Abstract
Uterine fibroids (UF) are common benign tumors in women. The course of UF is associated with troubling symptoms and the development of infertility and pregnancy pathology. Surgical treatment even implies hysterectomy, while pharmacological interventions are modestly effective. Classically, hypoxic metabolism is considered a hallmark of malignant tumor. However, the role of hypoxia-induced factor (HIF) is significant in benign tumors as well. Herein, we briefly review the basic biology of HIF-family proteins, outlining their possible roles in UF. Apart from theoretical justifications, we summarized 15 studies reporting increased expression of HIFs and downstream factors in UF samples. Altogether, data suggest that increased expression of the HIF-protein and altered expression of its dependent genes are presumed to be the factors leading to UF development. Thus, even without being a malignant tumor, UF is characterized by the strong involvement of HIF. This novel insight may give rise to further research in the direction of finding new prognostic markers and effective medicines against UF.
1. Introduction
Uterine fibroids (UF), also known as uterine myoma or uterine leiomyoma, are the widespread benign tumors of the female reproductive system, affecting approximately 70% of white women and 80% of women of African descent during their lifetime [1]. In approximately 30% of cases, UF manifest with abnormal uterine bleeding, pelvic pain, pressure, or discomfort, as well as anemia. Whereas malignant transformation of UF is a relatively rare phenomenon [2], the conditions associated with fibroids are still severe and might include infertility. Moreover, whereas UF could be the only cause of infertility in 2–3% of cases [3], these neoplasms cause complications during pregnancy and delivery [4]. Unfortunately, the management of patients with UF frequently relies on surgical approaches, potentially resulting in hysterectomy, which is one of the worst scenarios for women desiring future pregnancy. Moreover, while surgical interventions may decrease the chances of a healthy pregnancy, current pharmacological approaches demonstrate modest efficacy or serious adverse effects. For instance, currently available medications modulating different levels of the hypothalamic–pituitary–gonadal axis provide only short-term relief [5] and may cause adverse effects—bone loss [6] and a wide spectrum of other effects [7]. Additional pharmacological options such as nonsteroidal anti-inflammatory drugs and tranexamic acid help to reduce blood loss, but they do not have any proven effect on tumoral growth.
Obviously, further studies of the molecular pathways underlying UF development and malignant transformation may provide novel insights into more effective and safe therapies. In this regard, herein, we review the role of crosstalk between molecular pathways driving UF and HIFs, the family of well-known proteins orchestrating cellular oxygen sensing and response to hypoxia.
2. Methodology
Hypothesizing the role of HIF pathways in UF, we performed a literature analysis via PubMed and Google Scholar to identify relevant studies disclosing the role of HIF-proteins in benign myometrium/leiomyoma tumors. Outlining basic evidences bridging HIF and UF we also extracted studies revealing the contribution of HIF-responsive genes/proteins (the list was found in the Kegg pathway database). Combinations of the key words were as follows: uterine fibroids, leiomyoma, myoma, expression, names of chosen genes/proteins: HIF, ANGPT1, VEGF, FLT1, EGF, PAI-1, SEPRINE1, TIE-2, TEK, TIMP-1, Endothelin-1, EDN1, iNOS, eNOS, ANP, EPO, TF, HMOX1, TFRC, GLUT-1, SLC2A1, PDK-1, HK, PFKL, GAPDH, ALDOA, ENO1, PGK, PFK2, LDHA, Bcl-2, p21, cyclin-dependent kinase inhibitor 1A, and CD18. Bibliographies were cross-referenced to identify additional studies. All relevant articles and additional articles cited in primary references are included. To search for genes associated with the risk of UF at the whole-genome level of significance, we used the GWAS catalog (https://www.ebi.ac.uk/gwas/ (accessed on 1 June 2023)). Then, the STRING database online bioinformatics tool (https://string-db.org/ (accessed on 3 June 2023)) was used to determine which GWAS genes are in direct interaction with the genes encoding HIF subunits. The STRING database contains information about mechanisms of protein–protein association within a common framework. The search for common biological processes (the so-called overrepresented biological processes) involving the HIF genes and the GWAS genes directly interacting with them was carried out using the Gene Ontology online tool (http://geneontology.org/ (accessed on 4 June 2023)).
3. UF in Terms of Hypoxia Response
The benign nature of UF suggests that the cells maintain the main characteristics of normal myocytes. However, they are also distinguished by specific features. In tumors, metabolism always shifts to a hypoxic state due to two factors. The first one is that even under normoxic conditions, tumor cells fail to utilize pyruvate in the tricarbonic acids cycle but use it to produce lactate. This is one of the most typical phenomena called the Warburg effect [8]. The mechanisms underlying this effect have yet to be understood in detail, but it allows tumor cells to benefit by accelerating anabolic processes [9]. The second factor is that the rapid growth of the tumor cells overtakes its vascularization, causing imbalanced feeding and insufficient blood supply.
As a result, metabolic products are accumulated and the cells send neighbors “hypoxic” help signaling, which increases vascularization, Fe-accumulation, glucose uptake and inflammatory response [10]. Hypoxic signaling also affects cells of normal myometrium (NM), endowing them with traits inherent in UF such as aberrant cell cycle dynamics or abnormal metabolism [11]. UF is characterized by high oxidative stress due to impaired redox metabolism [12] and increased reactive oxygen species production in response to hypoxia [13]. Moreover, when the metabolism of UF is switched to the direction of hypoxia, the tumor obviously increases its chances of malignant transformation.
4. Basic Biology of HIF
Since aerobic metabolism is a universal sign of all Metazoa, the identification of molecular sensors of oxygen can be called one of the most important discoveries in the last 30 years [14].
This origin of this discovery goes back to the late 1970s with the identification of a hypoxia-responsive element of human EPO 3′ enchancer in liver and renal cells [15]. It was suggested that this specific oxygen-sensing mechanism is specific for EPO-producing cells. However, further studies revealed hypoxia-responsive element-binding activity in tissues not expressing EPO: lungs, aortic endothelium, etc. [16]. The next landmark was the identification of the main sensor of hypoxia, the HIF itself [17], as well as upstream and downstream members of its pathway: the complex of von Hippel–Lindau (VHL)–Elongin B and C-CUL2 [18,19]. Vascular endothelial growth factor (VEGF) [20], the prolyl-hydroxylase family [21], and factor inhibiting HIF [22,23].
There are three isoforms of HIF proteins, each consisting of alpha (α) and beta (β) subunits. HIF-1 and HIF-2 are the most studied members, characterized by similar protein structures. Notably, HIF-1 is a master regulator of the acute response to oxygen depletion, whereas HIF-2 is responsible for adaptation to prolonged hypoxia. Molecular biology and the particular role of HIF-3 have yet to be studied in detail; however, it regulates various pathways, including HIF-1 and HIF-2 [24,25].
The alpha subunit of each isoform is an inducible part normally located in the cytoplasm. The second one, beta, is a constitutively expressed part located in the nucleus. During normoxia, prolyl hydroxylases add hydroxyl residues to the α-subunit, making it a target for ubiquitin-dependent breakdown via proteasome degradation. Another regulator of HIF activity is the factor inhibiting HIF, which adds hydroxyl residues to the asparagine and thus interferes with the binding of HIF and transcriptional coactivators. Since both factors function in an oxygen-dependent manner, they are unfunctional during hypoxia. Thus, as one’s oxygen level decreases, factors inhibiting HIF and prolyl hydroxylase domain proteins are inhibited, enabling the alpha subunit to be stabilized with further translocation into the nucleus and assembly with the beta subunit. The formed HIF heterodimeric complex binds to the promoter of HIF-responsive genes in order to modulate their expression [26].
5. The Role of Hypoxia-Inducible Factor in Uterine Fibroids
In keeping with its essential physiological role, HIF-1 has been demonstrated to contribute in a widest spectrum of human pathologies, from oncological to neurodegenerative and infectious [15]. Not surprisingly, HIF-1 is also strongly involved in various gynecological diseases—preeclampsia [27], polycystic ovary syndrome [28], cancer [29], endometriosis [30] and others [31,32].
Several studies have expectedly reported increased HIF1-α expression in UF [33,34,35]. In order to further elucidate this link, we carried out an analysis of available data related to expression of genes involved in the HIF-1 signaling pathway in UF based on the KEGG pathway database. It is known that the HIF pathway includes many genes with major effects on the processes typical for UF pathogenesis: apoptosis, proliferation, metabolism, regulation of the cellular availability of oxygen and nutrients [36]. In particular, such proteins as VEGF-A, ANG-1 (angiopoietin-1), VEGFR-1, EGF, SERPINE1 (PAI1), TEK, and TIMP1 regulate the division, proliferation, and motility of vascular cells as well as the disorganization of the extracellular matrix, thus favoring angiogenesis [37]. Factors like HMOX1, EDN1, iNOS, and cNOS provide control of vascular tone by balancing nutrients and oxygen supply [38]. Additionally, NO-synthases are involved in the regulation of apoptosis in UF cells by nytrosylation of caspase 3, downregulating Caspase 3 activity, and increasing the Bcl2/Bax ratio [39]. Finally, the HIF-dependent response to oxygen depletion is critically dependent on the glucose carrier protein GLUT-1 and glycolytic enzymes: HK, ALDOA, ENO1, PGK1, PFK/FBPase 3, and LDHA [40] (Table 1).

Table 1.
Main HIF-responsive factors, imbalanced in UF.
6. HIF and GWAS-Identified Genes
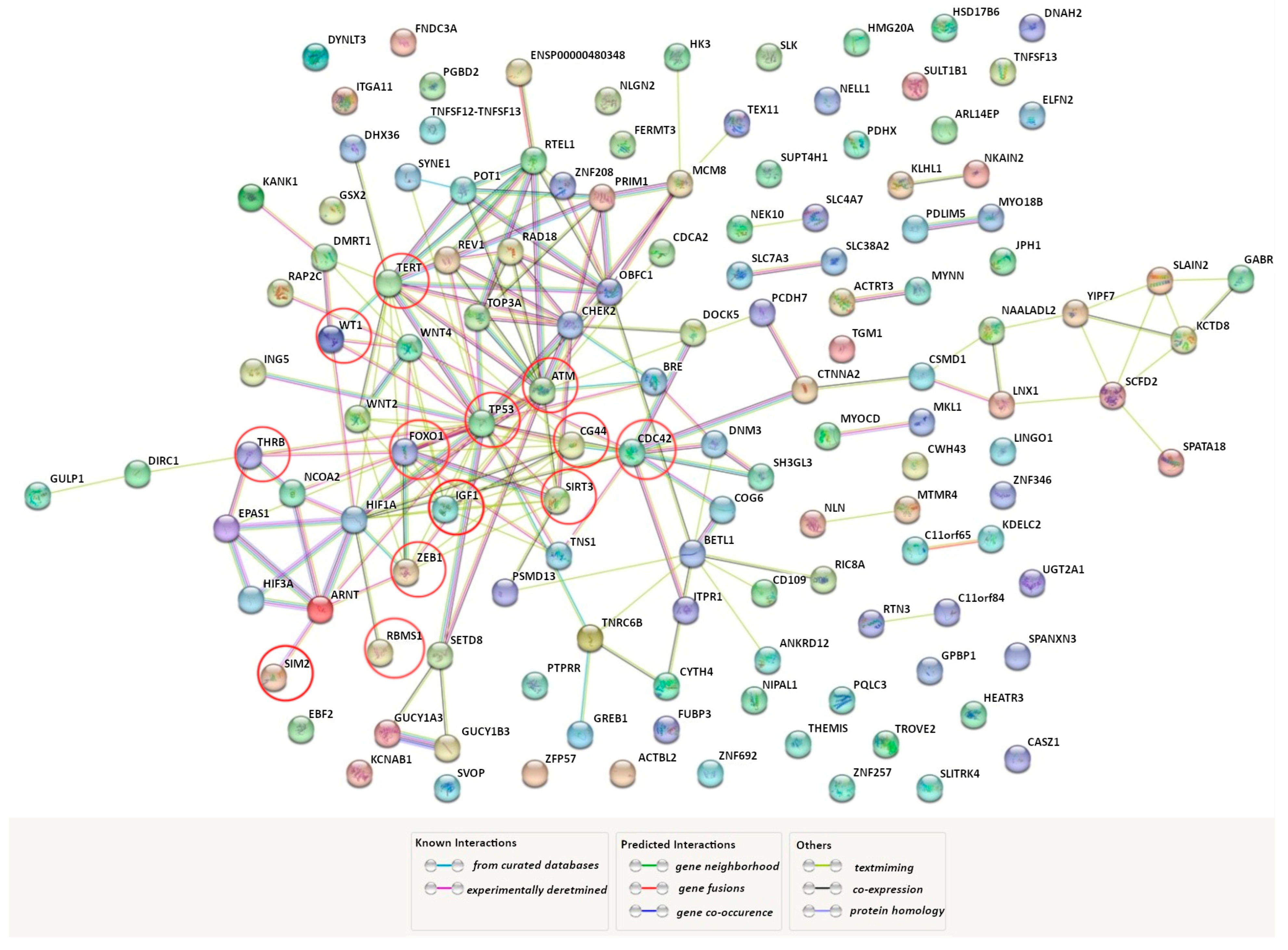
GWAS remains one of the most effective approaches to the search for “susceptibility” genes to human multifactorial diseases [69,70,71,72]. Using the GWAS catalog, we found that variations in 174 genes are associated with UF risk in different populations around the world (for instance, rs16991615 MCM8, rs17631680 DNMT3AP1, rs2306022 ITGA11, rs2456181 ZNF346, rs3820282 WNT4, rs4335411 ZNF692, rs5930554 AGKP2, rs66998222 HNRNPA1P48, rs78378222 TP53, rs7986407 FOXO1, rs8105767 ZNF257, and rs9548898 COG6). We found it interesting to analyze how genes that encode HIF subunits (HIF1A, HIF1B (ARNT), HIF2A (EPAS1), HIF3A, HIF2B, HIF3B) interact with those GWAS-selected genes in a functional way. For this purpose, we have used the bioinformatics tool STRING (https://string-db.org/ (accessed on 3 June 2023)). The interactome network of protein–protein interactions (PPI) obtained using the source is shown in Figure 1. HIF2B and HIF3B were not included in the analysis of the PPI network since the STRING database does not provide data on them. It turned out that 72 GWAS genes, directly or indirectly through other functional partners, are involved in the interaction with HIF1A, HIF1B, HIF2A, and HIF3A. During analysis of the STRING network, we identified 13 GWAS genes that directly interact with genes encoding HIF subunits: ATM, CD44, CDC42, FOXO1, IGF1, RBMS1, SIM2, SIRT3, TERT, THRB, TP53, WT1, ZEB1 (marked with red circles in Figure 1).
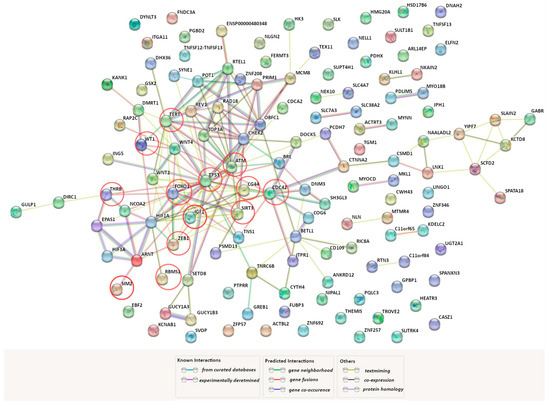
Figure 1.
Predicted functional partners of genes encoding HIF subunits across the genes associated with UF (https://string-db.org/ (accessed on 3 June 2023)). A network of protein–protein interactions among the genes encoding HIF subunits (HIF1A, HIF1B (ARNT), HIF2A (EPAS1), and HIF3A).and genes identified as a result of genome-wide association studies (GWAS) of UF. The factors, directly (without secondary mediators) interacting with the HIF subunits, are marked with red circles. PPI enrichment p-value: <1.0 × 10−16.
Together with HIF1A, HIF1B, HIF2A, and HIF3A, these genes jointly participate in a large number of pathogenetically significant UF biological processes, characterizing hypoxia, oxidative stress apoptosis, proliferation, cell growth, adhesion and migration, myoblast differentiation, metabolic changes, morphogenesis of vessels, and others (Table 2). In particular, we would like to note the participation of this set of genes in overrepresented biological processes involved in the mechanisms of cellular reply in response to hypoxia and oxidative stress; in the regulation of apoptosis directly and through the P53 signaling; in the regulation of vascular homeostasis through nitric-oxide synthase activity; participation in neoangiogenesis through the regulation of vascular associated smooth muscle cell proliferation; as well as participation in the processes that directly control the initiation and progression of the tumor: “myoblast differentiation”, “positive regulation of cell growth, “mesenchymal cell differentiation”.

Table 2.
Overrepresented biological processes, reflecting genes encoding HIF subunits (HIF1A, HIF1B (ARNT), HIF2A (EPAS1), HIF3A) and their main functional partners (ATM, CD44, CDC42, FOXO1, IGF1, RBMS1, SIM2, SIRT3, TERT, THRB, TP53, WT1, ZEB1).
Taking into account the fact that these processes are reflectors of high-functional HIFs’ interaction with genes, established as a result of human genome-wide association studies (GWAS), this provides conclusive evidence of the role of the HIF pathway genes we have studied in key molecular determinants of tumor growth.
Altogether, the summarized data reflecting previous research and our bioinformatic findings are interpretated in Figure 2.
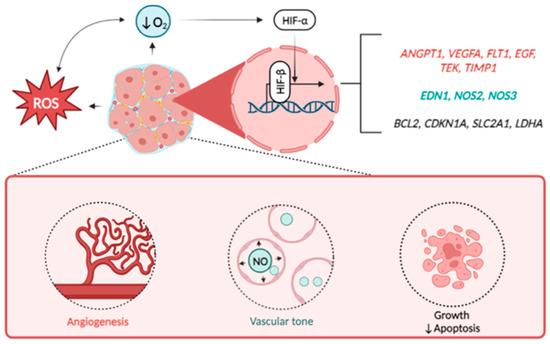
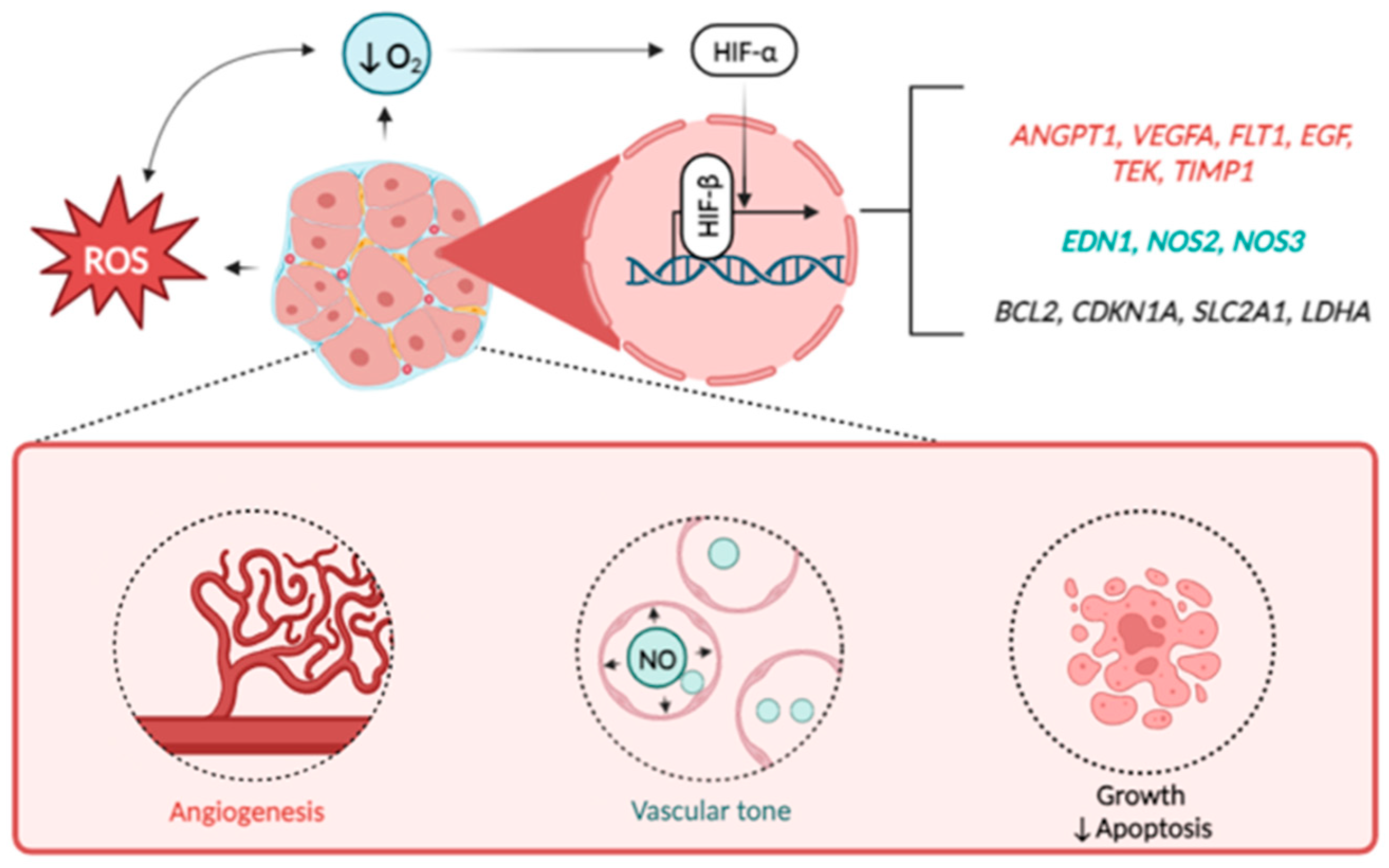
Figure 2.
HIF-downstream signaling in the orchestration of UF development. Note: Excessive production of reactive oxygen species (ROS) and decreased oxygen supply induce the activation of HIF alpha subunits (mostly HIF-1a) which heads to the nucleus and binds to beta subunit. Interacting, both subunits launch the transcription of wide spectrum of HIF-responsive genes of hypoxia response. Besides the others these genes include regulators of vascular growth (ANGPT1, VEGFA, FLT1, EGF, TEK, TIMP1), vascular tone (EDN1, NOS2, NOS3), apoptosis (BCL2), cell cycle (CDKN1A), as well as glucose transporter GLUT1 (SLC2A1) and lactate dehydrogenase (LDHA). Altogether, these factors promote the growth rate of UF thus playing negative role in disease pathogenesis.
7. Future Perspectives
Although the presented data provide a solid ground to bridge HIF and pathogenesis of UF, there is no rationale to distinguish whether HIF is involved in growth, onset, or both. As upregulated levels of HIF and its downstream effectors were detected in UF samples, HIF is involved in progression of the disease. However, future studies can help to elucidate its role in etiology of the disease.
Further investigation can also focus on identifying specific markers associated with HIF expression and downstream factors in UF. Understanding these markers could aid in predicting disease progression, treatment response, and potential complications. The recognition of HIF involvement in UF suggests that targeting HIF pathways could be a potential therapeutic strategy. Developing medications that effectively modulate HIF expression or its downstream factors may offer improved treatment options for UF patients. Further research are needed to elucidate the complete mechanisms by which HIF influences UF development. This sort of research includes studying the interactions between HIF and other molecular pathways implicated in UF pathogenesis, such as hormonal factors, epigenetic regulation and immune responses. Obtaining a comprehensive understanding of the complex biology underlying UF will contribute to improved diagnostic and therapeutic strategies.
In recent decades, there has been a growing interest in the study of HIF and its targets in oncology. This attention is of highest clinical significance first of all because of the opportunity to develop new pharmacological avenues for the treatment of various pathological conditions, first of all cancer [73]. In this respect, one may observe a number of HIF-targeted medications undergoing clinical trials in cancer patients. Most HIF inhibitors are characterized by suppressive activity against cancer. For instance, PX-478 promotes apoptosis, suppresses tumor proliferation, epithelial-mesenchymal transition, and arrests the cell cycle in G2 phase, thus inhibiting tumor growth [74].
There are also several HIF-2a inhibitors undergoing clinical studies. Results of completed phase II clinical trials of HIF-2a inhibitor PT2385 in treating patients suffered from recurrent glioblastoma were posted in 2020 (https://beta.clinicaltrials.gov/study/NCT03216499?tab=history (accessed on 10 June 2023)). A few clinical trials of HIF-2α inhibitors were started in 2021. NKT2152 is being studied in a phase I/II clinical trial in patients suffering from advanced clear-cell renal cell carcinoma. https://clinicaltrials.gov/ct2/show/study/NCT05119335?term=HIF+inhibitor&cond=cancer&draw=2&rank=4 (accessed on 10 June 2023) [75].
DFF332, Another HIF2-α inhibitor for patients with renal cell carcinoma and other tumors (such as HLRCC and VHL disease), is already undergoing phase I/Ib (https://clinicaltrials.gov/ct2/show/NCT04895748?term=DFF332&draw=2&rank=1 (accessed on 10 June 2023)).
Such a wide range of pharmacological tools gives a reason to hope for near-term applications targeting HIF in UF patients. Further studies can help to evaluate the long-term effects of HIF-suppressing therapy in order to find the most efficient and safest drugs. In this respect, one may probably expect the appearance of a rationale to use some of the mentioned medicines to treat UF. Our review gave a good reason to consider HIF-suppressing pathways to decelerate the growth rates of the UF. In this respect, potential application of HIF-inhibitors may serve a new avenue to prevent some of complications related to the disease. For instance, apart from bleeding UF can potentially cause infertility by blocking the fallopian tubes or stopping a fertilized egg from implanting in the uterus [76]. If the inhibition of HIF turns out successful therapy, women with poor fertility prognosis, miscarriages or complicated pregnancies in anamnesis would may be prescribed a new type of nonsurgical interventions to be treated. Noteworthy, that anatomical features of UF allow topical application of the medical compounds thus minimizing systemic side effects. Thus, we are assuming that the selective and local inhibition of HIF pathways may serve a novel approach to the treatment of UF.
8. Limitations
This work was based on the literature review and did not imply following classical principles of meta-analysis such as assessing of statistical power and strict inclusion/exclusion criteria. The authors admit the limitations related to a low number of studies directly evaluating the role of HIF protein in UF. All the theoretical justifications require experimental approval.
9. Conclusions
This article has demonstrated for the first time a growing understanding of molecular and genetic determinants of UF development, orchestrated by HIF. Moreover, as far as we are aware, this work is the first review emphasizing the role of HIF in benign tumors. The HIF pathway activates expression of a number of genes responsible for processes known to be imbalanced in tumors. Analysis of patterns of mRNA and protein expression of HIF-target genes, which play a key role in UF development, showed that UF cells displayed their altered status. Additionally, we revealed that genes encoding HIF subunits interact with genes associated with the development of UF in a functional way. Thirteen proteins encoded by those genes are directly interacting with HIFs: Additionally, we distinguished the main biological processes, provided by the revealed genes. These processes cover the whole spectrum of the UF-driving mechanisms: proliferation, cell growth, adhesion and migration, myoblast differentiation, metabolic changes, morphogenesis of vessels, and others. Summarizing the considerable role of hypoxia and HIF in UF pathogenesis, we expect that further studies of this disease narrowly focused on HIF-related pathways may bring new insight on more effective therapeutic options.
Author Contributions
Conceptualization, O.B.; investigation, M.F. and E.B.; writing—original draft preparation, M.F.; writing—review and editing, O.B.; visualization, E.B.; supervision, O.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| UF | uterine fibroids |
| NM | normal myometrium |
| HIF | hypoxia-inducible factor |
| EPO | erythropoietin |
| VHL | von Hippel–Lindau |
| CUL2 | cullin 2 |
| VEGF | vascular endothelial growing factor |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| ANG-1 | angiopoietin-1 |
| VEGFR-1 | vascular endothelial growing factor receptor 1 |
| EGF | epidermal growing factor |
| SERPINE1 (PAI1) | plasminogen activator inhibitor 1 |
| TEK | endothelial tyrosine kinase |
| TIMP1 | tissue inhibitor of metalloproteinases 1 |
| HMOX1 | heme oxygenase 1 |
| EDN1 | endothelin 1 |
| iNOS | inducible nitrogen oxide synthase |
| cNOS (eNOS) | constitutive (endothelial) nitric oxide synthase |
| GLUT-1 | glucose transporter type 1 |
| HK | hexokinase |
| ALDOA | aldolase, fructose-bisphosphate A |
| ENO1 | enolase 1 |
| PGK1 | phosphoglycerate kinase 1 |
| PFK/FBPase 3 | 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 |
| LDHA | lactate dehydrogenase A |
| HLRCC | hereditary leiomyomatosis and renal cell cancer |
| FH | fumarate hydratase |
| F2,6BP | fructose-2,6-bisphosphate |
| GWAS | genome-wide association studies |
| Bcl-2 | B-cell lymphoma 2 |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| ROS | reactive oxygen species |
| PPI | protein–protein interactions |
| ATM | ataxia telangiectasia mutated |
| CDC42 | cell division control protein 42 homolog |
| FOXO1 | forkhead box protein O1 |
| IGF1 | insulin-like growth factor I |
| RBMS1 | RNA-binding motif, single-stranded-interacting protein 1 |
| SIM2 | single-minded homolog 2 |
| SIRT3 | NAD-dependent protein deacetylase sirtuin-3, mitochondrial |
| TERT | telomerase reverse transcriptase |
| THRB | thyroid hormone receptor beta |
| TP53 | tumor protein P53 |
| WT1 | Wilms tumor 1 |
| ZEB1 | zinc finger e-box binding homeobox 1 |
References
- Giuliani, E.; As-Sanie, S.; Marsh, E.E. Epidemiology and Management of Uterine Fibroids. Int. J. Gynaecol. Obstet. 2020, 149, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Al Ansari, A.A.; Al Hail, F.A.; Abboud, E. Malignant Transformation of Uterine Leiomyoma. Qatar Med. J. 2012, 2012, 71–74. [Google Scholar] [CrossRef][Green Version]
- Freytag, D.; Günther, V.; Maass, N.; Alkatout, I. Uterine Fibroids and Infertility. Diagnostics 2021, 11, 1455. [Google Scholar] [CrossRef]
- Parazzini, F.; Tozzi, L.; Bianchi, S. Pregnancy Outcome and Uterine Fibroids. Best. Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Marjoribanks, J.; Lethaby, A.; Farquhar, C. Surgery versus Medical Therapy for Heavy Menstrual Bleeding. Cochrane Database Syst. Rev. 2016, 2016, CD003855. [Google Scholar] [CrossRef]
- Surrey, E.S.; Hornstein, M.D. Prolonged GnRH Agonist and Add-Back Therapy for Symptomatic Endometriosis: Long-Term Follow-Up. Obstet. Gynecol. 2002, 99 Pt 1, 709–719. [Google Scholar] [CrossRef]
- Lewis, T.D.; Malik, M.; Britten, J.; San Pablo, A.M.; Catherino, W.H. A Comprehensive Review of the Pharmacologic Management of Uterine Leiomyoma. Biomed. Res. Int. 2018, 2018, 2414609. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg Effect: Essential Part of Metabolic Reprogramming and Central Contributor to Cancer Progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Islam, M.S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular Matrix in Uterine Leiomyoma Pathogenesis: A Potential Target for Future Therapeutics. Hum. Reprod. Update 2018, 24, 59–85. [Google Scholar] [CrossRef]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular Adaptation to Hypoxia through Hypoxia Inducible Factors and Beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Kim, J.J.; Li, Y.; Xie, J.; Shao, C.; Wei, J.-J. Oxidative Stress-Induced MiRNAs Modulate AKT Signaling and Promote Cellular Senescence in Uterine Leiomyoma. J. Mol. Med. 2018, 96, 1095–1106. [Google Scholar] [CrossRef]
- Smith, K.A.; Waypa, G.B.; Schumacker, P.T. Redox Signaling during Hypoxia in Mammalian Cells. Redox Biol. 2017, 13, 228–234. [Google Scholar] [CrossRef]
- Soldatova, V.A.; Demidenko, A.N.; Soldatov, V.; Deykin, A.; Bushueva, O.; Puchenkova, O.A. Hypoxia-Inducible Factor: Basic Biology and Involvement in Cardiovascular Pathology. Asian J. Pharm. 2018, 12, S1173–S1178. [Google Scholar]
- Semenza, G.L.; Nejfelt, M.K.; Chi, S.M.; Antonarakis, S.E. Hypoxia-Inducible Nuclear Factors Bind to an Enhancer Element Located 3′ to the Human Erythropoietin Gene. Proc. Natl. Acad. Sci. USA 1991, 88, 5680–5684. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Pugh, C.W.; Ratcliffe, P.J. Inducible Operation of the Erythropoietin 3′ Enhancer in Multiple Cell Lines: Evidence for a Widespread Oxygen-Sensing Mechanism. Proc. Natl. Acad. Sci. USA 1993, 90, 2423–2427. [Google Scholar] [CrossRef]
- Wang, G.L.; Semenza, G.L. Purification and Characterization of Hypoxia-Inducible Factor 1. J. Biol. Chem. 1995, 270, 1230–1237. [Google Scholar] [CrossRef]
- Kibel, A.; Iliopoulos, O.; DeCaprio, J.A.; Kaelin, W.G. Binding of the von Hippel-Lindau Tumor Suppressor Protein to Elongin B and C. Science 1995, 269, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, K.M.; Iliopoulos, O.; Ohh, M.; Kamura, T.; Conaway, R.C.; Conaway, J.W.; Kaelin, W.G. Regulation of Hypoxia-Inducible MRNAs by the von Hippel-Lindau Tumor Suppressor Protein Requires Binding to Complexes Containing Elongins B/C and Cul2. Mol. Cell. Biol. 1998, 18, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of Vascular Endothelial Growth Factor Gene Transcription by Hypoxia-Inducible Factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef] [PubMed]
- Ivan, M.; Kondo, K.; Yang, H.; Kim, W.; Valiando, J.; Ohh, M.; Salic, A.; Asara, J.M.; Lane, W.S.; Kaelin, W.G. HIFalpha Targeted for VHL-Mediated Destruction by Proline Hydroxylation: Implications for O2 Sensing. Science 2001, 292, 464–468. [Google Scholar] [CrossRef]
- Mahon, P.C.; Hirota, K.; Semenza, G.L. FIH-1: A Novel Protein That Interacts with HIF-1alpha and VHL to Mediate Repression of HIF-1 Transcriptional Activity. Genes. Dev. 2001, 15, 2675–2686. [Google Scholar] [CrossRef]
- Lando, D.; Peet, D.J.; Gorman, J.J.; Whelan, D.A.; Whitelaw, M.L.; Bruick, R.K. FIH-1 Is an Asparaginyl Hydroxylase Enzyme That Regulates the Transcriptional Activity of Hypoxia-Inducible Factor. Genes. Dev. 2002, 16, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Jaskiewicz, M.; Moszynska, A.; Serocki, M.; Króliczewski, J.; Bartoszewska, S.; Collawn, J.F.; Bartoszewski, R. Hypoxia-Inducible Factor (HIF)-3a2 Serves as an Endothelial Cell Fate Executor during Chronic Hypoxia. EXCLI J. 2022, 21, 454–469. [Google Scholar] [CrossRef] [PubMed]
- Serocki, M.; Bartoszewska, S.; Janaszak-Jasiecka, A.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. MiRNAs Regulate the HIF Switch during Hypoxia: A Novel Therapeutic Target. Angiogenesis 2018, 21, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Corrado, C.; Fontana, S. Hypoxia and HIF Signaling: One Axis with Divergent Effects. Int. J. Mol. Sci. 2020, 21, 5611. [Google Scholar] [CrossRef]
- Albogami, S.M.; Al-Kuraishy, H.M.; Al-Maiahy, T.J.; Al-Buhadily, A.K.; Al-Gareeb, A.I.; Alorabi, M.; Alotaibi, S.S.; De Waard, M.; Sabatier, J.-M.; Saad, H.M.; et al. Hypoxia-Inducible Factor 1 and Preeclampsia: A New Perspective. Curr. Hypertens. Rep. 2022, 24, 687–692. [Google Scholar] [CrossRef]
- Fu, X.; Shi, L.; Liu, P.; Jiao, Y.; Guo, S.; Chen, Q.; Zheng, Q.; Chen, X.; Wang, Y. Expression and Clinical Significance of HIF-1α in Follicular Fluid and Granulosa Cells in Infertile PCOS Patients. Reprod. Sci. 2023, 30, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Chen, Q.; Xiao, J.; Yao, T.; Bian, L.; Liu, C.; Lin, Z. Overexpression of Hypoxia-Inducible Factor-1α Is a Predictor of Poor Prognosis in Cervical Cancer: A Clinicopathologic Study and a Meta-Analysis. Int. J. Gynecol. Cancer 2014, 24, 1054–1064. [Google Scholar] [CrossRef]
- Zhan, L.; Wang, W.; Zhang, Y.; Song, E.; Fan, Y.; Wei, B. Hypoxia-Inducible Factor-1alpha: A Promising Therapeutic Target in Endometriosis. Biochimie 2016, 123, 130–137. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, D.; Lu, X.; Zhang, Q.; Gu, R.; Sun, B.; Sun, Y. Hypoxia and Its Possible Relationship with Endometrial Receptivity in Adenomyosis: A Preliminary Study. Reprod. Biol. Endocrinol. 2021, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Seeber, L.M.S.; Horrée, N.; Vooijs, M.A.G.G.; Heintz, A.P.M.; van der Wall, E.; Verheijen, R.H.M.; van Diest, P.J. The Role of Hypoxia Inducible Factor-1alpha in Gynecological Cancer. Crit. Rev. Oncol. Hematol. 2011, 78, 173–184. [Google Scholar] [CrossRef]
- Miyashita-Ishiwata, M.; El Sabeh, M.; Reschke, L.D.; Afrin, S.; Borahay, M.A. Differential Response to Hypoxia in Leiomyoma and Myometrial Cells. Life Sci. 2022, 290, 120238. [Google Scholar] [CrossRef]
- Hou, P.; Zhao, L.; Li, Y.; Luo, F.; Wang, S.; Song, J.; Bai, J. Comparative Expression of Thioredoxin-1 in Uterine Leiomyomas and Myometrium. Mol. Hum. Reprod. 2014, 20, 148–154. [Google Scholar] [CrossRef]
- Ishikawa, H.; Xu, L.; Sone, K.; Kobayashi, T.; Wang, G.; Shozu, M. Hypoxia Induces Hypoxia-Inducible Factor 1α and Potential HIF-Responsive Gene Expression in Uterine Leiomyoma. Reprod. Sci. 2019, 26, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q.; Jiang, B.; Feng, J.; Li, J.; Gu, Y. PI3K/Akt Signaling Transduction Pathway, Erythropoiesis and Glycolysis in Hypoxia (Review). Mol. Med. Rep. 2019, 19, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular Endothelial Growth Factor (VEGF)—Key Factor in Normal and Pathological Angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Fletcher, N.M.; Abusamaan, M.S.; Memaj, I.; Saed, M.G.; Al-Hendy, A.; Diamond, M.P.; Saed, G.M. Oxidative Stress: A Key Regulator of Leiomyoma Cell Survival. Fertil. Steril. 2017, 107, 1387–1394.e1. [Google Scholar] [CrossRef]
- Tirpe, A.A.; Gulei, D.; Ciortea, S.M.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. Int. J. Mol. Sci. 2019, 20, 6140. [Google Scholar] [CrossRef]
- Hague, S.; Zhang, L.; Oehler, M.K.; Manek, S.; MacKenzie, I.Z.; Bicknell, R.; Rees, M.C. Expression of the Hypoxically Regulated Angiogenic Factor Adrenomedullin Correlates with Uterine Leiomyoma Vascular Density. Clin. Cancer Res. 2000, 6, 2808–2814. [Google Scholar] [PubMed]
- Korompelis, P.; Piperi, C.; Adamopoulos, C.; Dalagiorgou, G.; Korkolopoulou, P.; Sepsa, A.; Antsaklis, A.; Papavassiliou, A.G. Expression of Vascular Endothelial Factor-A, Gelatinases (MMP-2, MMP-9) and TIMP-1 in Uterine Leiomyomas. Clin. Chem. Lab. Med. 2015, 53, 1415–1424. [Google Scholar] [CrossRef]
- Wei, J.-J.; Zhang, X.-M.; Chiriboga, L.; Yee, H.; Perle, M.A.; Mittal, K. Spatial Differences in Biologic Activity of Large Uterine Leiomyomata. Fertil. Steril. 2006, 85, 179–187. [Google Scholar] [CrossRef]
- Asano, R.; Asai-Sato, M.; Matsukuma, S.; Mizushima, T.; Taguri, M.; Yoshihara, M.; Inada, M.; Fukui, A.; Suzuki, Y.; Miyagi, Y.; et al. Expression of Erythropoietin Messenger Ribonucleic Acid in Wild-Type MED12 Uterine Leiomyomas under Estrogenic Influence: New Insights into Related Growth Disparities. Fertil. Steril. 2019, 111, 178–185. [Google Scholar] [CrossRef]
- Joo, B.S.; Park, M.J.; Kim, C.-W.; Lee, K.S.; Joo, J.K. Differential Expression of Visfatin, Leptin, Stromal Cell Derived Factor-1α, Endothelial Nitric Oxide Synthase, and Vascular Endothelial Growth Factor in Human Leiomyomas. Gynecol. Endocrinol. 2017, 33, 306–310. [Google Scholar] [CrossRef]
- Sanci, M.; Dikis, C.; Inan, S.; Turkoz, E.; Dicle, N.; Ispahi, C. Immunolocalization of VEGF, VEGF Receptors, EGF-R and Ki-67 in Leiomyoma, Cellular Leiomyoma and Leiomyosarcoma. Acta Histochem. 2011, 113, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Tsiligiannis, S.E.; Zaitseva, M.; Coombs, P.R.; Shekleton, P.; Olshansky, M.; Hickey, M.; Vollenhoven, B.; Rogers, P.A.W. Fibroid-Associated Heavy Menstrual Bleeding: Correlation between Clinical Features, Doppler Ultrasound Assessment of Vasculature, and Tissue Gene Expression Profiles. Reprod. Sci. 2013, 20, 361–370. [Google Scholar] [CrossRef]
- Dixon, D.; He, H.; Haseman, J.K. Immunohistochemical Localization of Growth Factors and Their Receptors in Uterine Leiomyomas and Matched Myometrium. Environ. Health Perspect. 2000, 108 (Suppl. S5), 795–802. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Chae, B.; Kim, M.-R. The Potential of Transforming Growth Factor-Beta Inhibitor and Vascular Endothelial Growth Factor Inhibitor as Therapeutic Agents for Uterine Leiomyoma. Int. J. Med. Sci. 2022, 19, 1779–1786. [Google Scholar] [CrossRef]
- Sourla, A.; Polychronakos, C.; Zeng, W.R.; Nepveu, A.; Kukuvitis, A.; Naud, F.; Koutsilieris, M. Plasminogen Activator Inhibitor 1 Messenger RNA Expression and Molecular Evidence for Del(7)(Q22) in Uterine Leiomyomas. Cancer Res. 1996, 56, 3123–3128. [Google Scholar]
- Cheng, Z.; Xie, Y.; Dai, H.; Hu, L.; Zhu, Y.; Gong, J. Unequal Tissue Expression of Proteins from the PA/PAI System, Myoma Necrosis, and Uterus Survival after Uterine Artery Occlusion. Int. J. Gynaecol. Obstet. 2008, 102, 55–59. [Google Scholar] [CrossRef]
- Bogusiewicz, M.; Stryjecka-Zimmer, M.; Postawski, K.; Jakimiuk, A.J.; Rechberger, T. Activity of Matrix Metalloproteinase-2 and -9 and Contents of Their Tissue Inhibitors in Uterine Leiomyoma and Corresponding Myometrium. Gynecol. Endocrinol. 2007, 23, 541–546. [Google Scholar] [CrossRef]
- Governini, L.; Marrocco, C.; Semplici, B.; Pavone, V.; Belmonte, G.; Luisi, S.; Petraglia, F.; Luddi, A.; Piomboni, P. Extracellular Matrix Remodeling and Inflammatory Pathway in Human Endometrium: Insights from Uterine Leiomyomas. Fertil. Steril. 2021, 116, 1404–1414. [Google Scholar] [CrossRef]
- Pekonen, F.; Nyman, T.; Rutanen, E.M. Differential Expression of MRNAs for Endothelin-Related Proteins in Human Endometrium, Myometrium and Leiomyoma. Mol. Cell. Endocrinol. 1994, 103, 165–170. [Google Scholar] [CrossRef]
- Wallace, K.; Chatman, K.; Porter, J.; Scott, J.; Johnson, V.; Moseley, J.; LaMarca, B. Enodthelin 1 Is Elevated in Plasma and Explants from Patients Having Uterine Leiomyomas. Reprod. Sci. 2014, 21, 1196–1205. [Google Scholar] [CrossRef]
- Plewka, A.; Madej, P.; Plewka, D.; Kowalczyk, A.; Miskiewicz, A.; Wittek, P.; Leks, T.; Bilski, R. Immunohistochemical Localization of Selected Pro-Inflammatory Factors in Uterine Myomas and Myometrium in Women of Various Ages. Folia Histochem. Cytobiol. 2013, 51, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.-J.; Ryu, K.-Y.; Jung, C.-N.; Yi, S.Y.; Kim, S.-R. Expression of Endothelial Nitric Oxide Synthase in the Uterus of Patients with Leiomyoma or Adenomyosis. J. Obstet. Gynaecol. Res. 2013, 39, 536–542. [Google Scholar] [CrossRef]
- Gokdeniz, R.; Mizrak, B.; Ozen, S.; Bazoglu, N. Endothelial Nitric Oxide Synthase Expression in Leiomyoma and Parental Myometrium. Gynecol. Obstet. Investig. 2000, 49, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Asano, R.; Asai-Sato, M.; Miyagi, Y.; Mizushima, T.; Koyama-Sato, M.; Nagashima, Y.; Taguri, M.; Sakakibara, H.; Hirahara, F.; Miyagi, E. Aberrant Expression of Erythropoietin in Uterine Leiomyoma: Implications in Tumor Growth. Am. J. Obstet. Gynecol. 2015, 213, e1–e8. [Google Scholar] [CrossRef]
- Knapp, P.; Chabowski, A.; Posmyk, R.; Górski, J. Expression of the Energy Substrate Transporters in Uterine Fibroids. Prostaglandins Other Lipid Mediat. 2016, 123, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Cho, C.-H.; Cha, S.-D.; Back, W.-K.; Kim, M.-K.; Kim, J.-C. Gene Expression Analysis between Uterine Leiomyoma and Normal Myometrial Tissues by DNA Chip. Korean J. Obstet. Gynecol. 2003, 46, 701–706. [Google Scholar]
- Catherino, W.H.; Mayers, C.M.; Mantzouris, T.; Armstrong, A.Y.; Linehan, W.M.; Segars, J.H. Compensatory Alterations in Energy Homeostasis Characterized in Uterine Tumors from Hereditary Leiomyomatosis and Renal Cell Cancer. Fertil. Steril. 2007, 88 (Suppl. S4), 1039–1048. [Google Scholar] [CrossRef]
- Vanharanta, S.; Pollard, P.J.; Lehtonen, H.J.; Laiho, P.; Sjöberg, J.; Leminen, A.; Aittomäki, K.; Arola, J.; Kruhoffer, M.; Orntoft, T.F.; et al. Distinct Expression Profile in Fumarate-Hydratase-Deficient Uterine Fibroids. Hum. Mol. Genet. 2006, 15, 97–103. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Zhou, J. Expression of bcl-2 and bax protein in uterine leiomyosarcomas and leiomyomas. J. Cent. South University Med. Sci. 2005, 30, 183–186. [Google Scholar]
- Wu, X.; Blanck, A.; Olovsson, M.; Henriksen, R.; Lindblom, B. Expression of Bcl-2, Bcl-x, Mcl-1, Bax and Bak in Human Uterine Leiomyomas and Myometrium during the Menstrual Cycle and after Menopause. J. Steroid Biochem. Mol. Biol. 2002, 80, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kovács, K.A.; Lengyel, F.; Környei, J.L.; Vértes, Z.; Szabó, I.; Sümegi, B.; Vértes, M. Differential Expression of Akt/Protein Kinase B, Bcl-2 and Bax Proteins in Human Leiomyoma and Myometrium. J. Steroid Biochem. Mol. Biol. 2003, 87, 233–240. [Google Scholar] [CrossRef]
- Csatlós, É.; Máté, S.; Laky, M.; Rigó, J.; Joó, J.G. Role of Apoptosis in the Development of Uterine Leiomyoma: Analysis of Expression Patterns of Bcl-2 and Bax in Human Leiomyoma Tissue With Clinical Correlations. Int. J. Gynecol. Pathol. 2015, 34, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Salimi, S.; Hajizadeh, A.; Yaghmaei, M.; Rezaie, S.; Shahrakypour, M.; Teimoori, B.; Parache, M.; Naghavi, A.; Mokhtari, M. The Effects of P21 Gene C98A Polymorphism on Development of Uterine Leiomyoma in Southeast Iranian Women. Tumour Biol. 2016, 37, 12497–12502. [Google Scholar] [CrossRef]
- Polonikov, A.V.; Samgina, T.A.; Nazarenko, P.M.; Bushueva, O.Y.; Ivanov, V.P. Alcohol Consumption and Cigarette Smoking Are Important Modifiers of the Association Between Acute Pancreatitis and the PRSS1–PRSS2 Locus in Men. Pancreas 2017, 46, 230–236. [Google Scholar] [CrossRef]
- Novakov, V.; Novakova, O.; Sorokina, I. Genetic Markers of Knee Osteoarthritis in Women of the Central Chernozem Region of Russia. Res. Results Biomed. 2023, 9, 191–205. [Google Scholar] [CrossRef]
- Abramova, M. Genetic Markers of Severe Preeclampsia. Res. Results Biomed. 2022, 8, 305–316. [Google Scholar] [CrossRef]
- Malashenkova, I.K.; Ushakov, V.I.; Krynskiy, S.A.; Ogurtsov, D.P.; Khailov, N.A. The Association of Inflammatory Status and Immunological Parameters with Single-Nucleotide Polymorphisms of Cytokine and Toll-like Receptor Genes in Patients with Schizophrenia. Res. Results Biomed. 2022, 8, 148–163. [Google Scholar] [CrossRef]
- Rashid, M.; Zadeh, L.R.; Baradaran, B.; Molavi, O.; Ghesmati, Z.; Sabzichi, M.; Ramezani, F. Up-down Regulation of HIF-1α in Cancer Progression. Gene 2021, 798, 145796. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zang, Y.; Zhao, F.; Li, Z.; Zhang, J.; Fang, L.; Li, M.; Xing, L.; Xu, Z.; Yu, J. Inhibition of HIF-1α by PX-478 Suppresses Tumor Growth of Esophageal Squamous Cell Cancer In Vitro and In Vivo. Am. J. Cancer Res. 2017, 7, 1198–1212. [Google Scholar] [PubMed]
- Lu, J.; Wei, H.; Sun, W.; Geng, J.; Liu, K.; Liu, J.; Liu, Z.; Fu, J.; He, Y.; Wang, K. NKT2152: A Highly Potent HIF2α Inhibitor and Its Therapeutic Potential in Solid Tumors beyond CcRCC. Cancer Res. 2022, 82 (Suppl. S12), 6330. [Google Scholar] [CrossRef]
- Guo, X.C.; Segars, J.H. The Impact and Management of Fibroids for Fertility: An Evidence-Based Approach. Obstet. Gynecol. Clin. N. Am. 2012, 39, 521–533. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).