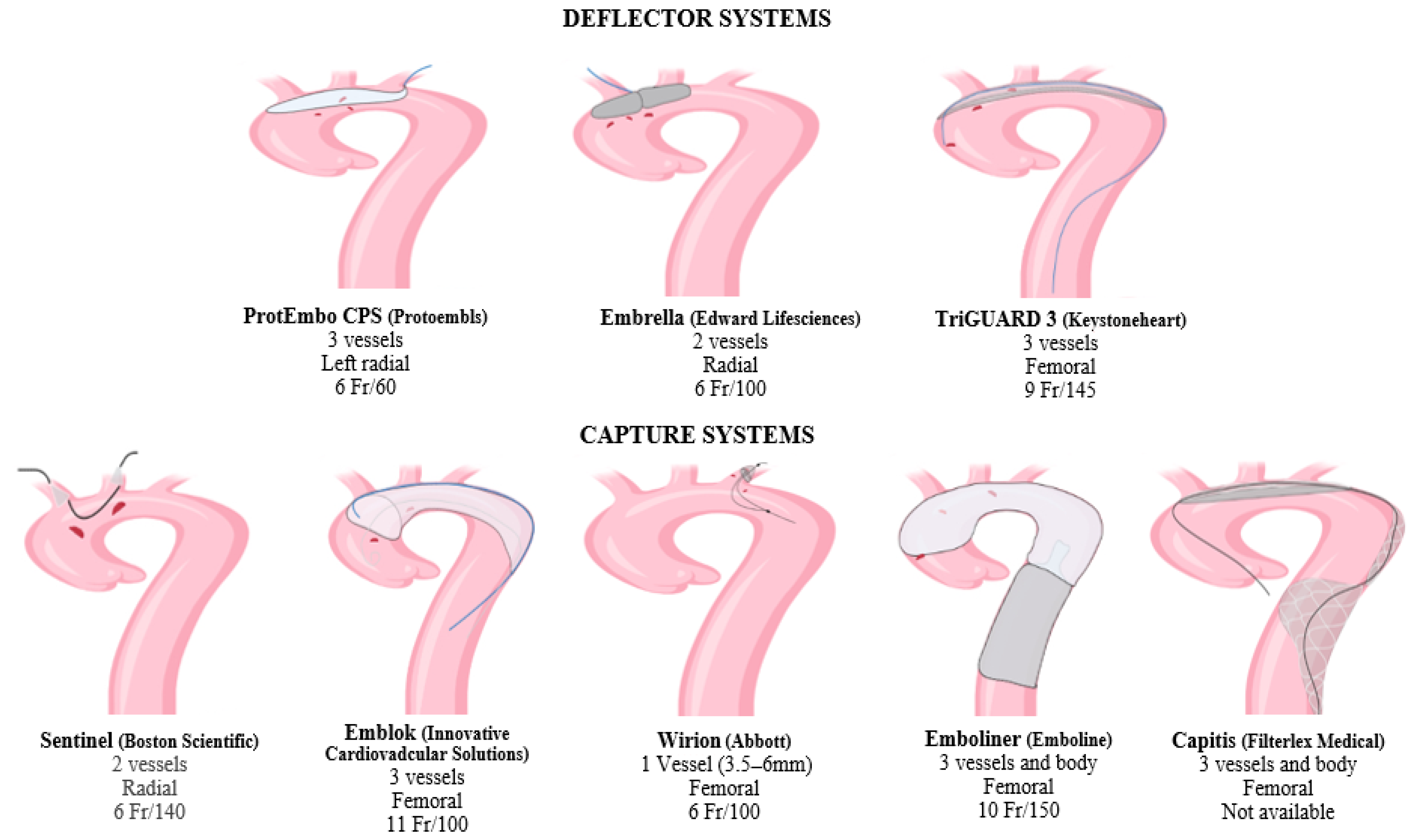
2. Cerebral Protection Devices
2.1. Deflector Systems
- –
- Embrella (Edwards Lifesciences, Irvine, CA, USA) received a European CE mark approval in 2010. It was developed to deflect embolic material during TAVR [29]. This device is inserted by right radial or brachial approach with a 6 Fr sheath. The distal end is an umbrella-like device with two heparin-coated polyurethane membranes (pore size: 100 μm). The CPD is placed through the greater curvature of the aorta, safeguarding the brachiocephalic and left common carotid artery. Since the left subclavian artery is not covered by the device, Embrella provides only partial protection to supra-aortic vessels. According to the pilot study PROTAVI-C, the device was successfully positioned in 100% of the TAVR procedures (N = 41) [30]. Although its use was associated with a reduction in lesion volume evaluated by DW-MRI, it did not prevent the occurrence of new cerebral microemboli.
- –
- TriGuard (Keystone Heart, Caesarea, Israel) received a European CE mark in 2014 [31]. It is advanced through a 9 Fr arterial sheath placed into the left femoral artery and deployed to cover the ostia of the three supra-aortic trunks. Its new generation, the TriGuard 3, incorporates a self-expanding deflection filter composed of a structural radiopaque nitinol frame and an ultra-thin polymer mesh (nominal pore size 115 × 145 μm). The device is heparin-coated to reduce thrombogenicity and increase lubricity. The full system includes a delivery subsystem for crimping and loading the device into an 8F sheath [32]. The device was primarily developed to provide cerebral protection during TAVR [33,34]. In recent years, its use in LAAC and VT ablation procedures has rapidly increased and provided encouraging results that could pave the way for new employment in electrophysiological procedures [35,36].
- –
- ProtEmbo CPS (Protembis, Aachen, Germany, EU) received a European CE mark in 2014. This device covers all three supra-aortic vessels, and its low-profile design provides delivery by left radial access. The heparin-coated mesh has the smallest pore size (60 μm) among all available CPDs. For this reason, it might even safeguard the cerebrum from smaller-sized debris [32,37]. The PROTEMBO C trial evaluated the safety and performance of the ProtEmbo CPS in TAVR patients [38]. The CPD met the primary safety and performance endpoints compared to prespecified historical performance goals. Enrolled patients had smaller brain lesion volumes on DW-MRI compared to prior series and no large single lesions (>150 mm3). The ongoing PROTEMBO SF (ClinicalTrials.gov Identifier: NCT03325283) is a prospective, observational, multicenter, intention-to-treat study of the safety and feasibility of the ProtEmbo CPS in subjects with severe symptomatic native aortic valve stenosis indicated for TAVR.
2.2. Filter Systems
2.2.1. Supra-Aortic Filters
- –
- Sentinel (Boston Scientific, Marlborough, MA, USA) received a European CE mark in 2014 and is the most widely used CPD so far. It is formed by a dual system filter basket containing two polyurethane mesh filters with 140 μm pores. It is advanced through a 6 Fr delivery catheter from the right radial over a 0.014 inch guidewire. It consists of a proximal filter (diameter of 9–15 mm) delivered in the brachiocephalic artery and a distal filter (diameter of 6.5–10 mm) delivered in the left common carotid artery. Through an articulating sheath, the device can be sealed into the aortic arch according to its anatomy [27]. Since the Sentinel device is deployed into supra-aortic vessels, the diameter of the supra-aortic vessels must be previously measured by CTA, because proximal and distal filters are developed to be accommodated within a brachiocephalic artery of 9 to 15 mm, and a common carotid of more than 3 mm [39]. The left vertebral artery remains unprotected. Sentinel devices have only one available size, so complete sealing might not be obtained in all aortic anatomies. Several uses of this device for LAAC and VT ablation have been reported [18,36].
- –
- The Wirion (Abbott, Chicago, IL, USA) is a single filter usually employed for carotid stenting and lower extremity endovascular interventions [40]. It consists of a distal filter (filter basket and locking mechanism) and a rapid exchange delivery catheter. The exchange catheter has a 1.1 mm crossing profile and can be mounted on any 0.014 inch guidewire and via 6F or greater guiding catheters. The filter basket is made of a self-expanding nitinol scaffold and a nylon filter membrane with 100 μm pores. The filter can efficiently be deployed in vessels with a diameter ranging from 3.5 to 6.0 mm and at any location along the guidewire, using a proprietary remote locking system (handle at the proximal end of the delivery catheter). Since this device protects only one vessel at a time, it cannot be used alone for TPs at high risk of cardioembolism. A study reported the utility of Wirion in combination with Sentinel to complete the protection of the left vertebral artery in patients undergoing TAVR [31].
- –
- Emblok Embolic Protection System (EPS, Innovative Cardiovascular Solutions, Grand Rapids, MI, USA) is currently only for investigational use. It is formed by an 11 F sheath device containing a 4 Fr pigtail catheter advanced through femoral access. The filter system is a 125 μm pore-size nitinol that allows the embolic filter and a radiopaque pigtail catheter to be advanced simultaneously through femoral access. It fits in various anatomies of the aorta with a diameter of up to 35 mm. The prospective, nonrandomized, multicenter, first-in-man pilot study was designed to evaluate the efficacy and safety of cerebral embolic protection utilizing the EPS-enrolled 20 patients undergoing TAVR [41]. The device was successfully placed and retrieved in all cases, and no neurological events were observed. Cerebral total new lesion volume was similar to other trials on cerebral protection during TAVR. An ongoing prospective, multicenter, single-blind, randomized controlled trial enrolling >500 patients aims to evaluate the safety, effectiveness, and performance of the EMBLOK EPS during TAVR by randomized comparison with a commercially available embolic protection device (ClinicalTrials.gov Identifier: NCT05295628).
2.2.2. Full Body Filters
- –
- Emboliner (Emboline, Santa Cruz, CA, USA) device system is currently only for investigational use. It is advanced from a 9 Fr transfemoral sheath used for the 6 Fr pigtail catheter for TAVR. It is engineered to protect all three cerebral vessels and the whole body. Early results from the SafePass 2 trial were presented in Transcatheter Cardiovascular Therapeutics 2019, reflecting no adverse events at 30 days with 100% procedural success.
- –
- Captis (Filterlex Medical, Caesarea, Israel) is currently under development and carries a deflector mechanism with ipsilateral transfemoral access. Positioned in the aortic arch and descending aorta, it promises to provide full cerebral and body protection. The results of the prospective, single-arm, first-in-human study presented at EuroPCR 2022 involving 20 patients who underwent TAVR showed 100% technical device performance success, including deploy and retrieve and any interferences with the TAVR procedure. There were neither device-related complications nor cerebrovascular events (ClinicalTrials.gov Identifier: NCT04659538).
3. Cerebral Protection in Cath Labs
4. Cerebral Protection in EP Labs
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davidson, L.J.; Davidson, C.J. Transcatheter Treatment of Valvular Heart Disease. JAMA 2021, 325, 2480–2494. [Google Scholar] [CrossRef] [PubMed]
- Latib, A.; Mustehsan, M.H.; Abraham, W.T.; Jorde, U.P.; Bartunek, J. Transcatheter interventions for heart failure. EuroIntervention 2023, 18, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Canfield, J.; Totary-Jain, H. 40 Years of Percutaneous Coronary Intervention: History and Future Directions. J. Pers. Med. 2018, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, C.; Pitschner, H.; Borggrefe, M. Evolution of ablation techniques: From WPW to complex arrhythmias. Eur. Hear. J. Suppl. 2007, 9, I116–I121. [Google Scholar] [CrossRef]
- Giustino, G.; Dangas, G.D. Stroke prevention in valvular heart disease: From the procedure to long-term management. EuroIntervention 2015, 14, W26–W31. [Google Scholar] [CrossRef]
- Carrena, O.; Young, R.; Tarrar, I.H.; Nelson, A.J.; Woidyla, D.; Wang, T.Y.; Mehta, R.H. Trends in the Incidence and Fatality of Peripercutaneous Coronary Intervention Stroke. J. Am. Coll. Cardiol. 2022, 80, 1772–1774. [Google Scholar] [CrossRef]
- Kogan, E.V.; Sciria, C.T.; Liu, C.F.; Wong, S.C.; Bergman, G.; Ip, J.E.; Thomas, G.; Markowitz, S.M.; Lerman, B.B.; Kim, L.K.; et al. Early Stroke and Mortality after Percutaneous Left Atrial Appendage Occlusion in Patients with Atrial Fibrillation. Stroke 2023, 54, 947–954. [Google Scholar] [CrossRef]
- Song, Z.-L.; Wu, S.-H.; Zhang, D.-L.; Jiang, W.-F.; Qin, M.; Liu, X. Clinical Safety and Efficacy of Ablation for Atrial Fibrillation Patients with a History of Stroke. Front. Cardiovasc. Med. 2021, 8, 630090. [Google Scholar] [CrossRef]
- Ma, V.Y.; Chan, L.; Carruthers, K.J. Incidence, Prevalence, Costs, and Impact on Disability of Common Conditions Requiring Rehabilitation in the United States: Stroke, Spinal Cord Injury, Traumatic Brain Injury, Multiple Sclerosis, Osteoarthritis, Rheumatoid Arthritis, Limb Loss, and Back Pain. Arch. Phys. Med. Rehabil. 2014, 95, 986–995.e1. [Google Scholar] [CrossRef]
- Lansky, A.J.; Brown, D.; Pena, C.; Pietras, C.G.; Parise, H.; Ng, V.G.; Meller, S.; Abrams, K.J.; Cleman, M.; Margolis, P.; et al. Neurologic Complications of Unprotected Transcatheter Aortic Valve Implantation (from the Neuro-TAVI Trial). Am. J. Cardiol. 2016, 118, 1519–1526. [Google Scholar] [CrossRef]
- Vermeer, S.E.; Prins, N.D.; Heijer, T.D.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M. Silent Brain Infarcts and the Risk of Dementia and Cognitive Decline. N. Engl. J. Med. 2003, 348, 1215–1222. [Google Scholar] [CrossRef]
- Bokura, H.; Kobayashi, S.; Yamaguchi, S.; Iijima, K.; Nagai, A.; Toyoda, G.; Oguro, H.; Takahashi, K. Silent Brain Infarction and Subcortical White Matter Lesions Increase the Risk of Stroke and Mortality: A Prospective Cohort Study. J. Stroke Cerebrovasc. Dis. 2006, 15, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Maleki, K.; Mohammadi, R.; Hart, D.; Cotiga, D.; Farhat, N.; Steinberg, J.S. Intracardiac Ultrasound Detection of Thrombus on Transseptal Sheath: Incidence, Treatment, and Prevention. J. Cardiovasc. Electrophysiol. 2005, 16, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.I.; I Mehta, R.; E Solis, O.; Jahan, R.; Salamon, N.; Tobis, J.M.; Yong, W.H.; Vinters, H.V.; Fishbein, M.C. Hydrophilic polymer emboli: An under-recognized iatrogenic cause of ischemia and infarct. Mod. Pathol. 2010, 23, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Aldenhoff, Y.B.; Hanssen, J.H.; Knetsch, M.L.; Koole, L.H. Thrombus Formation at the Surface of Guide-Wire Models: Effects of Heparin-releasing or Heparin-exposing Surface Coatings. J. Vasc. Interv. Radiol. 2007, 18, 419–425. [Google Scholar] [CrossRef]
- Frerker, C.; Schlüter, M.; Sanchez, O.D.; Reith, S.; Romero, M.E.; Ladich, E.; Schröder, J.; Schmidt, T.; Kreidel, F.; Joner, M.; et al. Cerebral Protection During MitraClip Implantation: Initial Experience at 2 Centers. JACC Cardiovasc. Interv. 2016, 9, 171–179. [Google Scholar] [CrossRef]
- Feld, G.K.; Tiongson, J.; Oshodi, G. Particle formation and risk of embolization during transseptal catheterization: Comparison of standard transseptal needles and a new radiofrequency transseptal needle. J. Interv. Card. Electrophysiol. 2011, 30, 31–36. [Google Scholar] [CrossRef][Green Version]
- Heeger, C.; Metzner, A.; Schlüter, M.; Rillig, A.; Mathew, S.; Tilz, R.R.; Wohlmuth, P.; Romero, M.E.; Virmani, R.; Fink, T.; et al. Cerebral Protection during Catheter Ablation of Ventricular Tachycardia in Patients with Ischemic Heart Disease. J. Am. Heart Assoc. 2018, 7, e009005. [Google Scholar] [CrossRef]
- Stachon, P.; Kaier, K.; Heidt, T.; Wolf, D.; Duerschmied, D.; Staudacher, D.; Zehender, M.; Bode, C.; Mühlen, C.v.Z. The Use and Outcomes of Cerebral Protection Devices for Patients Undergoing Transfemoral Transcatheter Aortic Valve Replacement in Clinical Practice. JACC Cardiovasc. Interv. 2021, 14, 161–168. [Google Scholar] [CrossRef]
- Ahmad, Y.; Howard, J.P. Meta-Analysis of Usefulness of Cerebral Embolic Protection during Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2021, 146, 69–73. [Google Scholar] [CrossRef]
- Zahid, S.; Ullah, W.; Uddin, M.F.; Rai, D.; Abbas, S.; Khan, M.U.; Hussein, A.; Salama, A.; Bandyopadhyay, D.; Bhaibhav, B.; et al. Cerebral Embolic Protection during Transcatheter Aortic Valve Implantation: Updated Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2023, 48, 101127. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Dhaliwal, A.; Sohal, S.; Kliger, C.; Velagapudi, P.; Basman, C.; Dominguez, A.C. TCT-327 Clinical and Radiographic Measures of Stroke-Related Outcomes with Cerebral Embolic Protection Devices during TAVR: A Meta-Analysis. J. Am. Coll. Cardiol. 2022, 80, B131. [Google Scholar] [CrossRef]
- Haussig, S.; Mangner, N.; Dwyer, M.G.; Lehmkuhl, L.; Lücke, C.; Woitek, F.; Holzhey, D.M.; Mohr, F.W.; Gutberlet, M.; Zivadinov, R.; et al. Effect of a Cerebral Protection Device on Brain Lesions following Transcatheter Aortic Valve Implantation in Patients with Severe Aortic Stenosis: The CLEAN-TAVI Randomized Clinical Trial. AMA 2016, 316, 592–601. [Google Scholar] [CrossRef]
- Sharma, S.P.; Cheng, J.; Turagam, M.K.; Gopinathannair, R.; Horton, R.; Lam, Y.-Y.; Tarantini, G.; D’Amico, G.; Rofastes, X.F.; Lange, M.; et al. Feasibility of Left Atrial Appendage Occlusion in Left Atrial Appendage Thrombus: A Systematic Review. JACC Clin. Electrophysiol. 2020, 6, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Isogai, T.; Shekhar, S.; Kapadia, S. Cerebral Embolic Protection Devices: Current State of the Art. US Cardiol. Rev. 2023, 17, e02. [Google Scholar] [CrossRef]
- Kang, G.; Lee, J.; Song, T.; Pantelic, M.; Reeser, N.; Keimig, T.; Nadig, J.; Villablanca, P.; Frisoli, T.; Eng, M.; et al. 3-Dimensional CT Planning for Cerebral Embolic Protection in Structural Interventions. JACC Cardiovasc. Imaging 2020, 13, 2673–2676. [Google Scholar] [CrossRef]
- Cubero-Gallego, H.; Pascual, I.; Rozado, J.; Ayesta, A.; Hernandez-Vaquero, D.; Diaz, R.; Alperi, A.; Avanzas, P.; Moris, C. Cerebral protection devices for transcatheter aortic valve replacement. Ann. Transl. Med. 2019, 7, 584. [Google Scholar] [CrossRef]
- Steinvil, A.; Benson, R.T.; Waksman, R.; Chhatriwalla, A.K.; Allen, K.B.; Saxon, J.T.; Cohen, D.J.; Aggarwal, S.; Hart, A.J.; Baron, S.J.; et al. Embolic Protection Devices in Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2016, 9, e003284. [Google Scholar] [CrossRef]
- Demir, O.M.; Iannopollo, G.; Mangieri, A.; Ancona, M.B.; Regazzoli, D.; Mitomo, S.; Colombo, A.; Weisz, G.; Latib, A. The Role of Cerebral Embolic Protection Devices During Transcatheter Aortic Valve Replacement. Front. Cardiovasc. Med. 2018, 5, 150. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; Kahlert, P.; Neumann, F.-J.; Schymik, G.; Webb, J.G.; Amarenco, P.; Brott, T.; Garami, Z.; Gerosa, G.; Lefèvre, T.; et al. Feasibility and Exploratory Efficacy Evaluation of the Embrella Embolic Deflector System for the Prevention of Cerebral Emboli in Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2014, 7, 1146–1155. [Google Scholar] [CrossRef]
- Nombela-Franco, L.; Armijo, G.; Tirado-Conte, G. Cerebral embolic protection devices during transcatheter aortic valve implantation: Clinical versus silent embolism. J. Thorac. Dis. 2018, 10, S3604–S3613. [Google Scholar] [CrossRef]
- Gasior, T.; Mangner, N.; Bijoch, J.; Wojakowski, W. Cerebral embolic protection systems for transcatheter aortic valve replacement. J. Interv. Cardiol. 2018, 31, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Baumbach, A.; Mullen, M.; Brickman, A.M.; Aggarwal, S.K.; Pietras, C.G.; Forrest, J.K.; Hildick-Smith, D.; Meller, S.M.; Gambone, L.; den Heijer, P.; et al. Safety and performance of a novel embolic deflection device in patients undergoing transcatheter aortic valve replacement: Results from the DEFLECT I study. EuroIntervention 2015, 11, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Samim, M.; van der Worp, B.; Agostoni, P.; Hendrikse, J.; Budde, R.P.; Nijhoff, F.; Ramjankhan, F.; Doevendans, P.A.; Stella, P.R. TriGuard™ HDH embolic deflection device for cerebral protection during transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2017, 89, 470–477. [Google Scholar] [CrossRef]
- Zachariah, D.; Limite, L.R.; Mazzone, P.; Marzi, A.; Radinovic, A.; Baratto, F.; Italia, L.; Ancona, F.; Paglino, G.; Della Bella, P. Use of Cerebral Protection Device in Patients Undergoing Ventricular Tachycardia Catheter Ablation. JACC Clin. Electrophysiol. 2022, 8, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Preda, A.; Fierro, N.; Marzi, A.; Radinovic, A.; Della Bella, P.; Mazzone, P. A Referral Center Experience with Cerebral Protection Devices: Challenging Cardiac Thrombus in the EP Lab. J. Clin. Med. 2023, 12, 1549. [Google Scholar] [CrossRef]
- Jagielak, D.; Targonski, R.; Ciecwierz, D. First-in-Human Use of the Next-generation ProtEmbo Cerebral Embolic Protection System During Transcatheter Aortic Valve-in-valve Implantation. Interv. Cardiol. Rev. Res. Resour. 2021, 16, 1–4. [Google Scholar] [CrossRef]
- Jagielak, D.; Targonski, R.; Frerker, C.; Abdel-Wahab, M.; Wilde, J.; Werner, N.; Lauterbach, M.; Leick, J.; Grygier, M.; Misterski, M.; et al. Safety and performance of a novel cerebral embolic protection device for transcatheter aortic valve implantation: The PROTEMBO C Trial. EuroIntervention 2022, 18, 590–597. [Google Scholar] [CrossRef]
- Vernikouskaya, I.; Rottbauer, W.; Gonska, B.; Rodewald, C.; Seeger, J.; Rasche, V.; Wöhrle, J. Image-guidance for transcatheter aortic valve implantation (TAVI) and cerebral embolic protection. Int. J. Cardiol. 2017, 249, 90–95. [Google Scholar] [CrossRef]
- Giannopoulos, S.; Armstrong, E.J. WIRION™ embolic protection system for carotid artery stenting and lower extremity endovascular intervention. Futur. Cardiol. 2020, 16, 527–538. [Google Scholar] [CrossRef]
- Latib, A.; Mangieri, A.; Vezzulli, P.; Spagnolo, P.; Sardanelli, F.; Fellegara, G.; Pagnesi, M.; Giannini, F.; Falini, A.; Gorla, R.; et al. First-in-Man Study Evaluating the Emblok Embolic Protection System During Transcatheter Aortic Valve Replacement. JACC: Cardiovasc. Interv. 2020, 13, 860–868. [Google Scholar] [CrossRef]
- Nazif, T.M.; Moses, J.; Sharma, R.; Dhoble, A.; Rovin, J.; Brown, D.; Horwitz, P.; Makkar, R.; Stoler, R.; Forrest, J.; et al. Randomized Evaluation of TriGuard 3 Cerebral Embolic Protection After Transcatheter Aortic Valve Replacement: REFLECT II. JACC Cardiovasc. Interv. 2021, 14, 515–527. [Google Scholar] [CrossRef]
- Kapadia, S.R.; Kodali, S.; Makkar, R.; Mehran, R.; Lazar, R.M.; Zivadinov, R.; Dwyer, M.G.; Jilaihawi, H.; Virmani, R.; Anwaruddin, S.; et al. Protection Against Cerebral Embolism During Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2017, 69, 367–377. [Google Scholar] [CrossRef]
- Mangieri, A.; Montalto, C.; Poletti, E.; Sticchi, A.; Crimi, G.; Giannini, F.; Latib, A.; Capodanno, D.; Colombo, A. Thrombotic Versus Bleeding Risk After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 74, 2088–2101. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, A.-O.M.; Gherasim, L.M.; Vinereanu, D.M. Risk of Stroke After Transcatheter Aortic Valve Implantation: Epidemiology, Mechanism, and Management. Am. J. Ther. 2021, 28, e560–e572. [Google Scholar] [CrossRef] [PubMed]
- Armijo, G.; Nombela-Franco, L.; Tirado-Conte, G. Cerebrovascular Events After Transcatheter Aortic Valve Implantation. Front. Cardiovasc. Med. 2018, 5, 104. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Leon, M.B.; Mehran, R.; Kuck, K.-H.; Alu, M.C.; Braumann, R.E.; Kodali, S.; Kapadia, S.R.; Linke, A.; Makkar, R.; et al. Debris Heterogeneity across Different Valve Types Captured by a Cerebral Protection System during Transcatheter Aortic Valve Replacement. JACC: Cardiovasc. Interv. 2018, 11, 1262–1273. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Barón-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; De Bonis, M.; Evangelista, A.; et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. J. Cardiothorac. Surg. 2012, 42, S1–S44. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef]
- Kapadia, S.R.; Makkar, R.; Leon, M.; Abdel-Wahab, M.; Waggoner, T.; Massberg, S.; Rottbauer, W.; Horr, S.; Sondergaard, L.; Karha, J.; et al. Cerebral Embolic Protection during Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2022, 387, 1253–1263. [Google Scholar] [CrossRef]
- Giustino, G.; Sorrentino, S.; Mehran, R.; Faggioni, M.; Dangas, G. Cerebral Embolic Protection During TAVR: A Clinical Event Meta-Analysis. J. Am. Coll. Cardiol. 2017, 69, 465–466. [Google Scholar] [CrossRef] [PubMed]
- Butala, N.M.; Makkar, R.; Secemsky, E.A.; Gallup, D.; Marquis-Gravel, G.; Kosinski, A.S.; Vemulapalli, S.; Valle, J.A.; Bradley, S.M.; Chakravarty, T.; et al. Cerebral Embolic Protection and Outcomes of Transcatheter Aortic Valve Replacement: Results from the Transcatheter Valve Therapy Registry. Circulation 2021, 143, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Ferket, B.S.; Morey, J.R.; Gelijns, A.C.; Moskowitz, A.J.; Giustino, G. Abstract 156: Cost-Effectiveness Analysis of the Sentinel Cerebral Embolic Protection Device During Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Qual. Outcomes 2019, 12, A156. [Google Scholar] [CrossRef]
- Alkhouli, M.; Ganz, M.; Mercaldi, K.; McGovern, A.; Griffiths, R.; Kapadia, S. TCT-305 Cost-Effectiveness of Cerebral Embolic Protection with the SENTINEL Device in Transcatheter Aortic Valve Replacement: A US Medicare Payer Perspective. J. Am. Coll. Cardiol. 2021, 78, B125. [Google Scholar] [CrossRef]
- Lansky, A.J.; Schofer, J.; Tchetche, D.; Stella, P.; Pietras, C.G.; Parise, H.; Abrams, K.; Forrest, J.K.; Cleman, M.; Reinöhl, J.; et al. A prospective randomized evaluation of the TriGuard™ HDH embolic DEFLECTion device during transcatheter aortic valve implantation: Results from the DEFLECT III trial. Eur. Hear. J. 2015, 36, 2070–2078. [Google Scholar] [CrossRef]
- Wendt, D.; Kleinbongard, P.; Knipp, S.; Al-Rashid, F.; Gedik, N.; El Chilali, K.; Schweter, S.; Schlamann, M.; Kahlert, P.; Neuhäuser, M.; et al. Intraaortic Protection from Embolization in Patients Undergoing Transaortic Transcatheter Aortic Valve Implantation. Ann. Thorac. Surg. 2015, 100, 686–691. [Google Scholar] [CrossRef]
- Van Mieghem, N.M.; van Gils, L.; Ahmad, H.; van Kesteren, F.; van der Werf, H.W.; Brueren, G.; Storm, M.; Lenzen, M.; Daemen, J.; Heuvel, A.F.v.D.; et al. Filter-based cerebral embolic protection with transcatheter aortic valve implantation: The randomised MISTRAL-C trial. EuroIntervention 2016, 12, 499–507. [Google Scholar] [CrossRef]
- Patel, D.; Bailey, S.M.; Furlan, A.J.; Ching, M.; Zachaib, J.; DI Biase, L.; Mohanty, P.; Horton, R.P.; Burkhardt, J.D.; Sanchez, J.E.; et al. Long-Term Functional and Neurocognitive Recovery in Patients Who Had an Acute Cerebrovascular Event Secondary to Catheter Ablation for Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2010, 21, 412–417. [Google Scholar] [CrossRef]
- Cappato, R.; Calkins, H.; Chen, S.-A.; Davies, W.; Iesaka, Y.; Kalman, J.; Kim, Y.-H.; Klein, G.; Natale, A.; Packer, D.; et al. Updated Worldwide Survey on the Methods, Efficacy, and Safety of Catheter Ablation for Human Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2010, 3, 32–38. [Google Scholar] [CrossRef]
- Santangeli, P.; Di Biase, L.; Horton, R.; Burkhardt, J.D.; Sanchez, J.; Al-Ahmad, A.; Hongo, R.; Beheiry, S.; Bai, R.; Mohanty, P.; et al. Ablation of atrial fibrillation under therapeutic warfarin reduces periprocedural complications: Evidence from a meta-analysis. Circ. Arrhythmia Electrophysiol. 2012, 5, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, M.; Stevenson, W.G.; Tedrow, U.B.; Michaud, G.F.; John, R.M.; Epstein, L.M.; Albert, C.M.; Koplan, B.A. Incidence and predictors of major complications from contemporary catheter ablation to treat cardiac arrhythmias. Heart Rhythm 2011, 8, 1661–1666. [Google Scholar] [CrossRef]
- Whitman, I.R.; Gladstone, R.A.; Badhwar, N.; Hsia, H.H.; Lee, B.K.; Josephson, S.A.; Meisel, K.M.; Dillon, W.P.; Hess, C.P.; Gerstenfeld, E.P.; et al. Brain Emboli After Left Ventricular Endocardial Ablation. Circulation 2017, 135, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.-H.; Schaumann, A.; Eckardt, L.; Willems, S.; Ventura, R.; Delacrétaz, E.; Pitschner, H.-F.; Kautzner, J.; Schumacher, B.; Hansen, P.S. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): A multicentre randomised controlled trial. Lancet 2010, 375, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Sievert, H.; Halperin, J.; Doshi, S.K.; Buchbinder, M.; Neuzil, P.; Huber, K.; Whisenant, B.; Kar, S.; Swarup, V.; et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: A randomized clinical trial. JAMA 2014, 312, 1988–1998. [Google Scholar] [CrossRef]
- Cresti, A.; García-Fernández, M.A.; De Sensi, F.; Miracapillo, G.; Picchi, A.; Scalese, M.; Severi, S. Prevalence of auricular thrombosis before atrial flutter cardioversion: A 17-year transoesophageal echocardiographic study. EP Eur. 2015, 18, 450–456. [Google Scholar] [CrossRef]
- Scherr, D.; Dalal, D.; Chilukuri, K.; Dong, J.U.N.; Spragg, D.; Henrikson, C.A.; Nazarian, S.; Cheng, A.; Berger, R.D.; Abraham, T.P.; et al. Incidence and Predictors of Left Atrial Thrombus Prior to Catheter Ablation of Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2009, 20, 379–384. [Google Scholar] [CrossRef]
- Fukuda, S.; Watanabe, H.; Shimada, K.; Aikawa, M.; Kono, Y.; Jissho, S.; Taguchi, H.; Umemura, J.; Yoshiyama, M.; Shiota, T.; et al. Left atrial thrombus and prognosis after anticoagulation therapy in patients with atrial fibrillation. J. Cardiol. 2011, 58, 266–277. [Google Scholar] [CrossRef][Green Version]
- Guarracini, F.; Bonvicini, E.; Preda, A.; Martin, M.; Muraglia, S.; Casagranda, G.; Mochen, M.; Coser, A.; Quintarelli, S.; Branzoli, S.; et al. Appropriate use criteria of left atrial appendage closure devices: Latest evidences. Expert Rev. Med. Devices 2023, 20, 493–503. [Google Scholar] [CrossRef]
- Preda, A.; Baroni, M.; Varrenti, M.; Vargiu, S.; Carbonaro, M.; Giordano, F.; Gigli, L.; Mazzone, P. Left Atrial Appendage Occlusion in Patients with Failure of Antithrombotic Therapy: Good Vibes from Early Studies. J. Clin. Med. 2023, 12, 3859. [Google Scholar] [CrossRef]
- Saw, J.; Holmes, D.R.; Cavalcante, J.L.; Freeman, J.V.; Goldsweig, A.M.; Kavinsky, C.J.; Moussa, I.D.; Munger, T.M.; Price, M.J.; Reisman, M.; et al. SCAI/HRS expert consensus statement on transcatheter left atrial appendage closure. Heart Rhythm 2023, 20, e1–e16. [Google Scholar] [CrossRef]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—An update. EuroIntervention 2020, 15, 1133–1180. [Google Scholar] [CrossRef] [PubMed]
- Seiffge, D.J.; De Marchis, G.M.; Koga, M.; Paciaroni, M.; Wilson, D.; Cappellari, M.; Macha, K.; Tsivgoulis, G.; Ambler, G.; Arihiro, S.; et al. Ischemic Stroke despite Oral Anticoagulant Therapy in Patients with Atrial Fibrillation. Ann. Neurol. 2020, 87, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Lowe, B.S.; Kusunose, K.; Motoki, H.; Varr, B.; Shrestha, K.; Whitman, C.; Tang, W.H.W.; Thomas, J.D.; Klein, A.L. Prognostic Significance of Left Atrial Appendage “Sludge” in Patients with Atrial Fibrillation: A New Transesophageal Echocardiographic Thromboembolic Risk Factor. J. Am. Soc. Echocardiogr. 2014, 27, 1176–1183. [Google Scholar] [CrossRef]
- Sebag, F.S.; Garot, P.; Galea, R.; De Backer, O.; Lepillier, A.; De Meesteer, A.; Hildick-Smith, D.; Armero, S.; Moubarak, G.; Ducrocq, G.; et al. Left atrial appendage closure for thrombus trapping: The international, multicentre TRAPEUR registry. EuroIntervention 2022, 18, 50–57. [Google Scholar] [CrossRef]
- Margonato, D.; Preda, A.; Ingallina, G.; Rizza, V.; Fierro, N.; Radinovic, A.; Ancona, F.; Patti, G.; Agricola, E.; Della Bella, P.; et al. Left atrial appendage occlusion after thromboembolic events or left atrial appendage sludge during anticoagulation therapy: Is two better than one? Real-world experience from a tertiary care hospital. J. Arrhythmia 2023, 39, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Boccuzzi, G.G.; Montabone, A.; D’Ascenzo, F.; Colombo, F.; Ugo, F.; Muraglia, S.; De Backer, O.; Nombela-Franco, L.; Meincke, F.; Mazzone, P. Cerebral protection in left atrial appendage closure in the presence of appendage thrombosis. Catheter. Cardiovasc. Interv. 2021, 97, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 3122–3135. [Google Scholar] [CrossRef]
- Lopez, E.M.; Malhotra, R. Ventricular Tachycardia in Structural Heart Disease. J. Innov. Card. Rhythm Manag. 2019, 10, 3762–3773. [Google Scholar] [CrossRef]
- Santangeli, P.; Rame, J.E.; Birati, E.Y.; Marchlinski, F.E. Management of Ventricular Arrhythmias in Patients with Advanced Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1842–1860. [Google Scholar] [CrossRef] [PubMed]
- Streitner, F.; Herrmann, T.; Kuschyk, J.; Lang, S.; Doesch, C.; Papavassiliu, T.; Streitner, I.; Veltmann, C.; Haghi, D.; Borggrefe, M. Impact of Shocks on Mortality in Patients with Ischemic or Dilated Cardiomyopathy and Defibrillators Implanted for Primary Prevention. PLoS ONE 2013, 8, e63911. [Google Scholar] [CrossRef] [PubMed]
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; Aguinaga, L.; Leite, L.R.; Al-Khatib, S.M.; Anter, E.; et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm 2020, 17, e2–e154. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, T.; Roumegou, P.; Sridi, S.; Mahida, S.; Bustin, A.; Duchateau, J.; Tixier, R.; Derval, N.; Pambrun, T.; Chniti, G.; et al. Prevalence and risk factors of cardiac thrombus prior to ventricular tachycardia catheter ablation in structural heart disease. Europace 2023, 25, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Medi, C.; Evered, L.; Silbert, B.; Teh, A.; Halloran, K.; Morton, J.; Kistler, P.; Kalman, J. Subtle Post-Procedural Cognitive Dysfunction After Atrial Fibrillation Ablation. J. Am. Coll. Cardiol. 2013, 62, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Valeri, Y.; Russo, A.D.; Falanga, U.; Volpato, G.; Compagnucci, P.; Barbarossa, A.; Cipolletta, L.; Parisi, Q.; Casella, M.; Piva, T. First report of cerebral embolic protection system use during combined atrial fibrillation Pulse Field Ablation and left atrial appendage closure. Authorea 2022. preprints. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Kapa, S.; Madhavan, M.; Van Houten, H.; Haas, L.; McLeod, C.; Friedman, P.; Asirvatham, S.; Shah, N.; Packer, D. Abstract 16200: Risk of Stroke After Catheter Ablation or Cardioversion for Atrial Fibrillation: Results from a Large Administrative Database, 2008–2012. Circulation 2014, 130, A16200. [Google Scholar]
- Di Biase, L.; Burkhardt, J.D.; Mohanty, P.; Sanchez, J.E.; Horton, R.; Gallinghouse, G.J.; Lakkireddy, D.; Verma, A.; Khaykin, Y.; Hongo, R.; et al. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation: The impact of periprocedural therapeutic international normalized ratio. Circulation 2010, 121, 2550–2556. [Google Scholar] [CrossRef]
- Scherr, D.; Sharma, K.; Dalal, D.; Spragg, D.; Chilukuri, K.; Cheng, A.; Dong, J.; Henrikson, C.A.; Nazarian, S.; Berger, R.D.; et al. Incidence and Predictors of Periprocedural Cerebrovascular Accident in Patients Undergoing Catheter Ablation of Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2009, 20, 1357–1363. [Google Scholar] [CrossRef]
- Calvert, P.; Kollias, G.; Pürerfellner, H.; Narasimhan, C.; Osorio, J.; Lip, G.Y.H.; Gupta, D. Silent cerebral lesions following catheter ablation for atrial fibrillation: A state-of-the-art review. EP Eur. 2023, 25, euad151. [Google Scholar] [CrossRef]
- Haines, D.E.; Stewart, M.T.; Ahlberg, S.; Barka, N.D.; Condie, C.; Fiedler, G.R.; Kirchhof, N.A.; Halimi, F.; Deneke, T. Microembolism and catheter ablation I: A comparison of irrigated radiofrequency and multielectrode-phased radiofrequency catheter ablation of pulmonary vein ostia. Circ. Arrhythm. Electrophysiol. 2013, 6, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.; Nagy-Baló, E.; Sándorfi, G.; Édes, I.; Csanádi, Z. Cerebral microembolization during atrial fibrillation ablation: Comparison of different single-shot ablation techniques. Int. J. Cardiol. 2014, 174, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Watanabe, T.; Kajiyama, T.; Iwasawa, J.; Ichijo, S.; Nakamura, H.; Taniguchi, H.; Hirao, K.; Iesaka, Y. Thromboembolic Risks of the Procedural Process in Second-Generation Cryoballoon Ablation Procedures. Circ. Arrhythm. Electrophysiol. 2017, 10, e005612. [Google Scholar] [CrossRef] [PubMed]
- Takami, M.; Lehmann, H.I.; Parker, K.D.; Welker, K.M.; Johnson, S.B.; Packer, D.L. Effect of Left Atrial Ablation Process and Strategy on Microemboli Formation during Irrigated Radiofrequency Catheter Ablation in an In Vivo Model. Circ. Arrhythm. Electrophysiol. 2016, 9, e003226. [Google Scholar] [CrossRef]
- Lasek-Bal, A.; Puz, P.; Wieczorek, J.; Nowak, S.; Wnuk-Wojnar, A.; Warsz-Wianecka, A.; Mizia-Stec, K. Cerebral microembolism during atrial fibrillation ablation can result from the technical aspects and mostly does not cause permanent neurological deficit. Arch. Med. Sci. 2020, 16, 1288–1294. [Google Scholar] [CrossRef]
- Braemswig, T.B.; Kusserow, M.; Kruppa, J.; Reinthaler, M.; Erdur, H.; Fritsch, M.; Curio, J.; Alushi, B.; Villringer, K.; Galinovic, I.; et al. Cerebral embolisation during transcatheter edge-to-edge repair of the mitral valve with the MitraClip system: A prospective, observational study. EuroIntervention 2022, 18, e160–e168. [Google Scholar] [CrossRef]
- Vella, C.; Preda, A.; Ferri, L.; Montorfano, M. Intravascular Coronary Lithotripsy for the Treatment of Iatrogenic Calcium Embolization: The “Block and Crack” Technique. In Catheterization and Cardiovascular Interventions; Wiley: Hoboken, NY, USA, 2023. [Google Scholar] [CrossRef]
- Hecker, F.; Arsalan, M.; Walther, T. Managing Stroke During Transcatheter Aortic Valve Replacement. Interv. Cardiol. Rev. Res. Resour. 2017, 12, 25–30. [Google Scholar] [CrossRef]
- Perry, T.E.; George, S.A.; Lee, B.; Wahr, J.; Randle, D.; Sigurðsson, G. A guide for pre-procedural imaging for transcatheter aortic valve replacement patients. Perioper. Med. 2020, 9, 36. [Google Scholar] [CrossRef]
- Jalal, Z.; Iriart, X.; Dinet, M.L.; Selly, J.B.; Tafer, N.; Renou, P.; Sibon, I.; Thambo, J.B. Extending percutaneous left atrial appendage closure indications using the AMPLATZER™ Cardiac Plug device in patients with persistent left atrial appendage thrombus: The thrombus trapping technique. Arch. Cardiovasc. Dis. 2016, 109, 659–666. [Google Scholar] [CrossRef]
- Tzikas, A.; Bergmann, M.W. Left atrial appendage closure: Patient, device and post-procedure drug selection. EuroIntervention 2016, 12, X48–X54. [Google Scholar] [CrossRef]
- Peichl, P.; Wichterle, D.; Čihák, R.; Aldhoon, B.; Kautzner, J. Catheter Ablation of Ventricular Tachycardia in the Presence of an Old Endocavitary Thrombus Guided by Intracardiac Echocardiography. Pacing Clin. Electrophysiol. 2016, 39, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.E.; Stewart, M.T.; Barka, N.D.; Kirchhof, N.; Lentz, L.R.; Reinking, N.M.; Urban, J.F.; Halimi, F.; Deneke, T.; Kanal, E.; et al. Microembolism and Catheter Ablation II. Circ. Arrhythm. Electrophysiol. 2013, 6, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, S.-J.; Du, X.; Jiang, C.; Lai, Y.-W.; Wang, Y.-F.; Jia, Z.-X.; He, L.; Tang, R.-B.; Dong, J.-Z.; et al. Incidence and risk factors of post-operative cognitive decline after ablation for atrial fibrillation. BMC Cardiovasc. Disord. 2021, 21, 341. [Google Scholar] [CrossRef] [PubMed]
- Sauren, L.D.; VAN Belle, Y.; DE Roy, L.; Pison, L.; LA Meir, M.; VAN DER Veen, F.H.; Crijns, H.J.; Jordaens, L.; Mess, W.H.; Maessen, J.G. Transcranial Measurement of Cerebral Microembolic Signals during Endocardial Pulmonary Vein Isolation: Comparison of Three Different Ablation Techniques. J. Cardiovasc. Electrophysiol. 2009, 20, 1102–1107. [Google Scholar] [CrossRef]
- van Oeveren, W.; Crijns, H.J.G.M.; Korteling, B.J.; Wegereef, E.W.; Haan, H.; Tigchelaar, I.; Hoekstra, A. Blood damage, platelet and clotting activation during application of radiofrequency or cryoablation catheters: A comparative in vitro study. J. Med Eng. Technol. 1999, 23, 20–25. [Google Scholar] [CrossRef]
- Neumann, T.; Kuniss, M.; Conradi, G.; Janin, S.; Berkowitsch, A.; Wojcik, M.; Rixe, J.; Erkapic, D.; Zaltsberg, S.; Rolf, A.; et al. MEDAFI-Trial (Micro-embolization during ablation of atrial fibrillation): Comparison of pulmonary vein isolation using cryoballoon technique vs. radiofrequency energy. Europace 2011, 13, 37–44. [Google Scholar] [CrossRef]
- Siklódy, C.H.; Deneke, T.; Hocini, M.; Lehrmann, H.; Shin, D.-I.; Miyazaki, S.; Henschke, S.; Fluegel, P.; Schiebeling-Römer, J.; Bansmann, P.M.; et al. Incidence of Asymptomatic Intracranial Embolic Events After Pulmonary Vein Isolation: Comparison of Different Atrial Fibrillation Ablation Technologies in a Multicenter Study. J. Am. Coll. Cardiol. 2011, 58, 681–688. [Google Scholar] [CrossRef]
- Gaita, F.; Leclercq, J.F.; Schumacher, B.; Scaglione, M.; Toso, E.; Halimi, F.; Schade, A.; Froehner, S.; Ziegler, V.; Sergi, D.; et al. Incidence of Silent Cerebral Thromboembolic Lesions After Atrial Fibrillation Ablation May Change According to Technology Used: Comparison of Irrigated Radiofrequency, Multipolar Nonirrigated Catheter and Cryoballoon. J. Cardiovasc. Electrophysiol. 2011, 22, 961–968. [Google Scholar] [CrossRef]
- Wasmer, K.; Foraita, P.; Leitz, P.; Güner, F.; Pott, C.; Lange, P.; Eckardt, L.; Mönnig, G. Safety profile of multielectrode-phased radiofrequency pulmonary vein ablation catheter and irrigated radiofrequency catheter. Europace 2016, 18, 78–84. [Google Scholar] [CrossRef]
- Wieczorek, M.; Hoeltgen, R.; Brueck, M. Does the number of simultaneously activated electrodes during phased RF multielectrode ablation of atrial fibrillation influence the incidence of silent cerebral microembolism? Heart Rhythm 2013, 10, 953–959. [Google Scholar] [CrossRef]
- Zellerhoff, S.; Ritter, M.A.; Kochhäuser, S.; Dittrich, R.; Köbe, J.; Milberg, P.; Korsukewitz, C.; Dechering, D.G.; Pott, C.; Wasmer, K.; et al. Modified phased radiofrequency ablation of atrial fibrillation reduces the number of cerebral microembolic signals. EP Eur. 2013, 16, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Grimaldi, M.; De Potter, T.; Vijgen, J.M.; Bulava, A.; Duytschaever, M.F.; Martinek, M.; Natale, A.; Knecht, S.; Neuzil, P.; et al. Pulmonary Vein Isolation with Very High Power, Short Duration, Temperature-Controlled Lesions: The QDOT-FAST Trial. JACC Clin. Electrophysiol. 2019, 5, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Dukkipati, S.R.; Neuzil, P.; Anic, A.; Petru, J.; Funasako, M.; Cochet, H.; Minami, K.; Breskovic, T.; Sikiric, I.; et al. Pulsed Field Ablation of Paroxysmal Atrial Fibrillation: 1-Year Outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin. Electrophysiol. 2021, 7, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Ekanem, E.; Reddy, V.Y.; Schmidt, B.; Reichlin, T.; Neven, K.; Metzner, A.; Hansen, J.; Blaauw, Y.; Maury, P.; Arentz, T.; et al. Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace 2022, 24, 1256–1266. [Google Scholar] [CrossRef]
| Device | Pros | Cons |
|---|---|---|
| ProtEmbo CPS [38] | Small size sheath (6 Fr); Left radial/brachial access; Mesh with the smallest pore size available; 100% successful device positioning. | Partial coverage of the supra-aortic trunk; New cerebral lesions were detected, but smaller; Available evidence only for TAVR. |
| Embrella [30] | Small size sheath (6 Fr); Right radial/brachial access; 100% successful device positioning. | Partial coverage of the supra-aortic trunk; New cerebral lesions were detected, but smaller; Available evidence only for TAVR. |
| TriGuard 3 [36,42] | Intermedium size sheath (9 Fr); Implantable through both the left and right femoral arteries; Full coverage of the supra-aortic trunk; Can be left in the aortic arch for days; 100% successful device positioning; Large amount of evidence; Available evidence for TAVR, LAAC, and VT ablation. | Femoral access; Procedural concerns if transcatheter procedure performed through the retro-aortic path; New cerebral lesions were detected, but smaller. |
| Sentinel [36,43] | Small size sheath (6 Fr); Right radial/brachial access; 94.4% successful device positioning; Largest amount of evidence; Available evidence for TAVR, LAAC, and VT ablation. | Partial coverage of the supra-aortic trunk; New cerebral lesions were detected but smaller. |
| Emblok [41] | Implantable through both the left and right femoral arteries; 100% successful device positioning. | Intermedium size sheath (11 Fr); Femoral access; Procedural concerns if transcatheter procedure performed through the retro-aortic path; New cerebral lesions were detected, but smaller; Available evidence only for TAVR. |
| Wirion [31] | Small size sheath (6 Fr); Right radial/brachial access; Very low amount of evidence. | Nonsufficient coverage of the supra-aortic trunk; Available evidence only for TAVR; |
| Emboliner | Coverage of the supra-aortic trunk and descending aorta; Implantable through both the left and right femoral arteries. | Data on the first-in-man study is not yet available. |
| Capitis | Coverage of the supra-aortic trunk and descending aorta; Implantable through both the left and right femoral arteries. | Data on the first-in-man study is not yet available. |
| RCT | Year | Sample Size | Prosthetic Valve | Endpoints | Results |
|---|---|---|---|---|---|
| DEFLECT III [56] | 2015 | TriGuard (n = 46) Controls (n = 39) | Balloon-expandable Self-expandable |
|
|
| EMBOL-X [57] | 2015 | Embol-x (n = 14) Controls (n = 16) | Balloon-expandable |
|
|
| MISTRAL-C [58] | 2016 | Sentinel (n = 32) Controls (n = 33) | Balloon-expandable Self-expandable |
|
|
| CLEAN-TAVI [23] | 2016 | Claret Montage Dual Filter System (n = 50) Controls (n = 50) | Self-expandable |
|
|
| SENTINEL [43] | 2017 | Sentinel (n = 123) Controls (n = 119) | Balloon-expandable Self-expandable |
|
|
| REFLECT II [42] | 2021 | TriGuard 3 (n = 121) Controls (n = 58) | Balloon-expandable Self-expandable |
|
|
| PROTECTED TAVR [51] | 2022 | Sentinel (n = 1501) Controls (n = 1499) | Balloon-expandable Self-expandable |
|
|
| Studies | Study Type | Year | Procedure Type | Sample Size | Results |
|---|---|---|---|---|---|
| Heeger et al. [18] | Retrospective study | 2018 | VT ablation | Sentinel (n = 11) |
|
| Sharma et al. [24] | Systematic review | 2020 | LAAC with LAA thrombosis | N = 58 CPD (not specified, n = 17) |
|
| Boccuzzi et al. [78] | Registry | 2021 | LAAC with LAA thrombosis | Sentinel (n = 27) |
|
| Zachariah et al. [35] | Research letter | 2022 | VT ablation | Sentinel (n = 6) TriGuard 3 (n = 1) |
|
| Trapeur [76] | Registry | 2022 | LAAC with LAA thrombosis | N = 53 Sentinel (n = 5) |
|
| Berg et al. [36] | Retrospective study | 2023 | LAAC with LAA thrombosis VT ablation | Sentinel (n = 14) TriGuard 3 (n = 21) Sentinel (n = 5) TriGuard 3 (n = 4) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preda, A.; Montalto, C.; Galasso, M.; Munafò, A.; Garofani, I.; Baroni, M.; Gigli, L.; Vargiu, S.; Varrenti, M.; Colombo, G.; et al. Fighting Cardiac Thromboembolism during Transcatheter Procedures: An Update on the Use of Cerebral Protection Devices in Cath Labs and EP Labs. Life 2023, 13, 1819. https://doi.org/10.3390/life13091819
Preda A, Montalto C, Galasso M, Munafò A, Garofani I, Baroni M, Gigli L, Vargiu S, Varrenti M, Colombo G, et al. Fighting Cardiac Thromboembolism during Transcatheter Procedures: An Update on the Use of Cerebral Protection Devices in Cath Labs and EP Labs. Life. 2023; 13(9):1819. https://doi.org/10.3390/life13091819
Chicago/Turabian StylePreda, Alberto, Claudio Montalto, Michele Galasso, Andrea Munafò, Ilaria Garofani, Matteo Baroni, Lorenzo Gigli, Sara Vargiu, Marisa Varrenti, Giulia Colombo, and et al. 2023. "Fighting Cardiac Thromboembolism during Transcatheter Procedures: An Update on the Use of Cerebral Protection Devices in Cath Labs and EP Labs" Life 13, no. 9: 1819. https://doi.org/10.3390/life13091819
APA StylePreda, A., Montalto, C., Galasso, M., Munafò, A., Garofani, I., Baroni, M., Gigli, L., Vargiu, S., Varrenti, M., Colombo, G., Carbonaro, M., Della Rocca, D. G., Oreglia, J., Mazzone, P., & Guarracini, F. (2023). Fighting Cardiac Thromboembolism during Transcatheter Procedures: An Update on the Use of Cerebral Protection Devices in Cath Labs and EP Labs. Life, 13(9), 1819. https://doi.org/10.3390/life13091819







