Adiponectin Modulates Smooth Muscle Cell Morpho-Functional Properties in Murine Gastric Fundus via Sphingosine Kinase 2 Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Gastric Fundus Muscle Strip Preparation and Treatments
2.2. Western Blot Analysis
2.3. Electrophysiological Experiments
Stimulation Protocols
2.4. Morphological Analyses
2.4.1. Hematoxylin and Eosin (H&E) Staining
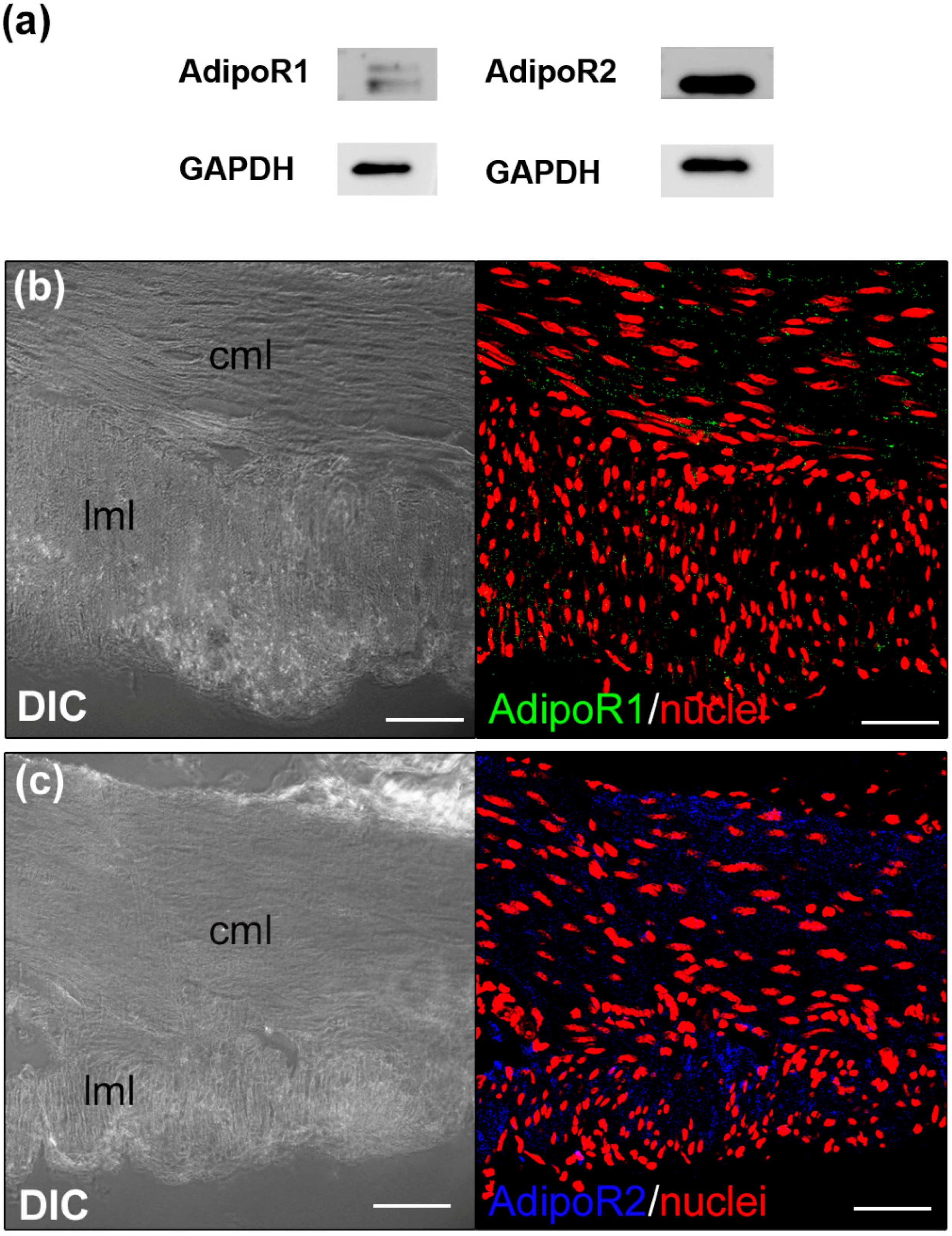
2.4.2. Confocal Immunofluorescence Analyses
2.4.3. Transmission Electron Microscopy (TEM)
2.5. Data Analysis and Statistical Tests
3. Results
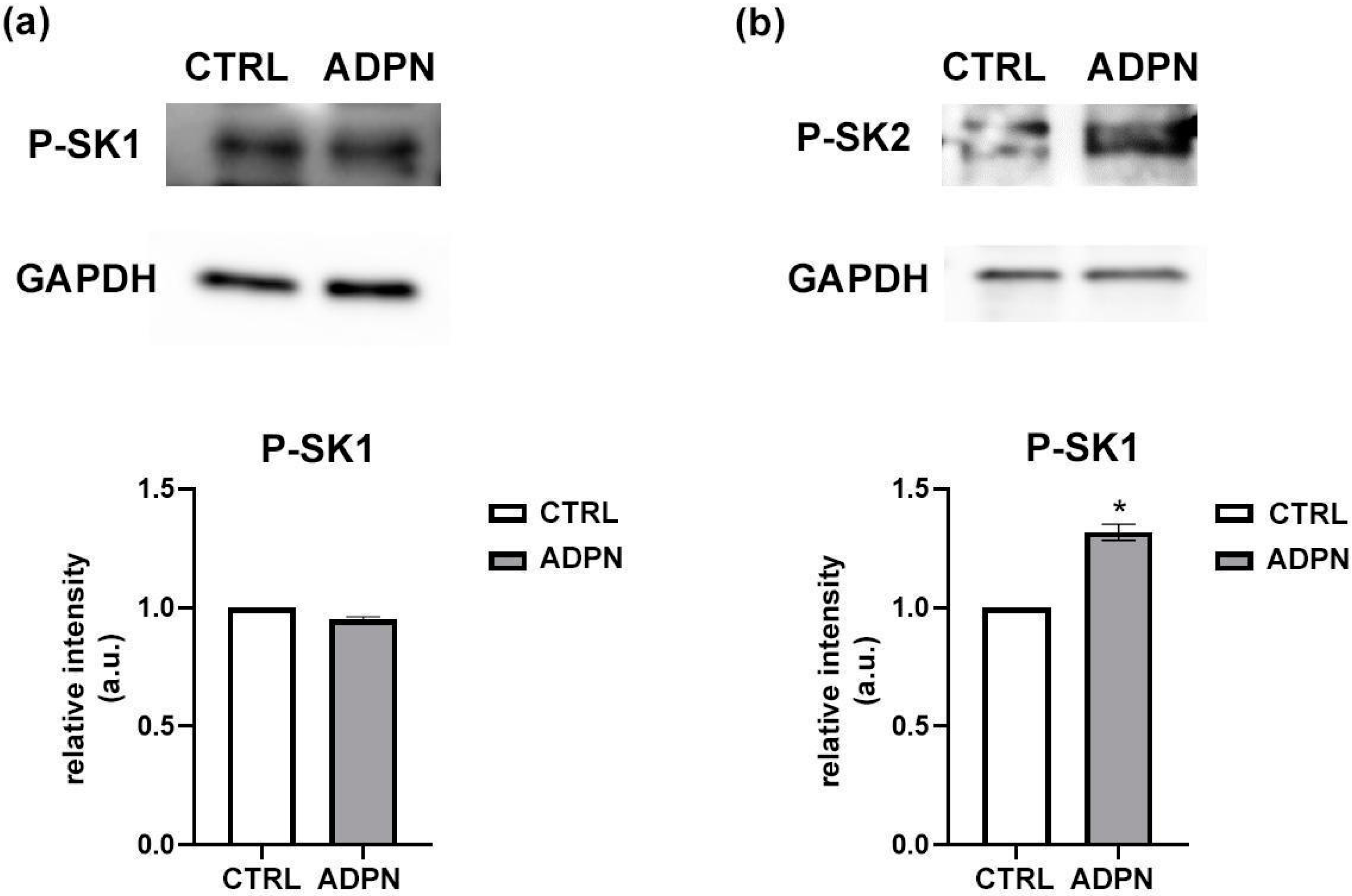
3.1. ADPN Activates Sphingosine Kinase 2 (SK2) in the Murine Gastric Fundus Smooth Muscle Samples
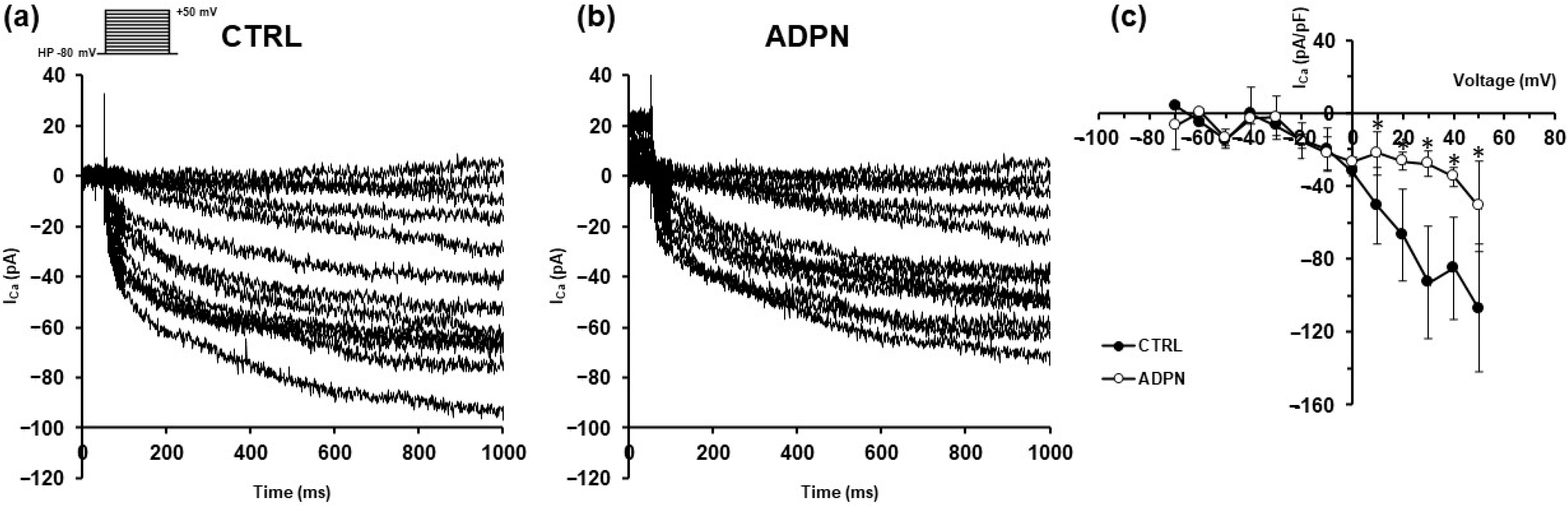
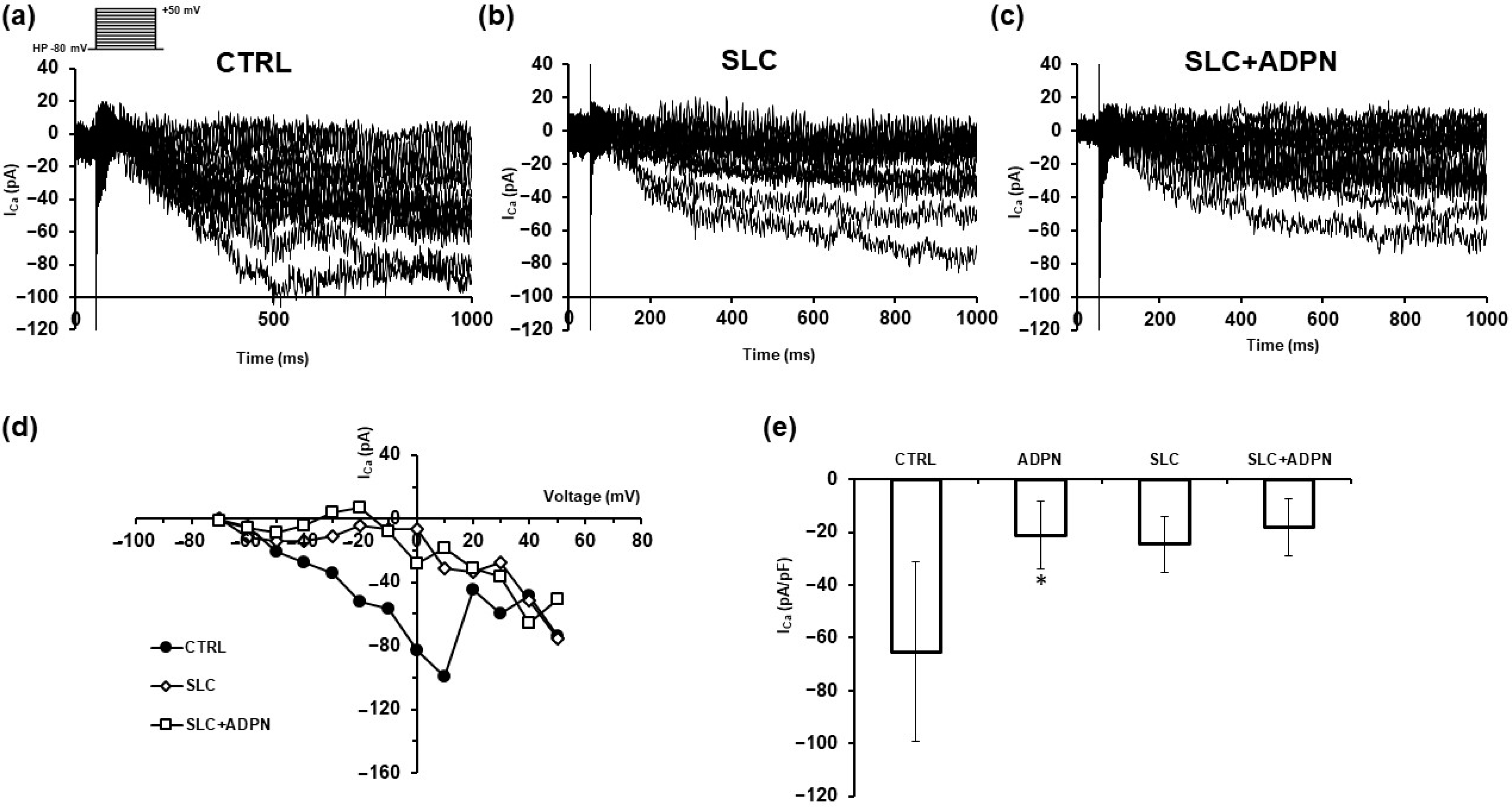
3.2. ADPN Effects on Membrane Properties Involve SK2 Signaling Pathway in the Murine Gastric Fundus SMCs
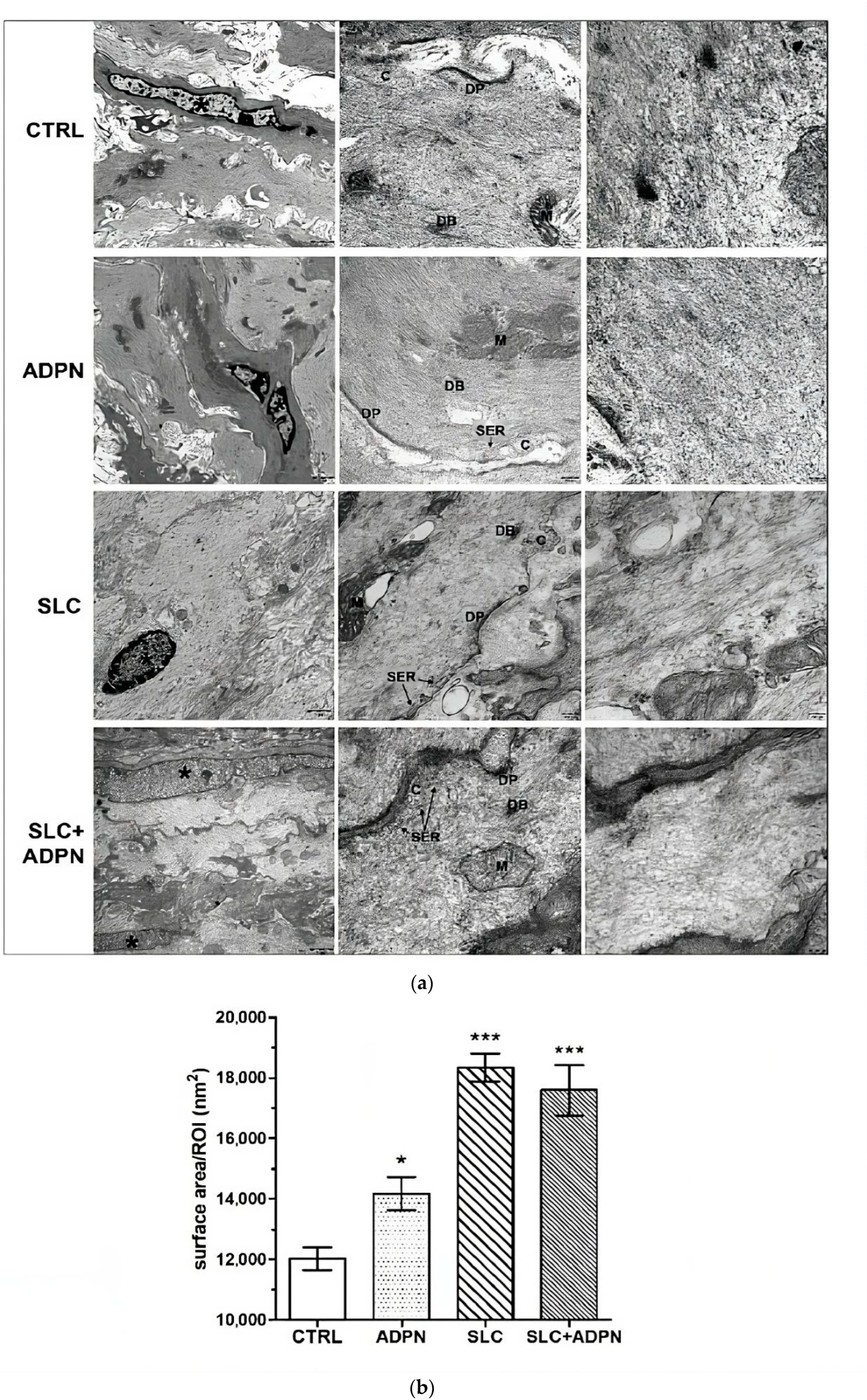
3.3. Cotreatment with ADPN and SK2 Inhibitor Modifies Myofilament Network Organization of the Contractile Apparatus Leading to a More Relaxed State
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rozwadowski, J.; Borodzicz-Jażdżyk, S.; Czarzasta, K.; Cudnoch-Jędrzejewska, A. A Review of the Roles of Apelin and ELABELA Peptide Ligands in Cardiovascular Disease, Including Heart Failure and Hypertension. Med. Sci. Monit. 2022, 28, e938112. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhang, G.; Cheng, Y.; Wang, B. Leptin in skin disease modulation. Clin. Chim. Acta 2021, 516, 8–14. [Google Scholar] [CrossRef]
- Schüler-Toprak, S.; Ortmann, O.; Buechler, C.; Treeck, O. The Complex Roles of Adipokines in Polycystic Ovary Syndrome and Endometriosis. Biomedicines 2022, 10, 2503. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Gallistl, J.; Schüler-Toprak, S.; Fritsch, J.; Buechler, C.; Ortmann, O.; Treeck, O. Anti-Tumoral Effect of Chemerin on Ovarian Cancer Cell Lines Mediated by Activation of Interferon Alpha Response. Cancers 2022, 14, 4108. [Google Scholar] [CrossRef] [PubMed]
- Shklyaev, S.S.; Melnichenko, G.A.; Volevodz, N.N.; Falaleeva, N.A.; Ivanov, S.A.; Kaprin, A.D.; Mokrysheva, N.G. Adiponectin: A pleiotropic hormone with multifaceted roles. Probl. Endocrinol. 2021, 67, 98–112. [Google Scholar] [CrossRef]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M.; et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef]
- Pilon, M. Paradigm shift: The primary function of the “Adiponectin Receptors” is to regulate cell membrane composition. Lipids Health Dis. 2021, 20, 43. [Google Scholar] [CrossRef]
- Suyama, S.; Maekawa, F.; Maejima, Y.; Kubota, N.; Kadowaki, T.; Yada, T. Glucose level determines excitatory or inhibitory effects of adiponectin on arcuate POMC neuron activity and feeding. Sci. Rep. 2016, 6, 30796. [Google Scholar] [CrossRef]
- Suyama, S.; Lei, W.; Kubota, N.; Kadowaki, T.; Yada, T. Adiponectin at physiological level glucose-independently enhances inhibitory postsynaptic current onto NPY neurons in the hypothalamic arcuate nucleus. Neuropeptides 2017, 65, 1–9. [Google Scholar] [CrossRef]
- Sun, J.; Gao, Y.; Yao, T.; Huang, Y.; He, Z.; Kong, X.; Yu, K.J.; Wang, R.T.; Guo, H.; Yan, J.; et al. Adiponectin potentiates the acute effects of leptin in arcuate Pomc neurons. Mol. Metab. 2016, 5, 882–891. [Google Scholar] [CrossRef]
- Idrizaj, E.; Garella, R.; Castellini, G.; Mohr, H.; Pellegata, N.S.; Francini, F.; Ricca, V.; Squecco, R.; Baccari, M.C. Adiponectin affects the mechanical responses in strips from the mouse gastric fundus. World J. Gastroenterol. 2018, 24, 4028–4035. [Google Scholar] [CrossRef] [PubMed]
- Idrizaj, E.; Garella, R.; Nistri, S.; Dell’Accio, A.; Cassioli, E.; Rossi, E.; Castellini, G.; Ricca, V.; Squecco, R.; Baccari, M.C. Adiponectin Exerts Peripheral Inhibitory Effects on the Mouse Gastric Smooth Muscle through the AMPK Pathway. Int. J. Mol. Sci. 2020, 21, 9617. [Google Scholar] [CrossRef]
- Garella, R.; Cassioli, E.; Chellini, F.; Tani, A.; Rossi, E.; Idrizaj, E.; Guasti, D.; Comeglio, P.; Palmieri, F.; Parigi, M.; et al. Defining the Molecular Mechanisms of the Relaxant Action of Adiponectin on Murine Gastric Fundus Smooth Muscle: Potential Translational Perspectives on Eating Disorder Management. Int. J. Mol. Sci. 2023, 24, 1082. [Google Scholar] [CrossRef]
- Idrizaj, E.; Garella, R.; Squecco, R.; Baccari, M.C. Adipocytes-released Peptides Involved in the Control of Gastrointestinal Motility. Curr. Protein Pept. Sci. 2019, 20, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Iwabu, M.; Okada-Iwabu, M.; Kadowaki, T. Adiponectin receptors: A review of their structure, function and how they work. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 15–23. [Google Scholar] [CrossRef]
- Clain, J.; Couret, D.; Planesse, C.; Krejbich-Trotot, P.; Meilhac, O.; Lefebvre d’Hellencourt, C.; Viranaicken, W.; Diotel, N. Distribution of Adiponectin Receptors in the Brain of Adult Mouse: Effect of a Single Dose of the Adiponectin Receptor Agonist, AdipoRON, on Ischemic Stroke. Brain Sci. 2022, 12, 680. [Google Scholar] [CrossRef]
- Choubey, M.; Ranjan, A.; Krishna, A. Adiponectin/AdipoRs signaling as a key player in testicular aging and associated metabolic disorders. Vitam. Horm. 2021, 115, 611–634. [Google Scholar] [CrossRef]
- Polito, R.; Nigro, E.; Pecoraro, A.; Monaco, M.L.; Perna, F.; Sanduzzi, A.; Genovese, A.; Spadaro, G.; Daniele, A. Adiponectin Receptors and Pro-inflammatory Cytokines Are Modulated in Common Variable Immunodeficiency Patients: Correlation With Ig Replacement Therapy. Front. Immunol. 2019, 10, 2812. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kadowaki, T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int. J. Obes. 2008, 32 (Suppl. S7), S13–S18. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, M.; Tölle, M.; Prüfer, J.; van der Giet, M. Pharmacological relevance and potential of sphingosine 1-phosphate in the vascular system. Br. J. Pharmacol. 2011, 163, 1140–1162. [Google Scholar] [CrossRef]
- Watterson, K.R.; Ratz, P.H.; Spiegel, S. The role of sphingosine-1-phosphate in smooth muscle contraction. Cell. Signal. 2005, 17, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Donati, C.; Meacci, E.; Nuti, F.; Becciolini, L.; Farnararo, M.; Bruni, P. Sphingosine 1-phosphate regulates myogenic differentiation: A major role for S1P2 receptor. FASEB J. 2005, 19, 449–451. [Google Scholar] [CrossRef]
- Calise, S.; Blescia, S.; Cencetti, F.; Bernacchioni, C.; Donati, C.; Bruni, P. Sphingosine 1-phosphate stimulates proliferation and migration of satellite cells: Role of S1P receptors. Biochim. Biophys. Acta 2012, 1823, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Bernacchioni, C.; Cencetti, F.; Blescia, S.; Donati, C.; Bruni, P. Sphingosine kinase/sphingosine 1-phosphate axis: A new player for insulin-like growth factor-1-induced myoblast differentiation. Skelet. Muscle 2012, 2, 15. [Google Scholar] [CrossRef]
- Le Stunff, H.; Milstien, S.; Spiegel, S. Generation and metabolism of bioactive sphingosine-1-phosphate. J. Cell. Biochem. 2004, 92, 882–899. [Google Scholar] [CrossRef]
- Palangi, A.; Shakhssalim, N.; Parvin, M.; Bayat, S.; Allameh, A. Differential expression of S1P receptor subtypes in human bladder transitional cell carcinoma. Clin. Transl. Oncol. 2019, 21, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Kharel, Y.; Morris, E.A.; Congdon, M.D.; Thorpe, S.B.; Tomsig, J.L.; Santos, W.L.; Lynch, K.R. Sphingosine Kinase 2 Inhibition and Blood Sphingosine 1-Phosphate Levels. J. Pharmacol. Exp. Ther. 2015, 355, 23–31. [Google Scholar] [CrossRef]
- Pitson, S.M.; Moretti, P.A.; Zebol, J.R.; Lynn, H.E.; Xia, P.; Vadas, M.A.; Wattenberg, B.W. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003, 22, 5491–5500. [Google Scholar] [CrossRef]
- Hait, N.C.; Bellamy, A.; Milstien, S.; Kordula, T.; Spiegel, S. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J. Biol. Chem. 2007, 282, 12058–12065. [Google Scholar] [CrossRef]
- Squecco, R.; Garella, R.; Luciani, G.; Francini, F.; Baccari, M.C. Muscular effects of orexin A on the mouse duodenum: Mechanical and electrophysiological studies. J. Physiol. 2011, 589 Pt 21, 5231–5246. [Google Scholar] [CrossRef]
- Formigli, L.; Francini, F.; Nistri, S.; Margheri, M.; Luciani, G.; Naro, F.; Silvertown, J.D.; Zecchi Orlandini, S.; Meacci, E.; Bani, D. Skeletal myoblasts overexpressing relaxin improve differentiation and communication of primary murine cardiomyocyte cell cultures. J. Mol. Cell. Cardiol. 2009, 47, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Aljafary, M.A.; Al-Suhaimi, E.A. Adiponectin System (Rescue Hormone): The Missing Link between Metabolic and Cardiovascular Diseases. Pharmaceutics 2022, 14, 1430. [Google Scholar] [CrossRef] [PubMed]
- Tyszkiewicz-Nwafor, M.; Jowik, K.; Paszynska, E.; Dutkiewicz, A.; Słopien, A.; Dmitrzak-Weglarz, M. Expression of immune-related proteins and their association with neuropeptides in adolescent patients with anorexia nervosa. Neuropeptides 2022, 91, 102214. [Google Scholar] [CrossRef]
- Nobis, S.; Morin, A.; Achamrah, N.; Belmonte, L.; Legrand, R.; Chan, P.; do Rego, J.L.; Vaudry, D.; Gourcerol, G.; Déchelotte, P.; et al. Delayed gastric emptying and altered antrum protein metabolism during activity-based anorexia. Neurogastroenterol. Motil. 2018, 30, e13305. [Google Scholar] [CrossRef]
- Ruan, H.; Dong, L.Q. Adiponectin signaling and function in insulin target tissues. J. Mol. Cell. Biol. 2016, 8, 101–109. [Google Scholar] [CrossRef]
- Perrotta, F.; Nigro, E.; Mollica, M.; Costigliola, A.; D’Agnano, V.; Daniele, A.; Bianco, A.; Guerra, G. Pulmonary Hypertension and Obesity: Focus on Adiponectin. Int. J. Mol. Sci. 2019, 20, 912. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Reibe-Pal, S.; Febbraio, M.A. Adiponectin serenades ceramidase to improve metabolism. Mol. Metab. 2017, 6, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef]
- Ahima, R.S.; Qi, Y.; Singhal, N.S.; Jackson, M.B.; Scherer, P.E. Brain adipocytokine action and metabolic regulation. Diabetes 2006, 55 (Suppl. S2), S145–S154. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Zhang, S.; Zhao, Y.; Yin, X.; Wang, W.; Ma, X.; Liu, H. The role of NO-cGMP pathway inhibition in vascular endothelial-dependent smooth muscle relaxation disorder of AT1-AA positive rats: Protective effects of adiponectin. Nitric Oxide 2019, 87, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, F.; Lonardi, S.; Bernardini, G.; Telfer, B.; Mandelli, G.E.; Santucci, A.; Vermi, W.; Giurisato, E. Tumor-Associated Macrophages in Osteosarcoma: From Mechanisms to Therapy. Int. J. Mol. Sci. 2020, 21, 5207. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Waki, H.; Iwabu, M.; Okada-Iwabu, M.; Nakamura, M. Adiponectin, adiponectin receptors, and epigenetic regulation of adipogenesis. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 257–265. [Google Scholar] [CrossRef]
- Bernacchioni, C.; Squecco, R.; Gamberi, T.; Ghini, V.; Schumacher, F.; Mannelli, M.; Garella, R.; Idrizaj, E.; Cencetti, F.; Puliti, E.; et al. S1P Signalling Axis Is Necessary for Adiponectin-Directed Regulation of Electrophysiological Properties and Oxidative Metabolism in C2C12 Myotubes. Cells 2022, 11, 713. [Google Scholar] [CrossRef]
- Holland, W.L.; Xia, J.Y.; Johnson, J.A.; Sun, K.; Pearson, M.J.; Sharma, A.X.; Quittner-Strom, E.; Tippetts, T.S.; Gordillo, R.; Scherer, P.E. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol. Metab. 2017, 6, 267–275. [Google Scholar] [CrossRef]
- Hla, T.; Venkataraman, K.; Michaud, J. The vascular S1P gradient-cellular sources and biological significance. Biochim. Biophys. Acta 2008, 1781, 477–482. [Google Scholar] [CrossRef]
- Mizuguchi, T.; Mitaka, T.; Katsuramaki, T.; Hirata, K. Hepatocyte transplantation for total liver repopulation. J. Hepato-Biliary-Pancreat. Surg. 2005, 12, 378–385. [Google Scholar] [CrossRef]
- Kase, H.; Hattori, Y.; Sato, N.; Banba, N.; Kasai, K. Symptoms of gastroesophageal reflux in diabetes patients. Diabetes Res. Clin. Pract. 2008, 79, e6–e7. [Google Scholar] [CrossRef]
- Chen, H.; Montagnani, M.; Funahashi, T.; Shimomura, I.; Quon, M.J. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 2003, 278, 45021–45026. [Google Scholar] [CrossRef]
- Zhou, H.; Murthy, K.S. Distinctive G protein-dependent signaling in smooth muscle by sphingosine 1-phosphate receptors S1P1 and S1P2. Am. J. Physiol. Cell Physiol. 2004, 286, C1130–C1138. [Google Scholar] [CrossRef]
- Kim, Y.; Park, C.W. Mechanisms of Adiponectin Action: Implication of Adiponectin Receptor Agonism in Diabetic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 1782. [Google Scholar] [CrossRef]
- Harraz, O.F.; Brett, S.E.; Welsh, D.G. Nitric oxide suppresses vascular voltage-gated T-type Ca2+ channels through cGMP/PKG signaling. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H279–H285. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, W.; Qu, L.; Cang, C. Sphingosine kinase 2 inhibitor ABC294640 suppresses neuronal excitability and inhibits multiple endogenously and exogenously expressed voltage-gated ion channels in cultured cells. Channels 2020, 14, 216–230. [Google Scholar] [CrossRef]
- Shi, W.; Zhai, C.; Feng, W.; Wang, J.; Zhu, Y.; Li, S.; Wang, Q.; Zhang, Q.; Yan, X.; Chai, L.; et al. Resveratrol inhibits monocrotaline-induced pulmonary arterial remodeling by suppression of SphK1-mediated NF-κB activation. Life Sci. 2018, 210, 140–149. [Google Scholar] [CrossRef]
- Kitazawa, T.; Semba, S.; Huh, Y.H.; Kitazawa, K.; Eto, M. Nitric oxide-induced biphasic mechanism of vascular relaxation via dephosphorylation of CPI-17 and MYPT1. J. Physiol. 2009, 587 Pt 14, 3587–3603. [Google Scholar] [CrossRef]
- Nincheri, P.; Luciani, P.; Squecco, R.; Donati, C.; Bernacchioni, C.; Borgognoni, L.; Luciani, G.; Benvenuti, S.; Francini, F.; Bruni, P. Sphingosine 1-phosphate induces differentiation of adipose tissue-derived mesenchymal stem cells towards smooth muscle cells. Cell. Mol. Life Sci. 2009, 66, 1741–1754. [Google Scholar] [CrossRef]
- Wang, T.; Brown, M.E.; Kelly, G.T.; Camp, S.M.; Mascarenhas, J.B.; Sun, X.; Dudek, S.M.; Garcia, J.G.N. Myosin light chain kinase (MYLK) coding polymorphisms modulate human lung endothelial cell barrier responses via altered tyrosine phosphorylation, spatial localization, and lamellipodial protrusions. Pulm. Circ. 2018, 8, 2045894018764171. [Google Scholar] [CrossRef]
- Lockman, K.; Hinson, J.S.; Medlin, M.D.; Morris, D.; Taylor, J.M.; Mack, C.P. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J. Biol. Chem. 2004, 279, 42422–42430. [Google Scholar] [CrossRef]
- Kraft, M.; Zettl, U.K.; Noack, T.; Patejdl, R. The sphingosine analog fingolimod (FTY720) enhances tone and contractility of rat gastric fundus smooth muscle. Neurogastroenterol. Motil. 2018, 30, e13372. [Google Scholar] [CrossRef] [PubMed]


| Parameter | CTRL | ADPN | SLC | SLC + ADPN |
|---|---|---|---|---|
| RMP (mV) | −34.6 ± 5.1 (n = 7) | −46.5 ± 8.6 * (n = 7) | −31.7 ± 1.9 # (n = 5) | −30.8 ± 0.8 # (n = 5) |
| Cm (pF) | 16.4 ± 7.1 (n = 38) | 25.6 ± 4.2 * (n = 6) | 14.3 ± 1.6 # (n = 7) | 13.6 ± 1.3 # (n = 7) |
| Gm (nS) | 7.7 ± 3.8 (n = 13) | 18.1 ± 8.7 * (n = 18) | 6.7 ± 1.8 # (n = 7) | 6.8 ± 1.4 # (n = 7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garella, R.; Bernacchioni, C.; Chellini, F.; Tani, A.; Palmieri, F.; Parigi, M.; Guasti, D.; Cassioli, E.; Castellini, G.; Ricca, V.; et al. Adiponectin Modulates Smooth Muscle Cell Morpho-Functional Properties in Murine Gastric Fundus via Sphingosine Kinase 2 Activation. Life 2023, 13, 1812. https://doi.org/10.3390/life13091812
Garella R, Bernacchioni C, Chellini F, Tani A, Palmieri F, Parigi M, Guasti D, Cassioli E, Castellini G, Ricca V, et al. Adiponectin Modulates Smooth Muscle Cell Morpho-Functional Properties in Murine Gastric Fundus via Sphingosine Kinase 2 Activation. Life. 2023; 13(9):1812. https://doi.org/10.3390/life13091812
Chicago/Turabian StyleGarella, Rachele, Caterina Bernacchioni, Flaminia Chellini, Alessia Tani, Francesco Palmieri, Martina Parigi, Daniele Guasti, Emanuele Cassioli, Giovanni Castellini, Valdo Ricca, and et al. 2023. "Adiponectin Modulates Smooth Muscle Cell Morpho-Functional Properties in Murine Gastric Fundus via Sphingosine Kinase 2 Activation" Life 13, no. 9: 1812. https://doi.org/10.3390/life13091812
APA StyleGarella, R., Bernacchioni, C., Chellini, F., Tani, A., Palmieri, F., Parigi, M., Guasti, D., Cassioli, E., Castellini, G., Ricca, V., Bani, D., Sassoli, C., Donati, C., & Squecco, R. (2023). Adiponectin Modulates Smooth Muscle Cell Morpho-Functional Properties in Murine Gastric Fundus via Sphingosine Kinase 2 Activation. Life, 13(9), 1812. https://doi.org/10.3390/life13091812









