D-Penicillamine-Induced Myasthenia Gravis—A Probable Complication of Wilson’s Disease Treatment—A Case Report and Systematic Review of the Literature
Abstract
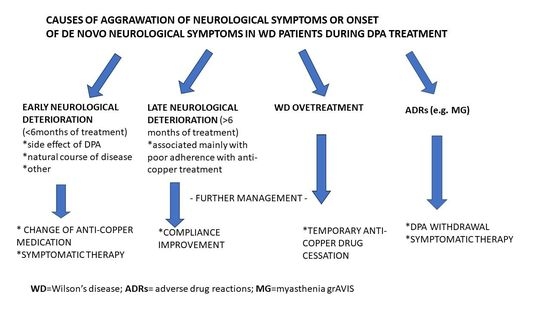
1. Introduction
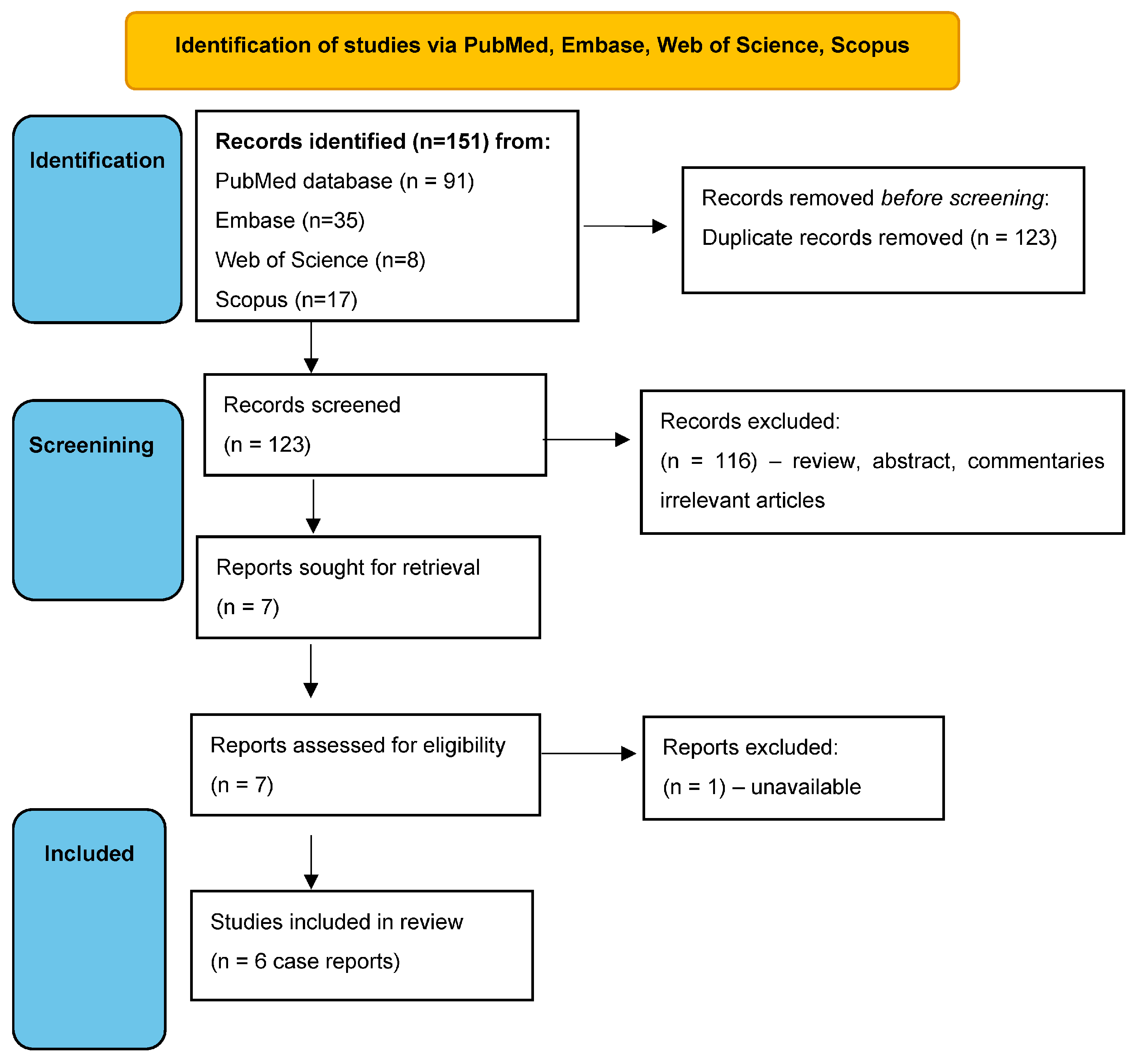
2. Materials and Methods
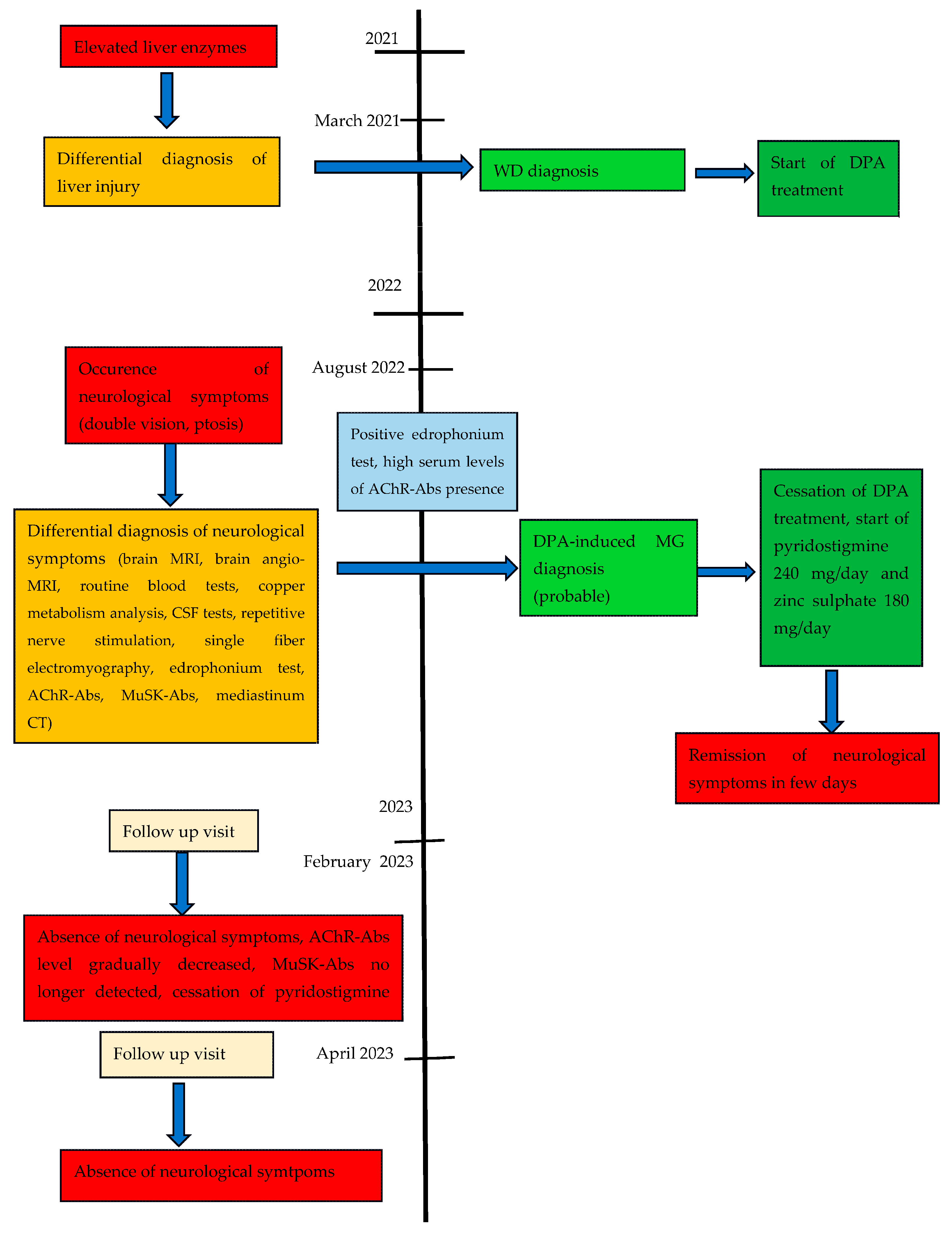
2.1. Case Report
2.2. Systematic Review of DPA-Induced MG in WD Patients
3. Discussion
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Association for The Study of The Liver. EASL Clinical Practice Guidelines: Wilson’s disease. J. Hepatol. 2012, 56, 671–685. [Google Scholar] [CrossRef]
- Członkowska, A.; Litwin, T.; Dusek, P.; Ferenci, P.; Lutsenko, S.; Medici, V.; Rybakowski, J.K.; Weiss, K.H.; Schilsky, M.L. Wilson disease. Nat. Rev. Dis. Primers. 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Czlonkowska, A.; Litwin, T. Wilson disease—Currently used anticopper therapy. Handb. Clin. Neurol. 2017, 142, 181–191. [Google Scholar]
- Weiss, K.H.; Askari, F.K.; Czlonkowska, A.; Ferenci, P.; Bronstein, J.M.; Bega, D.; Ala, A.; Nicholl, D.; Flint, S.; Olsson, L.; et al. Bis-choline tetrathiomolybdate in patients with Wilson’s disease: An open label, multicentre, phase 2 study. Lancet Gastroenterol. Hepatol. 2017, 2, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Litwin, T.; Dzieżyc, K.; Czlonkowska, A. Wilson disease—Treatment perspectives. Ann. Transl. Med. 2019, 7, S68. [Google Scholar] [CrossRef]
- Schilsky, M.L. Long-term outcome for Wilson disease: 85% good. Clin. Gastroenterol. Hepatol. 2014, 12, 690–691. [Google Scholar]
- Antos, A.; Członkowska, A.; Smolinski, L.; Bembenek, J.; Przybyłkowski, A.; Skowronska, M.; Kurkowska-Jastrzębska, I.; Litwin, T. Early neurological deterioraion in Wilson’s disease: A systematic literature review and meta-analysis. Neurol. Sci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Litwin, T.; Antos, A.; Bembenek, J.; Przybyłkowski, A.; Kurkowska-Jastrzębska, I.; Skowrońska, M.; Członkowska, A. Copper deficeicny as Wilson’s disease overtreatment: A systematic review. Diagnostics 2023, 13, 2424. [Google Scholar] [CrossRef]
- Antos, A.J.; Litwin, T.; Przybylkowski, A.; Skowronska, M.; Kurkowska-Jastrzebska, I.; Czlonkowska, A. D-penicillamine-induced lupus erythematosus as an adverse reaction of treatment of Wilson’s disease. Neurol. Neurochir. Pol. 2021, 55, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Litwin, T.; Czlonkowska, A.; Socha, P. Oral chelator treatment of Wilson Disease: D-penicillamine. In Clinical and Translational Perspectives on Wilson Disease; Litwin, T., Czlonkowska, A., Socha, P., Eds.; Academic Press: Cambridge, UK, 2019; pp. 357–364. [Google Scholar]
- Beinhardt, S.; Leiss, W.; Stättermayer, A.F.; Graziadei, I.; Zoller, H.; Stauber, R.; Maieron, A.; Datz, C.; Steindl-Munda, P.; Hofer, H.; et al. Long-term outcomes of patients with Wilson disease in a large Austrian cohort. Clin. Gastroenterol. Hepatol. 2014, 12, 683–689. [Google Scholar] [CrossRef]
- Bruha, R.; Marecek, Z.; Pospisilova, L.; Nevsimalova, S.; Vitek, L.; Martasek, P.; Nevoral, J.; Petrtyl, J.; Urbanek, P.; Jiraskova, A.; et al. Long-term follow-up of Wilson disease: Natural history, treatment, mutations analysis and phenotypic correlation. Liver Int. 2011, 31, 83–91. [Google Scholar] [PubMed]
- Poujois, A.; Trocello, J.-M.; Djebrani-Oussedik, N.; Poupon, J.; Collet, C.; Girardot-Tinant, N.; Sobesky, R.; Habès, D.; Debray, D.; Vanlemmens, C.; et al. Exchangeable copper: A reflection of the neurological severity in Wilson’s disease. Eur. J. Neurol. 2017, 24, 154–160. [Google Scholar] [PubMed]
- Członkowska, A.; Litwin, T.; Karliński, M.; Dziezyc, K.; Chabik, G.; Czerska, M. D-penicillamine versus zinc sulfate s first-line therapy for Wilson’s Disease. Eur. J. Neurol. 2014, 21, 599–606. [Google Scholar] [PubMed]
- Vincent, A.; Palace, J.; Hilton-Jones, D. Myasthenia gravis. Lancet 2001, 357, 2122–2128. [Google Scholar] [PubMed]
- Gilhus, N.E.; Skeie, G.O.; Romi, F.; Lazaridis, K.; Zisimopoulou, P.; Tzartos, S. Myasthenia gravis–autoantibody characteristics and their implications for therapy. Nat. Rev. Neurol. 2016, 12, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Hu, X.; Lu, Z.; Hackett, M.L. Prognostic factors of remission in myasthenia gravis after thymectomy. Eur. J. Cardiothorac. Surg. 2015, 48, 18–24. [Google Scholar] [CrossRef]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions. Explanation and elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef]
- Thapa, L.; Thapa, M.; Bhattarai, S.; Shrestha, A.M.; Sharma, N.; Rai, N.; Pokharel, M.; Paudel, R. D-penicillamine induced myasthenia gravis in Wilson’s disease: A case report. JNMA J. Nepal. Med. Assoc. 2022, 60, 644–647. [Google Scholar]
- Reuner, U.; Stremmel, W.; Weiskirchen, R. The interesting case-orphan diseases-double trouble. Ann. Transl. Med. 2019, 7, S74. [Google Scholar] [CrossRef]
- Tan, S.S.; Latif, S.A.; Poh, W.Y. Concurrent massive breast enlargement, myasthenia gravis and dermopathy as manifestations of penicillamine toxicity in a Wilson’s disease patient. Med. J. Malaysia 2012, 67, 323–325. [Google Scholar]
- Varghese, T.; Ahmed, R.; Sankaran, J.D.; Al-Khusaiby, S.M. D-Penicillamine induced myasthenia gravis. Neurosciences 2002, 7, 293–295. [Google Scholar] [PubMed]
- Narayanan, C.S.; Behari, M. Generalized myasthenia gravis following use of D-pencillamine in Wilson’s disease. J. Assoc. Physician India 1999, 47, 648. [Google Scholar]
- Masters, C.L.; Dawkins, R.L.; Zilko, P.J.; Simpson, J.A.; Leedman, R.J. Penicillamine-associated myasthenia gravis, antiacetylcholine receptor and antistriational antibodies. Am. J. Med. 1977, 63, 689–694. [Google Scholar] [CrossRef]
- Czlonkowska, A. Myasthenia syndrome during penicillamine treatment. Br. Med. J. 1975, 28, 726–727. [Google Scholar] [CrossRef][Green Version]
- Poulas, K.; Koutsouraki, E.; Kordas, G.; Kokla, A.; Tzartos, S.J. Anti-MuSK and anti-AChR-positive myasthenia gravis induced by d-penicillamine. J. Neuroimmunol. 2012, 250, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Adelman, H.M.; Winters, P.R.; Mahan, C.S.; Wallach, P.M. D-penicillamine-induced myasthenia gravis: Diagnosis obscured by coexisting chronic obstructive pulmonary disease. Am. J. Med. Sci. 1995, 309, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Marchiori, P.E.; Scaff, M.; Cossermelli, W.; De Assis, J.L. Myasthenia gravis induced by D-penicillamine in a patient with progressive systemic sclerosis. Arq. Neuropsiquiatr. 1984, 42, 380–383. [Google Scholar] [CrossRef]
- Essigman, W.K. Multiple side effects of penicillamine therapy in one patient with rheumatoid arthritis. Ann. Rheum. Dis. 1982, 41, 617–620. [Google Scholar] [CrossRef]
- Kimbrough, R.L.; Mewis, L.; Stewart, R.H. D-penicillamine and the ocular myasthenic syndrome. Ann. Ophthalmol. 1981, 13, 1171–1172. [Google Scholar]
- Hill, M.; Moss, P.; Wordsworth, P.; Newsom-Davis, J.; Willcox, N. T cell responses to D-penicillamine in drug-induced myasthenia gravis: Recognition of modified DR1:peptide complexes. J. Neuroimmunol. 1999, 97, 146–153. [Google Scholar] [CrossRef]
- Rodolico, C.; Bonanno, C.; Toscano, A.; Vita, G. Mu-SK-associated myasthenia gravis: Clinical features and management. Front. Neurol. 2020, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.; Alvi, U.; Soliven, B.; Rezania, K. Drugs that induce or cause deterioration of Myasthenia Gravis: An update. J. Clin. Med. 2021, 10, 1537. [Google Scholar] [CrossRef] [PubMed]
- Antczak-Kowalska, M.; Członkowska, A.; Eyileten, C.; Palejko, A.; Cudna, A.; Wolska, M.; Piechal, A.; Litwin, T. Autoantibodies in Wilson disease: Impact on clinical course. JIMD Rep. 2022, 63, 508–517. [Google Scholar] [CrossRef]
- Seessle, J.; Gotthardt, D.N.; Schäfer, M.; Gohdes, A.; Pfeiffenberger, J.; Ferenci, P.; Stremmel, W.; Weiss, K.H. Concomitant immune-related events in Wilson disease: Implications for monitoring chelator therapy. J. Inherit. Metab. Dis. 2016, 39, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Czlonkowska, A.; Milewski, B. Immunological observations on patients with Wilson’s disease. J. Neurol. Sci. 1976, 29, 411–421. [Google Scholar] [CrossRef]
- Członkowska, A. The influence of prolonged treatment with D-penicillamine on the immune response in Wilson’s disease. Eur. J. Clin. Pharmacol. 1977, 12, 265–271. [Google Scholar] [CrossRef]
- Komal Kumar, R.N.; Patil, S.A.; Taly, A.B.; Nirmala, M.; Sinha, S.; Arunodaya, G.R. Effect of D-penicillamine on neuromuscular junction in patients with Wilson disease. Neurology 2004, 63, 935–936. [Google Scholar] [CrossRef]
- Antos, A.; Członkowska, A.; Bembenek, J.; Skowrońska, M.; Kurkowska-Jastrzębska, I.; Litwin, T. Blood based biomarkers of central nervous system involvement in Wilson’s disease. Diagnostics 2023, 13, 1554. [Google Scholar] [CrossRef]
| Reference | Patient Characteristics (Age, Gender, WD Symptoms) | Duration, Kind and Dose of WD Treatment | MG Clinical Presentation and Diagnostic Tests | MG Abs Induced by DPA | Treatment and Outcome |
|---|---|---|---|---|---|
| Thapa et al. 2022 [20] | 15-year-old male with neurological WD (generalized weakness, spastic limbs, hand tremor) | Duration of WD treatment with DPA: 6 years Since diagnosis aged 9, patient was treated with DPA 500 mg/day and zinc 60 mg/day | Bilateral facial weakness (dropping jaw and both upper eyelids) and dysphagia RNS from the facial nerve (orbicularis oculi muscle) showed >10% decremental response Normal chest CT | Positive serum AChR-Abs (14.3 nmol/L; normal < 0.4) Negative for MuSK-Abs | Withdrawal of DPA Pyridostigmine introduced (dose not known) Remarkable improvement within 2 months |
| Reuner et al. 2019 [21] | 17-year-old female with hepatic WD (moderately elevated transaminases) | Duration of WD treatment with DPA: 6 years Since diagnosis aged 11, patient was treated with DPA 300 mg/day with pyridoxine supplementation 20 mg/week | Exercise-induced speech difficulties, mouth and tongue motility and dysphagia RNS showed significant decrement in the orbicularis oris (38%) and trapezius (25%) muscle Chest magnetic resonance imaging visualized thymus hyperplasia | Positive serum AChR-Abs (>5 nM) MuSK-Abs not tested | Continuation of DPA Pyridostigmine introduced (180 mg/day) Robot-assisted endoscopic thymectomy showed marked lymphofolicular hyperplasia (no thymoma) Remarkable improvement, 2 years after cessation of antimyasthenic therapy patient remains asymptomatic |
| Tan et al. 2012 [22] | 16-year-old female with WD (clinically asymptomatic—diagnosed during family screening) | Duration of WD treatment with DPA: 4 months Since diagnosis aged 15 (10 months earlier), patient was treated with DPA 750 mg/day | Diplopia, bilateral ptosis, jaw weakness with difficulties in smiling and chewing, dysarthria and dysphagia, proximal limb weakness (with massive bilateral breast enlargement, galactorrhea, and dermopathy) RNS showed a decremental response of the right deltoid and orbicularis muscles Chest CT not done | AChR-Abs and MuSK-Abs not tested | Withdrawal of DPA; trientine 900 mg/day introduced with clobetasone butyrate for skin lesions (indication dermopathy) One month later disappearance of the visual and skin complaints At follow-up at 7 months, complete resolution of all neurological and dermatological DPA-induced side effects was observed |
| Varghese et al. 2002 [23] | 12-year-old female with hepatic WD (prior hepatomegaly and jaundice) | Duration of WD treatment with DPA: 4 years Since diagnosis aged 8, patient was treated with DPA 1000 mg/day and pyridoxine 1 mg/day | Left-sided ptosis (cranial nerves normal, systemic examination unremarkable) RNS showed decremental response of more than 20% in both deltoid muscles and the left orbicularis oculi Positive edrophonium test Normal chest CT | Positive serum AChR-Abs (29.1 nmol/L; normal < 0.4) MuSK-Abs not tested | Withdrawal of DPA and trientine 1500 mg/day introduced Remarkable improvement within 3 months, all symptoms disappeared After 1 year, AChR-Abs became negative |
| Masters et al. 1976 [25] | 18-year-old female with hepatic WD | Duration of WD treatment with DPA: 8 years Since diagnosis aged 10, patient was treated with DPA 1000 mg/day | Progressive muscular weakness with bilateral ptosis, diplopia, bilateral facial and palatal weakness, dysphagia, dysarthria and marked fatigability RNS showed a decremental response of the right deltoid and orbicularis muscles Positive edrophonium test Normal chest CT | Serum AChR-Abs titer 19 U (normal 1 U) MuSK-Abs not tested | DPA was continued Pyridostigmine (120 mg/day) and neostigmine (15 mg as required) was introduced Thymectomy (enlarged thymus, with thymic hyperplasia) but no thymoma MG symptoms disappeared over 5 months; however, patient was diagnosed as MG and treated with pyridostigmine as increased serum AChR-Abs were observed during follow-up |
| Czlonkowska et al. 1975 [26] | 14-year-old male with WD (clinically asymptomatic—family screening) | Duration of WD treatment with DPA: 13 months Since diagnosis aged 13, patient was treated with DPA 1000 mg/day and pyridoxine 1 mg/day | Initial right-sided ptosis (cranial nerves normal, systemic examination unremarkable). After 10 days, left ptosis also occurred RNS not determined Positive edrophonium test Chest CT not done | Serum AChR-Abs positive MuSK-Abs not tested | Withdrawal of DPA Neostigmine was introduced (dose not known) Remarkable improvement with cessation of all symptoms within 6 weeks After 8 months, DPA 750 mg/day was reintroduced without adverse events during next 10 months |
| Affected System | Symptoms and Their Estimated Frequency | Type of ADR (Early/Late) |
|---|---|---|
| Skin | Degenerative dermatoses (elastosis perforans serpiginosa a, cutis laxa (skin laxity) a, anterodermax, pseudo-pseudoxanthoma elasticum a) Bullous dermatosesx (pemphigus and bullous disease) Miscellaneous cutaneous conditions (lichen planus-like eruptionsx, aphtous stomatitis or glossitis a, oral ulcerations a, alopecia a, psoriasiform dermatitisx, seborrheic dermatitis-like picturex, yellow-nail syndromex) | late early late |
| Nervous system | Paradoxical neurological deterioration b, myasthenia-like syndromesx, peripheral sensory-motor neuropathiesx, optic nerve neuropathyx, serous retinitisx, hypogeusia (diminution in taste perception)x, deafnessx | early/late late |
| Connective tissue disease | Lupus-like syndrome, rheumatoid arthritisx, polymyositisx, arthralgiax, | late early/late |
| Renal | Proteinuria b, hematuria a, Goodpasture syndromex, severe fatal glomerulonephritis associated with intra-alveolar hemorrhagex, renal vasculitisx | early/late late |
| Respiratory | Bronchiolitisx, pulmonary fibrosisx, pneumonitisx, pleural effusionx, dyspneax | late |
| Gastrointestinal | Nauseax, vomitingx, diarrheax, cholestatic jaundicex, liver siderosisx | early/late late |
| Hematologic | Thrombocytopenia c, neutropeniax, hemolytic anemiax Agranulocytosisx, aplastic anemiax, | early/late late |
| Immunologic | Immunoglobulin deficiencyx | late |
| Reproductive system and breast disorders | Breast enlargement a | late |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antos, A.; Członkowska, A.; Bembenek, J.; Kurkowska-Jastrzębska, I.; Litwin, T. D-Penicillamine-Induced Myasthenia Gravis—A Probable Complication of Wilson’s Disease Treatment—A Case Report and Systematic Review of the Literature. Life 2023, 13, 1715. https://doi.org/10.3390/life13081715
Antos A, Członkowska A, Bembenek J, Kurkowska-Jastrzębska I, Litwin T. D-Penicillamine-Induced Myasthenia Gravis—A Probable Complication of Wilson’s Disease Treatment—A Case Report and Systematic Review of the Literature. Life. 2023; 13(8):1715. https://doi.org/10.3390/life13081715
Chicago/Turabian StyleAntos, Agnieszka, Anna Członkowska, Jan Bembenek, Iwona Kurkowska-Jastrzębska, and Tomasz Litwin. 2023. "D-Penicillamine-Induced Myasthenia Gravis—A Probable Complication of Wilson’s Disease Treatment—A Case Report and Systematic Review of the Literature" Life 13, no. 8: 1715. https://doi.org/10.3390/life13081715
APA StyleAntos, A., Członkowska, A., Bembenek, J., Kurkowska-Jastrzębska, I., & Litwin, T. (2023). D-Penicillamine-Induced Myasthenia Gravis—A Probable Complication of Wilson’s Disease Treatment—A Case Report and Systematic Review of the Literature. Life, 13(8), 1715. https://doi.org/10.3390/life13081715