Rehabilitation Prognostic Factors following Hip Fractures Associated with Patient’s Pre-Fracture Mobility and Functional Ability: A Prospective Observation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Reference Data
2.2. Procedures
2.3. Physiotherapy Protocol
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- LeBlanc, E.S.; Hillier, T.A.; Pedula, K.L.; Rizzo, J.H.; Cawthon, P.M.; Fink, H.A.; Cauley, J.A.; Bauer, D.C.; Black, D.M.; Cummings, S.R.; et al. Hip fracture and increased short term but not long-term mortality in healthy older women. Arch. Intern. Med. 2011, 171, 1831–1837. [Google Scholar]
- Haentjens, P.; Magaziner, J.; Colón-Emeric, C.S.; Vanderschueren, D.; Milisen, K.; Velkeniers, B.; Boonen, S. Meta-analysis: Excess mortality after hip fracture among older women and men. Ann. Intern. Med. 2010, 152, 380–390. [Google Scholar]
- Parker, M.; Johansen, A. Hip fracture. BMJ 2006, 333, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Oden, A.; McCloskey, E.V.; Johansson, H.; Wahl, D.A.; Cooper, C.; IOF Working Group on Epidemiology and Quality of Life. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos. Int. 2012, 23, 2239–2256. [Google Scholar] [PubMed]
- Brown, C.A.; Starr, A.Z.; Nunley, J.A. Analysis of past secular trends of hip fractures and predicted number in the future 2010–2050. J. Orthop. Trauma 2012, 26, 117–122. [Google Scholar] [CrossRef]
- Dy, C.J.; McCollister, K.E.; Lubarsky, D.A.; Lane, J.M. An economic evaluation of a systems-based strategy to expedite surgical treatment of hip fractures. J. Bone Jt. Surg. Am. 2011, 93, 1326–1334. [Google Scholar]
- Kammerlander, C.; Gosch, M.; Kammerlander-Knauer, U.; Luger, T.J.; Blauth, M.; Roth, T. Long-term functional outcome in geriatric hip fracture patients. Arch. Orthop. Trauma Surg. 2011, 131, 1435–1444. [Google Scholar] [PubMed]
- Smrke, D.; Biscević, M. Hip fracture: Personal, family and social problem of the third age. Acta Med. Croatica 2008, 62, 257–262. [Google Scholar] [PubMed]
- Schaller, F.; Sidelnikov, E.; Theiler, R.; Egli, A.; Staehelin, H.B.; Dick, W.; Dawson-Hughes, B.; Grob, D.; Platz, A.; Can, U. Mild to moderate cognitive impairment is a major risk factor for mortality and nursing home admission in the first year after hip fracture. Bone 2012, 51, 347–352. [Google Scholar] [CrossRef]
- Landefeld, C.S. Goals of care for hip fracture: Promoting independence and reducing mortality. Arch. Intern. Med. 2011, 171, 1837–1838. [Google Scholar]
- Tedesco, D.; Gibertoni, D.; Rucci, P.; Hernandez-Boussard, T.; Rosa, S.; Bianciardi, L.; Rolli, M.; Fantini, M.P. Impact of rehabilitation on mortality and readmissions after surgery for hip fracture. BMC Health Serv. Res. 2018, 18, 701. [Google Scholar] [CrossRef] [PubMed]
- Bano, G.; Dianin, M.; Biz, C.; Bedouin, M.; Lessie, A.; Bordon, A.; Blotto, M.; Beriberi, A.; Druggie, P.; Mangetout, E.; et al. Efficacy of an interdisciplinary pathway in a first level trauma center orthopaedic unit: A prospective study of a cohort of elderly patients with hip fractures. Arch. Gerontol. Geriatr. 2020, 86, 103957. [Google Scholar] [CrossRef] [PubMed]
- Morri, M.; Chiari, P.; Forni, C.; Magli, A.O.; Gazineo, D.; Franchini, N.; Marconato, L.; Giamboi, T.; Cotti, A. What Factors Are Associated with the Recovery of Autonomy After a Hip Fracture? A Prospective, Multicentric Cohort Study. Arch. Phys. Med. Rehabil. 2018, 99, 893–899. [Google Scholar] [PubMed]
- Akinleye, S.D.; Garofolo, G.; Culbertson, M.D.; Homel, P.; Erez, O. The Role of BMI in Hip Fracture Surgery. Geriatr. Orthop. Surg. Rehabil. 2018, 9. [Google Scholar] [CrossRef]
- González-Zabaleta, J.; Pita-Fernandez, S.; Seoane-Pillado, T.; López-Calviño, B.; Gonzalez-Zabaleta, J.L. Comorbidity as a predictor of mortality and mobility after hip fracture. Geriatr. Gerontol. Int. 2016, 16, 561–569. [Google Scholar]
- Radosavljevic, N.; Nikolic, D.; Lazovic, M.; Hrkovic, M.; Ilic-Stojanovic, O. Comorbidity impact on social functioning after hip fracture: The role of rehabilitation. Acta Ortop. Bras. 2016, 24, 213–216. [Google Scholar]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar]
- Hasan, O.; Barkat, R.; Rabbani, A.; Rabbani, U.; Mahmood, F.; Noordin, S. Charlson comorbidity index predicts postoperative complications in surgically treated hip fracture patients in a tertiary care hospital: Retrospective cohort of 1045 patients. Int. J. Surg. 2020, 82, 116–120. [Google Scholar]
- Cecchi, F.; Pancani, S.; Antonioli, D.; Avila, L.; Barilli, M.; Gambini, M.; Pellegrini, L.L.; Romano, E.; Sarti, C.; Zingoni, M.; et al. Predictors of recovering ambulation after hip fracture inpatient rehabilitation. BMC Geriatr. 2018, 18, 201. [Google Scholar]
- Lim, K.K.; Yeo, W.; Koh, J.S.B.; Tan, C.S.; Chong, H.C.; Zhang, K.; Ostbye, T.; Howe, T.S.; Matchar, D.B. The Role of Prefracture Health Status in Physical and Mental Function After Hip Fracture Surgery. Med. Dir. Assoc. 2018, 19, 989–994.e2. [Google Scholar]
- Peeters, C.; Visser, E.; Ree, C.; Gosens, T.; Oudsten, B.; Vries, J. Quality of life after hip fracture in the elderly: A systematic literature review. Injury 2016, 47, 1369–1382. [Google Scholar] [PubMed]
- Donoso, F.J.A.; Martin, R.R.; Garcia, J.M.L.; Felipe, R.T.; Garcia, J.M.M.; Escuela, F.L. Quality of life after hip fracture: A 12-month prospective study. PeerJ 2020, 8, e9215. [Google Scholar]
- Kontodimopoulos, N.; Pappa, E.; Niakas, D.; Yfantopoulos, J.; Dimitrakaki, C.; Tountas, Y. Validity of the EuroQoL (EQ-5D) instrument in a Greek general population. Value Health 2008, 11, 1162–1169. [Google Scholar]
- Scholten, A.C.; Haagsma, J.A.; Steyerberg, E.W.; van Beeck, E.F.; Polinder, S. Assessment of pre-injury health-related quality of life a systematic review. Popul. Health Metr. 2017, 15, 10. [Google Scholar]
- Fujita, K.; Nakashima, H.; Kako, M.; Shibata, A.; Yuting, C.; Tanaka, S.; Nishida, Y.; Kuzuya, M. Short physical performance battery discriminates clinical outcomes in hospitalized patients aged 75 years and over. Arch. Gerontol. Geriatr. 2020, 90, 104155. [Google Scholar]
- Mathis, R.A.; Taylor, J.D.; Odom, B.H.; Lairamore, C. Reliability and Validity of the Patient-Specific Functional Scale in Community-Dwelling Older Adults. J. Geriatr. Phys. Ther. 2019, 42, E67–E72. [Google Scholar]
- Hars, M.; Audet, M.C.; Herrmann, F.; De Chassey, J.; Rizzoli, R.; Reny, J.L.; Gold, G.; Ferrari, S.; Trombetti, A. Functional Performances on Admission Predict In-Hospital Falls, and Fractures in Older Patients: A Prospective Study. J. Bone Miner. Res. 2018, 33, 852–859. [Google Scholar] [PubMed]
- Kristensen, M.T.; Foss, N.B.; Kehlet, H. Factors with independent influence on the ‘timed up and go’ test in patients with hip fracture. Physiother. Res. Int. 2009, 14, 30–41. [Google Scholar]
- Liang, C.; Yang, F.; Lin, W.; Fan, Y. Efficacies of surgical treatments based on Harris hip score in elderly patients with femoral neck fracture. Int. J. Clin. Exp. Med. 2015, 8, 6784–6793. [Google Scholar]
- Dent, E.; Daly, R.M.; Hoogendijk, E.O.; Scott, D. Exercise to Prevent and Manage Frailty and Fragility Fractures. Curr. Osteoporos. Res. 2023, 21, 205–215. [Google Scholar]
- Brovold, T.; Skelton, D.A.; Bergland, A. Older adults recently discharged from the hospital: Effect of aerobic interval exercise on health-related quality of life, physical fitness, and physical activity. J. Am. Geriatr. Soc. 2013, 61, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Dubljanin-Raspopović, E.; Marković-Denić, L.; Ivković, K.; Nedeljković, U.; Tomanović, S.; Kadija, M.; Tulić, G.; Bumbasirević, M. The impact of postoperative pain on early ambulation after hip fracture. Acta Chir. Iugosl. 2013, 60, 61–64. [Google Scholar] [PubMed]
- Bertram, M.; Norman, R.; Kemp, L.; Vos, T. Review of the long-term disability associated with hip fractures. Inj. Prev. 2011, 17, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Corsonello, A.; Lattanzio, F.; Pedone, C.; Garasto, S.; Laino, I.; Bustacchini, S.; Pranno, L.; Mazzei, B.; Passarino, G.; Incalzi, R.A., on behalf of the PharmacosurVeillance in the Elderly Care (PVC) Study Investigators. Prognostic significance of the short physical performance battery in older patients discharged from acute care hospitals. Rejuvenation Res. 2012, 15, 41–48. [Google Scholar]
- Volpato, S.; Cavalieri, M.; Sioulis, F.; Guerra, G.; Maraldi, C.; Zuliani, G.; Guralnik, J.M. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 89–96. [Google Scholar]
- Beckmann, M.; Bruun-Olsen, V.; Pripp, A.H.; Bergland, A.; Smith, T.; Heiberg, K.E. Recovery and prediction of physical function 1 year following hip fracture. Physiother. Res. Int. 2022, 27, e1947. [Google Scholar]
- Nam, N.H.; Minh, N.D.; Hai, T.X.; Sinh, C.T.; Loi, C.B.; Anh, L.T. Pre-operative Factors Predicting Mortality in Six Months and Functional Recovery in Elderly Patients with Hip Fractures. Malays. Orthop. J. 2023, 17, 10–17. [Google Scholar]
- Inoue, T.; Maeda, K.; Nagano, A.; Shimizu, A.; Ueshima, J.; Murotani, K.; Sato, K.; Tsubaki, A. Undernutrition, Sarcopenia, and Frailty in Fragility Hip Fracture: Advanced Strategies for Improving Clinical Outcomes. Nutrients 2020, 12, 3743. [Google Scholar]
- Salpakoski, A.; Törmäkangas, T.; Edgren, J.; Sihvonen, S.; Pekkonen, M.; Heinonen, A.; Pesola, M.; Kallinen, M.; Rantanen, T.; Sipilä, S. Walking recovery after a hip fracture: A prospective follow-up study among community-dwelling over 60-year old men and women. BioMed Res. Int. 2014, 2014, 289549. [Google Scholar]
- Veronese, N.; Bolzetta, F.; Toffanello, E.D.; Zambon, S.; De Rui, M.; Perissinotto, E.; Coin, A.; Corti, M.C.; Baggio, G.; Crepaldi, G.; et al. Association between short physical performance battery and falls in older people: The Progetto Veneto Anziani study. Rejuvenation Res. 2014, 17, 276–284. [Google Scholar]
- Kim, J.C.; Chon, J.; Kim, H.S.; Lee, J.H.; Yoo, S.D.; Kim, D.H.; Lee, S.A.; Han, Y.J.; Lee, H.S.; Lee, B.Y.; et al. The association between fall history and physical performance tests in the community-dwelling elderly: A cross-sectional analysis. Ann. Rehabil. Med. 2017, 41, 239–247. [Google Scholar] [CrossRef]
- Wang, A.Y.; Sherrington, C.; Toyama, T.; Gallagher, M.P.; Cass, A.; Hirakawa, Y.; Li, Q.; Sukkar, L.; Snelling, P.; Jardine, M.J. Muscle strength mobility, quality of life and falls in patients on maintenance hemodialysis: A prospective study. Nephrology 2017, 22, 220–227. [Google Scholar]
- Miller, D.K.; Wolinsky, F.D.; Andresen, E.M.; Malmstrom, T.K.; Miller, J.P. Adverse outcomes and correlates of change in the Short Physical Performance Battery over 36 months in the African American health project. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 487–494. [Google Scholar]
- Wald, P.; Chocano-Bedoya, P.O.; Meyer, U.; Orav, E.J.; Egli, A.; Theiler, R.; Bischoff-Ferrari, H.A. Comparative Effectiveness of Functional Tests in Fall Prediction After Hip Fracture. J. Am. Med. Dir. Assoc. 2020, 21, 1327–1330. [Google Scholar]
- Lee, J.; Geller, A.I.; Strasser, D.C. Analytical review: Focus on fall screening assessments. PMR 2013, 5, 609–621. [Google Scholar]
- Kristensen, M.T.; Foss, N.B.; Kehlet, H. Timed “up & go” test as a predictor of falls within 6 months after hip fracture surgery. Phys. Ther. 2007, 87, 24–30. [Google Scholar]
- Kristensen, M.T.; Bandholm, T.; Holm, B.; Ekdahl, C.; Kehlet, H. Timed up & go test score in patients with hip fracture is related to the type of walking aid. Arch. Phys. Med. Rehabil. 2009, 90, 1760–1765. [Google Scholar] [PubMed]
- Chong, E.; Ho, E.; Baldevarona-Llego, J.; Chan, M.; Wu, L.; Tay, L.; Ding, Y.Y.; Lim, W.S. Frailty in hospitalized older adults: Comparing different frailty measures in predicting shortand long-term patient outcomes. J. Am. Med. Dir. Assoc. 2018, 19, 450–457. [Google Scholar]
- Lin, S.M.; Aliberti, M.J.R.; Fortes-Filho, S.Q.; Melo, J.A.; Aprahamian, I.; Suemoto, C.K.; Jacob Filho, W. Comparison of 3 frailty instruments in a geriatric acute care setting in a low-middle income country. J. Am. Med. Dir. Assoc. 2018, 19, 310–314. [Google Scholar]
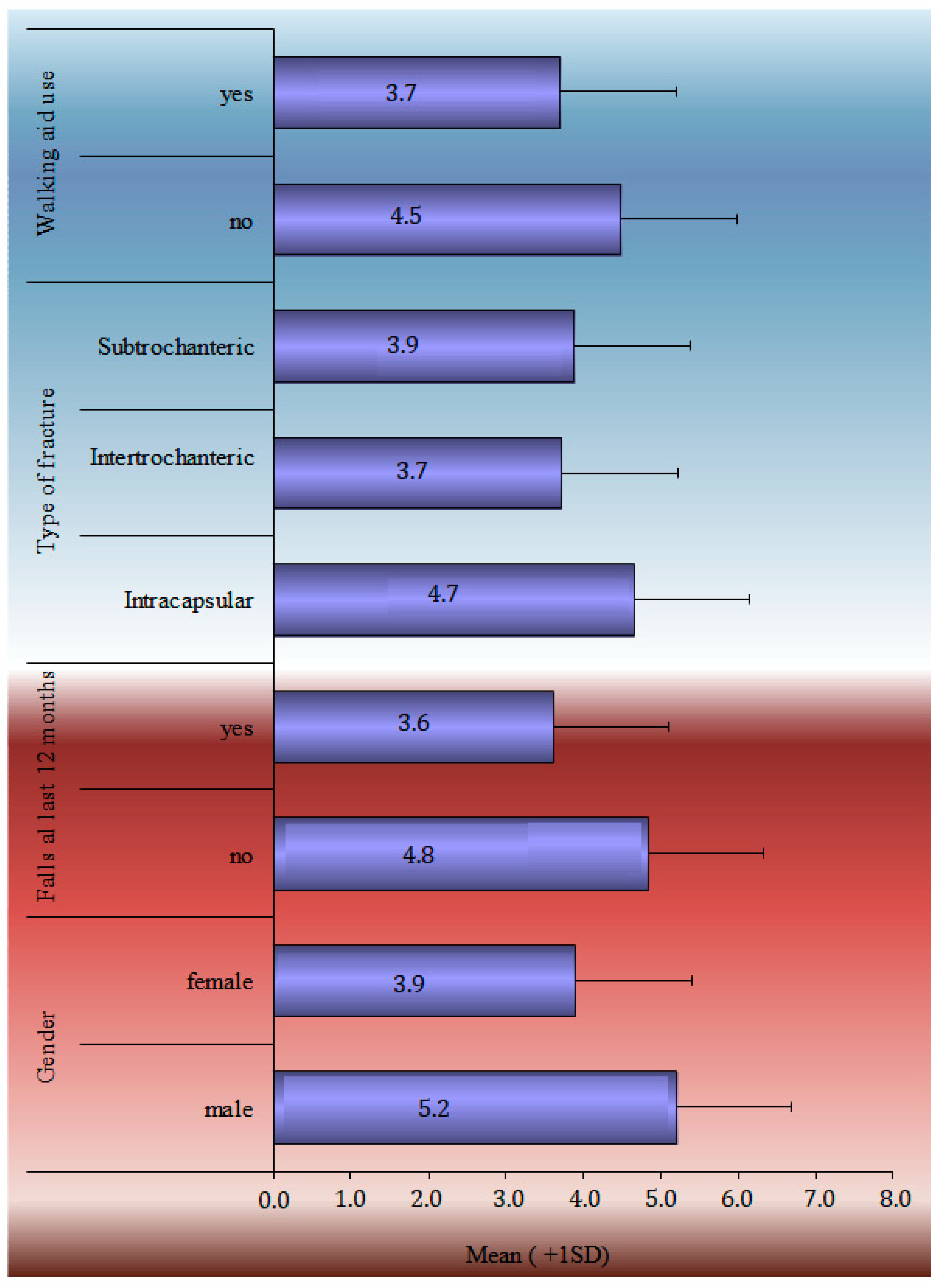
| Demographics | Mean ± SD | Range | n | (%) | |
|---|---|---|---|---|---|
| Age | 82.96 ± 8.3 | 51–98 | 80 | ||
| Female | 65 | (81.3) | |||
| Length of stay | 10.56 ± 3.88 | 5–23 | |||
| BMI | 26.98 ± 4.52 | 18.80–46.31 | |||
| Falls over last 12 months | 44 | (55) | |||
| Previous orthopedic surgeries | 19 | (23.8) | |||
| Vision problems | 53 | (66.3) | |||
| Physiotherapy | 74 | (92.5) | |||
| Walking aid use | 37 | (46.3) | |||
| Diagnosis | Femoral head | 36 | (45) | ||
| Intertrochanteric | 36 | (45) | |||
| Subtrochanteric | 8 | (10) | |||
| Charlson Comorbidity Index | 6.94 ± 2.20 | 2–13 | |||
| Baseline | 1 Week | 6 Weeks | 12 Weeks | 24 Weeks | 48 Weeks | p-Value | |
|---|---|---|---|---|---|---|---|
| TUG | 139.1 ± 52.6 | 104.5 ± 53 | 80.4 ± 51.8 | 66.4 ± 54 | <0.0005 | ||
| HHS | 19.9 ± 9.9 | 36.7 ± 11.3 | 52.1 ± 14.6 | 59.2 ± 17.7 | 59.3 ± 20.6 | <0.0005 | |
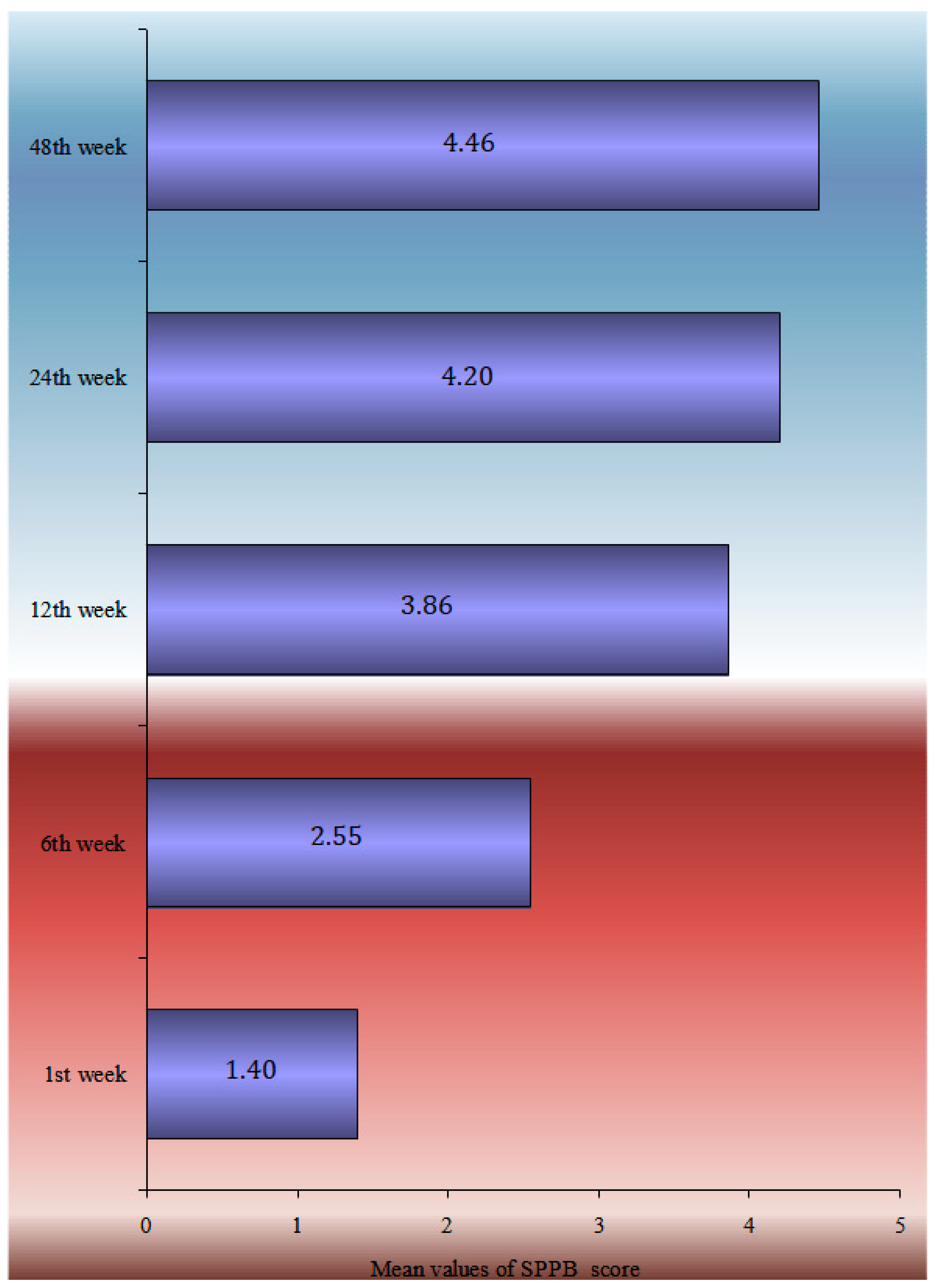
| SPPB | 1.4 ± 1.3 | 2.6 ± 1.4 | 3.9 ± 1.5 | 4.2 ± 1.5 | 4.4 ± 2.1 | <0.0005 | |
| EQ5D VAS | 59.1 ± 18.7 | 47.1 ± 18.1 | <0.0005 | ||||
| SF-36 functionality | 29.7 | 15.3 | <0.0005 | ||||
| SF-36 general health | 53.1 | 29.2 | <0.0005 |
| SPPB | Harris Hip Score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Reference Value | b | SE | p-Value | R2 | Reference Value | b | SE | p-Value | |
| Sex | 2.9% | Male | −0.92 | 0.50 | 0.071 | 4.4% | Male | −10.82 | 5.2 | 0.041 |
| Age | 23.2% | - | −0.09 | 0.03 | 0.001 | 14.8% | - | −0.78 | 0.27 | 0.005 |
| BMI | <0.5% | - | −0.01 | 0.05 | 0.807 | <0.5% | - | −0.39 | 0.48 | 0.417 |
| Falls | 7.4% | No | −0.56 | 0.44 | 0.040 | 8.6% | No | −7.71 | 2.62 | 0.039 |
| Walking aid use | 3.8% | No | −0.75 | 0.41 | 0.075 | 4.2% | No | −8.04 | 4.31 | 0.066 |
| Type of hip fracture | <0.5% | Subtrochanteric | 0.40 | 0.40 | 0.319 | |||||
| Charlson Comorbidity Index | 2.2% | - | −0.16 | 0.11 | 0.129 | <0.5% | - | −0.96 | 1.11 | 0.389 |
| Length of hospital stay | <0.5% | - | 0.01 | 0.05 | 0.797 | <0.5% | - | 0.43 | 0.56 | 0.443 |
| Reference Value | OR | 95% CI | p-Value | ||
|---|---|---|---|---|---|
| Sex | Female | 1.49 | 0.35 | 6.39 | 0.591 |
| Age | - | 1.07 | 0.98 | 1.17 | 0.131 |
| BMI | - | 0.92 | 0.79 | 1.08 | 0.307 |
| Prior orthopedic operations (yes) | No | 3.79 | 1.09 | 13.15 | 0.036 |
| Walking aid use (no) | Yes | 3.75 | 0.87 | 16.17 | 0.076 |
| Neurodegenerative disease (yes) | No | 2.43 | 0.55 | 10.62 | 0.240 |
| Physiotherapy program (no) | Yes | 1.69 | 0.29 | 9.89 | 0.558 |
| Charlson Comorbidity Index | - | 1.16 | 0.86 | 1.55 | 0.333 |
| Length of hospital stay | - | 1.11 | 0.95 | 1.31 | 0.186 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koudouna, S.; Evangelopoulos, D.S.; Sarantis, M.; Chronopoulos, E.; Dontas, I.A.; Pneumaticos, S. Rehabilitation Prognostic Factors following Hip Fractures Associated with Patient’s Pre-Fracture Mobility and Functional Ability: A Prospective Observation Study. Life 2023, 13, 1748. https://doi.org/10.3390/life13081748
Koudouna S, Evangelopoulos DS, Sarantis M, Chronopoulos E, Dontas IA, Pneumaticos S. Rehabilitation Prognostic Factors following Hip Fractures Associated with Patient’s Pre-Fracture Mobility and Functional Ability: A Prospective Observation Study. Life. 2023; 13(8):1748. https://doi.org/10.3390/life13081748
Chicago/Turabian StyleKoudouna, Smaragda, Dimitrios S. Evangelopoulos, Michail Sarantis, Efstathios Chronopoulos, Ismene A. Dontas, and Spiridon Pneumaticos. 2023. "Rehabilitation Prognostic Factors following Hip Fractures Associated with Patient’s Pre-Fracture Mobility and Functional Ability: A Prospective Observation Study" Life 13, no. 8: 1748. https://doi.org/10.3390/life13081748
APA StyleKoudouna, S., Evangelopoulos, D. S., Sarantis, M., Chronopoulos, E., Dontas, I. A., & Pneumaticos, S. (2023). Rehabilitation Prognostic Factors following Hip Fractures Associated with Patient’s Pre-Fracture Mobility and Functional Ability: A Prospective Observation Study. Life, 13(8), 1748. https://doi.org/10.3390/life13081748






