Abstract
With the increasing demand for medicinal plants and the increasing shortage of resources, improving the quality and yield of medicinal plants and making more effective use of medicinal plants has become an urgent problem to be solved. During the growth of medicinal plants, various adversities can lead to nutrient loss and yield decline. Using traditional chemical pesticides to control the stress resistance of plants will cause serious pollution to the environment and even endanger human health. Therefore, it is necessary to find suitable pesticide substitutes from natural ingredients. As an important part of the microecology of medicinal plants, endophytes can promote the growth of medicinal plants, improve the stress tolerance of hosts, and promote the accumulation of active components of hosts. Endophytes have a more positive and direct impact on the host and can metabolize rich medicinal ingredients, so researchers pay attention to them. This paper reviews the research in the past five years, aiming to provide ideas for improving the quality of medicinal plants, developing more microbial resources, exploring more medicinal natural products, and providing help for the development of research on medicinal plants and endophytes.
1. Introduction
Medicinal plants refer to plants used in medicine to prevent and treat diseases [1]. All or part of medicinal plants are used in medicine and will also be used as raw materials for the pharmaceutical industry, which have a wide range of medicinal and economic uses [2]. Especially in the field of traditional medicine represented by traditional Chinese medicine and Indian folk medicine, medicinal plants, as the main source of natural drugs, provide very important health care services for the population of developing countries [3,4,5]. With the rapid development of modern medicine, many clinical drugs still come from natural products extracted from medicinal plants [2]. Although many kinds of medicinal plants have been used in clinical treatment, due to environmental stress, overexploitation, low reproductive capacity, and other factors, some rare, high-demand, and wild medicinal plant resources cannot meet the market demand, so how to improve the germplasm resources of medicinal plants has become an urgent problem to be solved.
In recent years, researchers have gradually realized that endophytes can play an important role in affecting the quality and yield of medicinal plants through special microbe-plant interactions [6,7]. Plant endophytes are microbial groups that widely exist in healthy medicinal plant tissues, coexist harmoniously with host plants, and do not cause significant damage to hosts [8]. They are also an important part of theplant micro-ecosystem, which is rich in species, mainly including endophytic fungi, endophytic bacteria, and endophytic actinomycetes [7,9,10]. At present, endophytes have been isolated from a variety of medicinal plants, and many endophytes have been verified to secrete plant hormones, growth factors, etc., which are conducive to plant growth and development and can also regulate the accumulation and production of active ingredients in medicinal plants [10,11]. They increase the active ingredients of the host by producing the same or similar active products as the active ingredients in the host [11,12,13]. The most interesting property of endophytes is that they can convert the original active ingredients of the plant into new compounds. In 1993, Stierle et al. isolated an endophytic fungus from Taxus brevifolia and found that it can produce paclitaxel, an anti-tumor substance similar to the host plant, which inspired researchers to find bioactive substances from endophytes of medicinal plants [14]. Endophytes provide more resources of new bioactive metabolites, especially alkaloids, saponins, quinones, flavonoids, terpenoids, etc., which have a lot of biological activities and have also become research hotspots in the composition and production of natural drugs [8].
In order to improve the quality of medicinal plants, more needs to be known about the special relationship between endophytes and medicinal plants. In this review, Pubmed and Endnote were used to explore articles using the keywords ‘medicinal plants’, ‘endophyte’, ‘metabolites’, ‘growth promotion’, ‘stress resistance’, and ‘Biocontrol’ and summarize the research on the function of culturable endophytes of medicinal plants in the past five years (2019–2023). The development and utilization of endophytes in medicinal plants were prospected to provide references for the development of endophyte products and improving the quality of medicinal plants.
2. Medicinal Plants and Their Cultivable Endophyte Resources
Endophytes in medicinal plants mainly include endophytic fungi, endophytic bacteria, and endophytic actinomycetes, and they are rich in species diversity [6,7]. It is found that the biological functions of these endophytes have a great influence on medicinal plants [10,11], so obtaining more microbial resources, especially those with biological activity, can greatly promote the development of the medicinal plant industry.
2.1. Culturable Endophytic Bacteria Diversity in Medicinal Plants
Atractylodes macrocephala Koidz., called Baizhu in Chinese, is a medicinal plant used in traditional Chinese medicine theoretical systems to treat gastrointestinal dysfunction, cancer, osteoporosis, obesity and other symptoms, and has various pharmacological activities [15]. Wu et al. [16] explored the cultivable endophytic bacteria in the stems, leaves, roots, and rhizomes of Atractylodes macrocephala Koidz. in four different regions and their potential correlation with plant bioactives. A total of 118 endophytic bacteria belonging to 3 phyla, 5 classes, 11 orders, 26 families and 48 genera were identified from four Atractylodes macrocephala Koidz. tissues. Among them, Bacillus sp. is the most widely distributed. Dendrobium is one of the largest genera of Orchidaceae, with more than 1500 species distributed all over the world [17]. As a medicinal plant, dendrobium has greatly. contributed to the medical industry with its anticancer, antifatigue, and gastrointestinal protective effects [18]. In addition, there are also many microbial resources in dendrobium. Wang et al. [19] isolated and cultured endophytic bacteria from Dendrobium officinale samples of six different sources and cultivars. A total of 165 cultivable endophytic bacteria were isolated from sterilized Dendrobium officinale stems and classified into 43 species based on 16S rRNA gene sequence analysis, of which 14 strains had anti-plant-pathogenic activity. Mulberry, which belongs to the genus Morus of the Moraceae family, is an aggregated berry that is oval-shaped, palatable, and also rich in nutrients; it is regarded as a very important medicinal and edible plant due to its rich, effective chemical composition and wide range of biological activities [20,21,22]. Xu et al. [23] isolated a total of 608 endophytic bacteria from four mulberry cultivars, belonging to 4 phyla and 36 genera.
Bacteria, as the largest group of plant endophytes, have been isolated from many kinds of medicinal plants and widely studied due to their biocontrol functions [24,25,26,27,28,29,30,31,32]. By reviewing the recent literature on most of the endophytic bacteria of medicinal plants including Bacillus sp., Pseudomonas sp., Enterobacter sp., Agrobacterium sp., etc., and a large number of endophytic bacteria in the roots, stems, and leaves, we collated some of the relevant data of the isolated endophytic bacteria in Table 1.

Table 1.
Endophytic bacteria resources isolated from medicinal plants in recent years.
2.2. Culturable Endophytic Fungal Diversity in Medicinal Plants
Aconitum heterophyllum is an alkaloid-rich medicinal plant which is widely used in traditional Chinese medicine clinics [38,39]. A total of 328 fungal isolates were found in leaf, stem and root tissues of plants by Hafeez et al., and 12 endophytic fungal species were identified by molecular characterization [39]. Crocus sativus L. (family Iridaceae) has been widely used as an antimicrobial, antidepressant, digestive, anticancer, and anticonvulsant medicine due to its abundant natural products as well as antioxidant activity [40,41]. Lu et al. [42] isolated endophytic fungi from five different locations in Crocus sativus tissues (corm, scape, leaf, petal, and stigma) and identified a total of 32 endophytic fungal groups, assigned to seven orders within four classes. Wang et al. [43] isolated 34 endophytic fungi from Salvia miltiorrhiza, a traditional Chinese medicine, belonging to 10 genera and 16 species, and Epicoccus sp. SX19 and Colletotrichum gloeosporioids showed strong inhibitory effects on five pathogens. Ogbe et al. [30] isolated a total of 11 endophytic fungi from the roots and leaves of a drought tolerant mint species Endostemon obtusifolius. Similarly, five endophytic fungi were isolated from the leaf segments of wild Dendrobium nobile and identified as Colletotrichum tropicicola, Fusarium keratoplasticum, Fusarium oxysporum, Fusarium solani, and Trichoderma longibrachiatum [44]. Codonopsis pilosula, as a famous medicinal and food homologous plant, has functions such as strengthening the spleen, tonifying the lungs, and engendering liquid in traditional Chinese medicine [45]. Fan et al. [46] obtained 205 strains of endophytic fungi from the roots of Codonopsis pilosula, collected from six regions in Gansu Province, China, of which Fusarium sp., Aspergillus sp., Alternaria sp., Penicillium sp., and Plectosphaerella sp. were the dominant genera. Vernonia anthelmintica (L.) Willd has a long history in the treatment of several diseases related to skin, central nervous system, kidney, gynecology, gastrointestinal, metabolism, and general health [47]. Researchers have isolated more than 30 types of endophytic fungi from Vernonia anthelmintica. [48]
From the research in recent years, it can be seen that Fusarium sp., Aspergillus sp., and Penicillium sp. can be isolated from most medicinal plants, and because of their many biological functions, they are regarded as the key research objects of endophytic fungi in medicinal plants. The recent research results are summarized in Table 2.

Table 2.
Endophytic fungal resources isolated from medicinal plants in recent years.
2.3. Culturable Endophytic Actinomycetes Diversity in Medicinal Plants
Dioscorea has powerful medicinal functions and is a potential source of bioactive substances for combating various diseases [49]. Zhou et al. [50] isolated 116 actinomycetes from the tissues of Dioscorea opposita Thunb. and found a new Streptomyces sp. with strong biocontrol function. As a traditional Chinese medicine, Eucommia ulmoides Oliv. has been used to treat various diseases since ancient times [51]. The research group led by Mo et al. [52,53] isolated two new species of Nocardia sp. from the leaves and roots of Eucommia ulmoides Oliv. Thymus roseus schipcz is one of the traditional Chinese herbs belonging to Lamiaceae and has been proven to have anti-inflammatory, antioxidant, anti-cancer and other functions [54]. Musa et al. [54] isolated 128 strains from the roots, stems and leaves of Thymus roseus schipcz, with a predominance of Streptomyces sp., followed by Nocardiopsis sp., Micrococcus sp., Kocuria sp., and others. Viola odorata grows in the high altitude area of the Himalayas and is used as a natural medicine because of its antidiabetes, anti-inflammatory, and other functions [55,56]. Salwan et al. [56] isolated a Streptomyces strain with antioxidant and antibacterial activity from the medicinal plant Viola odorata collected in the Himalayas, which has the potential to produce antibacterial and antioxidant components. Xanthium sibiricum is a well-known Chinese herbal medicine commonly used to treat autoimmune and inflammatory diseases [57]. Hu et al. [58] isolated two new Streptomyces strains from healthy leaves and seeds of Xanthium sibiricum.
According to the research on endophytic actinomycetes of medicinal plants in recent years, actinomycetes are mainly distributed in the roots of medicinal plants, and their number is greater than that in other tissues of plants. Streptomyces sp. is the main research object of actinomycetes, and Streptomyces sp. has received extensive attention because of its strong biological activity [59]. Actinomycetes of other genera can also be isolated from medicinal plants, but the number is relatively small compared with Streptomyces. Many studies have isolated new species of bioactive endophytic actinomycetes from medicinal plants, which greatly expanded microbial resources and laid a foundation for the industrial application of actinomycetes [52,53,58,60,61]. The results of endophyte isolation from medicinal plants in recent years are shown in Table 3.

Table 3.
Endophytic actinomycete resources isolated from medicinal plants in recent years.
3. Beneficial Effects of Endophytes from Medicinal Plants on the Host
In recent years, many studies have shown that endophytes have made important contributions in promoting the growth of medicinal plants, enhancing the stress tolerance of medicinal plants and biological control of plant diseases.
3.1. Promoting the Growth of Medicinal Plants
Khan et al. [65] isolated an endophytic fungus Acremonium sp. Ld-03, which has antibacterial activity and can produce indoleacetic acid and siderophores, from the medicinal plant Lilium davidii. After diluting the culture medium to different concentrations and conducting liquid culture on the host, it was found that with the application of 40% culture dilution of Acremonium sp., the root and bud length of Allium tuberosum can be significantly increased. Zou et al. [66] isolated a Bacillus subtilis strain from the medicinal plant Aconitum carmichaelii DEBX., which can produce gluconase, cellulase, protease, indole acetic acid, siderophore, antifungal lipopeptides, and polyketides and significantly increase the fresh weight and dry weight of the host stem, main root, and lateral root. Tao et al. [67] isolated four strains of endophytic bacteria with indole acetic acid production, phosphate solubilization, and nitrogen fixation abilities from the precious traditional Chinese medicine Pairs polyphylla var. yunnanensis and significantly increased the host’s biomass. Mathur et al. [68] isolated Aspergillus niger which can produce gibberellin from Albizia lebbeck (L.) Benth, effectively promoting the seed germination of wheat, barley, and millet. Purushotham et al. [69] isolated an endophytic actinomycete Nocardia sp. TP1BA1B from the native medicinal plant Pseudowitera colorata (horopito) in New Zealand, which has the function of dissolving phosphate, producing siderophores, and promoting the growth of host seedlings.
The growth of medicinal plants is related to various factors, such as light, temperature and microorganisms, among which endophytes play a crucial role in the host’s growth process [70]. Endophytes promote the growth and development of plants in different ways, such as secreting siderophore to improve the utilization rate of iron in plants. Endophytes with nitrogen fixation, phosphate solubilization, and potassium solubilization abilities promote the growth of medicinal plants by promoting the absorption of nitrogen, phosphorus, and potassium. Endophytes can also promote plant growth by providing growth hormone to the host, such as indole-3-acetic acid, indole-3-acetonitrile, gibberellin, and cytokinins [10,71,72].
3.2. Enhance the Stress Tolerance of Medicinal Plants
Li et al. [73] isolated a strain of Streptomyces from Glycyrrhiza uralensis and confirmed through inoculation that this strain can enhance the tolerance of the host to drought, salt, and drought salt conditions. Studies have found that under drought stress, the growth of Helianthus tuberosus L. (Jerusalem artichoke) can be better promoted by the inoculation of endophytic bacteria [74]. Sphingomonas paucimobilis, an endophytic bacterium in the rare medicinal plant Dendrobium officinale, has good resistance to stresses of salt, drop and cadmium, and this strain is the only one with growth promoting ability reported in this species [75]. Some researchers isolated Endophytes from Astragalus mongholicus and co-inoculated them with Trichoderma strains under drought conditions, which significantly improved the root biomass, root length, calycosin-7-O-β-D-glucoside content of the host and activities of nitrate reductase and soil urease [76].
Endophytes can enhance the environmental adaptability of host plants by enhancing the expression of stress resistance related genes in host plants and increasing the activity of related enzymes [77]. In addition, some endophytes may also produce antibiotic compounds, antimicrobial peptides, or alkaloids to help the host resist pests and diseases [78,79].
3.3. Promoting the Accumulation of Secondary Metabolites in Medicinal Plants
Salvia miltiorrhiza is widely used in East Asia because of its anti-tumor, anti-inflammatory, and cardiovascular protection, and tanshinone and salvianolic acid are important medicinal components [80,81]. Endophytic fungus Cladosporium tenuissimum DF11 isolated from Salvia miltiorrhiza by Chen et al. [80] promoted the biosynthesis and accumulation of tanshinone in roots by upregulating the expression of HMGR, DXS, DXR, GGPPS, CPS, KSL and CYP76AH1, which are key enzyme genes of the tanshinone biosynthesis pathway. Other researchers used endophytes of Salvia miltiorrhiza to prepare elicitors, which affected the accumulation of metabolites in hairy roots of Salvia miltiorrhiza by inducing the expression of key genes (SmAACT, SmGGPPS, and SmPAL) [81]. Researchers have also isolated two strains of fungi from Salvia abrotanoides that can increase host cryptotanshinone and tanshinone IIA production [82]. Ye et al. [83] isolated three Endophytes from Houttuynia cordata, named Ilyonectria liriodendra, unidentified fungal sp., and Penicillium citrinum, which can respectively increase the phenolic compounds of the host, increase the components such as afzelin, decanal, and 2-undecanone, and increase the biomass of the host. Xie et al. [84] isolated an endophytic fungus, Schizophyllum commune from Panax ginseng and significantly enhanced the expression of key enzyme genes involved in ginsenoside biosynthesis pathways such as pgHMGR, pgSS, pgSE, and pgSD under co-culture conditions, promoting the accumulation of specific ginsenosides.
Endophytes can directly participate in the synthesis of secondary metabolites of medicinal plants and can also induce the formation of secondary metabolites of medicinal plants [85]. Selecting appropriate endophytes to act on medicinal plants can improve the content of secondary metabolites, which is of great significance for improving the quality of medicinal plants, protecting endangered medicinal plants, and synthesizing and developing new drugs.
3.4. Helping the Host Resist Pathogens
Streptomyces dioscori isolated from Glycyrrhiza uralensis exhibited inhibitory effects on three pathogenic fungi: Rhizoctonia solani, Fusarium acuminatum, and Sclerotinia scrotiorum [73]. An endophytic fungus, Diaporthe sp., was isolated from the leaves of the Indian medicinal plant Chloranthus elator Sw., and its camphor odor volatiles showed inhibitory effects on eight fungal pathogens in vitro [86]. Burkholderia gladioli, an endophytic bacterium from Crocus sativus Linn., can reduce corm rot and increase endogenous jasmonic acid (JA) level and expression of JA-regulated and other plant defense genes through antibacterial effects and improve the host’s resistance to Fusarium oxysporum [87].
Many endophytes can inhibit the occurrence of plant diseases caused by pathogenic bacteria. Endophytes can inhibit the activity of pathogens by inducing host resistance to resist the infection of pathogens and competing with pathogenic bacteria to produce antibiotics, hydrolytic enzymes, alkaloids, and other secondary metabolites and signal interference to resist disease caused by pathogens in host plants [88].
4. Medicinal Components Produced by Endophytes in Medicinal Plants
An endophytic fungus, Xylaria feejeensis, was derived from the medicinal plant Geophila repens in Sri Lanka. Integrated acids, derived from fungal metabolites, have strong antibacterial activity and are a potential resource of antibiotics [89]. Saikosaponin d (SSd) is an important medicinal component of the medicinal plant Bupleurum scorzonerifolium Willd. Some researchers isolated two endophytic Fungi from Bupleurum scorzonerifolium Willd., which can produce saikosaponin through UPLC/Q-TOF-MS detection [90]. The metabolite 7-methoxy-13-dehydroxypaxilline of Penicillium sp., an endophytic fungus isolated from the leaves of the traditional medicinal plant Baphicacanthus cusia (Nees) bremek., is a new indole diterpenoid, which exhibits anticancer activity [91]. Gu et al. [92] isolated a new compound, phomopolide G, from the fermentation broth of endophytic fungi of Artemisia argyi, which showed a wide range of antibacterial activities. Cochliobolus sp., an endophytic fungus of the Indian medical herb Andrographis paniculata, can metabolize the alkaloid aziridine, 1-(2-aminoethyl) and can be antibacterial and insect resistant [93]. A new crystalline compound 5-(1-hydroxybutyl)-4-methoxy-3-methyl-2h-pyran-2-one (c-hmmp) in the endophytic fungus Colletotrichum acutatum in the medicinal plant Angelica sinensis shows antibacterial, antimalarial, anticancer, antioxidant, and other activities [94]. The antibacterial compound 1,4-dihydroxy-2-methyl-anthraquinone was also isolated from the endophytic bacteria of Archidendron pauciflorum [32]. The structures of the above compounds are shown in Figure 1.
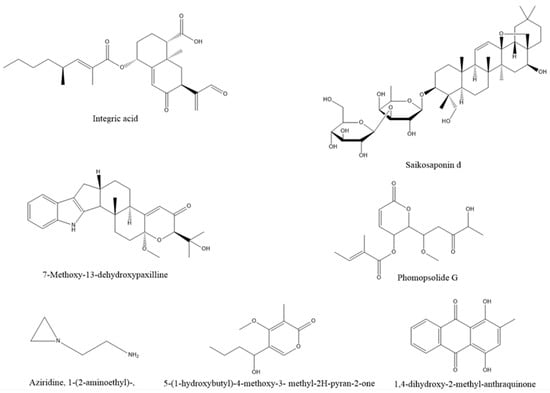
Figure 1.
Some active ingredients from endophytes of medicinal plants.
At present, many alkaloids, flavonoids, phenolic acids, terpenoids, coumarins, and other substances with antioxidant activity have been isolated from endophytes and their secondary metabolites of medicinal plants [10,11,32,95,96,97]. These natural antioxidant active substances often have anti-inflammatory, antioxidant, antibacterial, anti-tumor, antiviral and other functions [10,11,98]. Through searching the literature in the past five years, it was found that the bioactive natural products of endophytes in medicinal plants were metabolized by fungi, while the metabolites of bacteria and actinomycetes were mainly antibiotics [10]. More and more studies have found that natural product produced by host plants may be produced by endophytes or metabolites closely related to endophytes [99]. Further research on the metabolites of endophytes will be of great significance for the development of medicinal plants and clinical drugs.
5. Discussion
In recent years, people have gradually realized that endophytes play an important role in affecting the yield and quality of crude drugs by interacting with the host in a specific way. The traditional method of endophyte research is to use the artificial medium to culture, isolate, and purify microorganisms to obtain pure culture strains and use microscopic technology to observe and classify their morphology. According to the physiological and biochemical characteristics of the strain, 16S rRNA, its gene sequencing, and other molecular biological methods were used for gene identification [10].
The rapid development of gene sequencing technology, especially the emergence of high-throughput sequencing and other technical means, has brought unprecedented development to microbiology research [100]. High throughput sequencing technology has been applied to the study of the structure and diversity of a variety of plant endophytes and avoids the process of endophyte culturing in order to explore more microbial resources [100,101,102,103]. High throughput sequencing technology can explore the richness of microbial resources in medicinal plants, but only a small part can be isolated, which also means that there is still a great research potential for endophytes in medicinal plants. Therefore, it is necessary to optimize cultivation methods to obtain more cultivable microbial resources. In the process of endophytes’ isolation, the method of surface disinfection and the formula of the medium will affect the isolation of endophytes [104,105,106]. Excessive disinfection can cause damage to the endophytes of the plant, while incomplete disinfection can lead to contamination by other microbes [104,105]. Different carbon sources, nitrogen sources, and nutrients can also cause great differences in the isolation results of endophytes. The use of antibiotics in the culture medium can effectively improve the isolation efficiency of endophytes [104]. Some researchers have also added plant extracts to the culture medium to greatly increase the number of culturable endophytes [107].
Medicinal plants are the foundation of the development of the pharmaceutical industry. With the understanding and utilization of the cultivation, growth, and various functions of medicinal plants, the quality of medicinal plants has attracted great attention in society [108]. The quality and yield of medicinal plant raw materials are largely affected by many factors, such as plant genetic background, ecological habitat of plants, and soil nutrients [109,110]. Endophytes live in medicinal plants and have the functions of promoting the growth of host plants, enhancing the stress resistance of host plants, and regulating the synthesis of secondary metabolites and being able to metabolize medicinal compounds [111], as shown in Figure 2. Utilization of endophytes can reduce the use of fertilizers and pesticides, which plays an important role in protecting the environment [112,113].
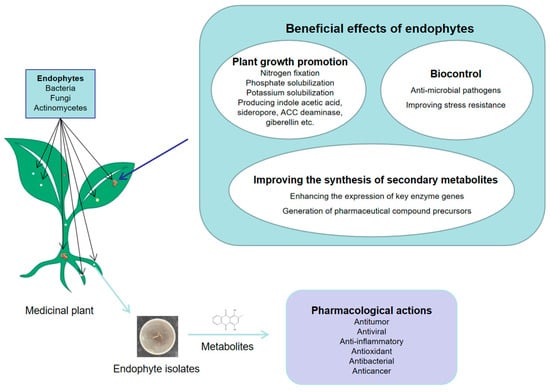
Figure 2.
Potential functions of endophytes in medicinal plants.
The secondary metabolites of endophytes from most medicinal plants have medicinal activities, which have great potential in the development of new drugs [11,113]. If endophytes of medicinal plants can be used to produce drugs in the future, it will make up for the demand for some rare medicinal plants to a certain extent [114]. In the case of taxol, as a diterpene with anticancer activity isolated from Taxus chinensis, the content of taxol in Taxus chinensis is very low, and its synthesis is complicated [66,115]. The slow growth rate and scarce resources of Taxus chinensis also limit the application of taxol. Since the isolation of taxol producing endophytes from Taxus chinensis, a new pathway for taxol production has been developed [116,117,118,119]. The endophytes, especially endophytic funji of Taxus chinensis have high growth rate, low cultivation cost, and are not affected by climate change [116]. Therefore, they have excellent prospects as taxol producers. The research methods of secondary metabolites of endophytes in plants are usually based on the isolation and purification strategies of natural products after fermentation of endophytes [120]. However, when endophytes leave the host and are cultured for multiple generations, the down-regulation of synthetic coding genes results in decreased metabolic capacity and biological functions [116,121,122]. This also causes losses to the benefits of using endophytic fungi to produce paclitaxel in industry. Fortunately, these problems can be overcome by optimizing culture methods, co-culturing, silencing rate-limiting genes and transcription factors, and activating the taxol synthesis gene cluster in fungi [115,116]. Therefore, optimizing the culture method of endophytes, promoting the metabolism of endophytes of medicinal plants, producing more metabolites with medicinal effects, and maintaining the biological function of endophytes are also urgent problems to be solved. Similarly, for other rare medicinal plants, we can also find endophytes with the potential of metabolizing active products, and through optimization of cultivation methods, genetic optimization and other methods, replace the rare medicinal plants to become producers of natural product and allow endophytes to maintain their biological functions, which is research with great potential and significance.
The use of chemical pesticides cause serious pollution to the environment and even endangers human health [123], so it is necessary to put forward more sustainable development strategies for the environment and human health and find suitable pesticide substitutes. Endophytes come from plants and act on plants, and they are rich in species and have strong biological control functions [67,124]. Therefore, they are the most suitable to replace chemical pesticides in the cultivation and protection of medicinal plants, and are an important environmental protection strategy. The metabolites of endophytes in medicinal plants are also a huge treasure house for the discovery of medicinal ingredients, which can greatly make up for the shortage of natural resources, which also makes the research of endophytes attract more researchers’ attention [8,125]. In order to fully develop the research and application of endophytes in medicinal plants, the following problems need to be solved urgently: 1. How to make the biocontrol strains survive in the environment outside the plant for a long time? 2. Can the reduced metabolic capacity and biological function of endophytes after multi-generation culturing be overcome by optimizing culture regulation? 3. In vitro endophytes can produce a variety of secondary metabolites to antagonize pathogenic bacteria and inhibit their growth. Can the development of new green pesticides be produced in large quantities with high efficiency? 4. How can we improve the fermentation efficiency of endophytes in medicinal plants to tap more abundant potential medicinal ingredients? To solve the above problems, exploring the interaction mechanism between endophytic bacteria and hosts as well as the metabolic mechanism of medicinal components and conducting more research on the application of endophytes in production will be of great help to the production of medicinal plants and the development of medicinal components.
Author Contributions
Conceptualization, J.J. and X.C.; methodology, X.C.; software, Y.W.; formal analysis, X.C.; investigation, J.J.; resources, J.J.; writing—original draft preparation, Y.W.; writing—review and editing, Y.W, X.C., Y.Z., H.C., L.L. and J.W.; visualization, C.L.; supervision, J.J.; project administration, J.J.; funding acquisition, J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 31770613) and the postgraduate research and practice innovation program of Jiangsu Province (Grant No. KYCX22_2822).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aye, M.M.; Aung, H.T.; Sein, M.M.; Armijos, C. A Review on the Phytochemistry, Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants. Molecules 2019, 24, 293. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Shen, T.; Wang, H.; Zhang, R.; Zhang, X.; Li, X.; Xiao, W. Challenges and opportunities for improving the druggability of natural product: Why need drug delivery system? Biomed. Pharmacother. 2023, 164, 114955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Wang, Y.L.; Yan, K.; Deng, Q.Q.; Li, F.Z.; Liang, X.J.; Hua, Q. Nanostructures in Chinese herbal medicines (CHMs) for potential therapy. Nanoscale Horiz. 2023, 8, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Noman, M.; Bano, U.; Akhtar, J.; Shaikh, Y.; Yar, M.S. Global uses of traditional herbs for hepatic diseases and other pharmacological actions: A comprehensive review. Polim. Med. 2023, 53, 81–89. [Google Scholar] [CrossRef]
- Wu, S.; Wang, C.; Bai, D.; Chen, N.; Hu, J.; Zhang, J. Perspectives of international multi-center clinical trials on traditional Chinese herbal medicine. Front. Pharmacol. 2023, 14, 1195364. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, P.; Dahiya, P.; Maheshwari, R.; Dang, A.S.; Suneja, P. Endophytism: A Multidimensional Approach to Plant-Prokaryotic Microbe Interaction. Front. Microbiol. 2022, 13, 861235. [Google Scholar] [CrossRef]
- Choudhary, M.; Gupta, S.; Dhar, M.K.; Kaul, S. Endophytic Fungi-Mediated Biocatalysis and Biotransformations Paving the Way Toward Green Chemistry. Front. Bioeng. Biotechnol. 2021, 9, 664705. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.S.; Patra, J.K. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef]
- Nalini, M.S.; Prakash, H.S. Diversity and bioprospecting of actinomycete endophytes from the medicinal plants. Lett. Appl. Microbiol. 2017, 64, 261–270. [Google Scholar] [CrossRef]
- Tshikhudo, P.P.; Ntushelo, K.; Mudau, F.N. Sustainable Applications of Endophytic Bacteria and Their Physiological/Biochemical Roles on Medicinal and Herbal Plants: Review. Microorganisms 2023, 11, 453. [Google Scholar] [CrossRef]
- Hashem, A.H.; Attia, M.S.; Kandil, E.K.; Fawzi, M.M.; Abdelrahman, A.S.; Khader, M.S.; Khodaira, M.A.; Emam, A.E.; Goma, M.A.; Abdelaziz, A.M. Bioactive compounds and biomedical applications of endophytic fungi: A recent review. Microb. Cell Fact. 2023, 22, 107. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.H.; Si, J.P.; Wu, L.S. Metabolites of medicine food homology-derived endophytic fungi and their activities. Curr. Res. Food Sci. 2022, 5, 1882–1896. [Google Scholar] [CrossRef]
- Tripathi, A.; Pandey, P.; Tripathi, S.N.; Kalra, A. Perspectives and potential applications of endophytic microorganisms in cultivation of medicinal and aromatic plants. Front. Plant Sci. 2022, 13, 985429. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhang, Q.L.; Hua, J.W.; Cheng, W.L.; Qin, L.P. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: A review. J. Ethnopharmacol. 2018, 226, 143–167. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, S.; Wu, J.; He, B.; Zhu, B.; Qin, L. Influence of tissue and geographic locality on culturable endophytic bacteria of Atractylodes macrocephala. Microbiology 2021, 167, 001109. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, M.; Cui, H.; Li, J.; Wang, M. Transcriptomic Landscape of Medicinal Dendrobium Reveals Genes Associated With the Biosynthesis of Bioactive Components. Front. Plant Sci. 2020, 11, 391. [Google Scholar] [CrossRef]
- Wu, W.; Lin, Y.; Farag, M.A.; Li, Z.; Shao, P. Dendrobium as a new natural source of bioactive for the prevention and treatment of digestive tract diseases: A comprehensive review with future perspectives. Phytomedicine 2023, 114, 154784. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Liu, J.M.; Sun, J.; Sun, Y.F.; Liu, J.N.; Jia, N.; Fan, B.; Dai, X.F. Diversity of culture-independent bacteria and antimicrobial activity of culturable endophytic bacteria isolated from different Dendrobium stems. Sci. Rep. 2019, 9, 10389. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit-A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-W.; Park, M.; Lee, H.-J. Mulberry (Morus alba L.) Leaf Extract and 1-Deoxynojirimycin Improve Skeletal Muscle Insulin Resistance via the Activation of IRS-1/PI3K/Akt Pathway in db/db Mice. Life 2022, 12, 1630. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Gao, Y.; Xue, J.; Yang, Y.; Yin, J.; Wu, T.; Zhang, M. Phytochemicals, Pharmacological Effects and Molecular Mechanisms of Mulberry. Foods 2022, 11, 1170. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, F.; Zhang, M.; Ou, T.; Wang, R.; Strobel, G.; Xiang, Z.; Zhou, Z.; Xie, J. Diversity of cultivable endophytic bacteria in mulberry and their potential for antimicrobial and plant growth-promoting activities. Microbiol. Res. 2019, 229, 126328. [Google Scholar] [CrossRef] [PubMed]
- Shurigin, V.; Alaylar, B.; Davranov, K.; Wirth, S.; Bellingrath-Kimura, S.D.; Egamberdieva, D. Diversity and biological activity of culturable endophytic bacteria associated with marigold (Calendula officinalis L.). AIMS Microbiol. 2021, 7, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, S.N.; Saikia, K.; Borah, A.; Thakur, D. Prospecting Endophytic Bacteria Endowed with Plant Growth Promoting Potential Isolated from Camellia sinensis. Front. Microbiol. 2021, 12, 738058. [Google Scholar] [CrossRef] [PubMed]
- Yarte, M.E.; Gismondi, M.I.; Llorente, B.E.; Larraburu, E.E. Isolation of endophytic bacteria from the medicinal, forestal and ornamental tree Handroanthus impetiginosus. Environ. Technol. 2022, 43, 1129–1139. [Google Scholar] [CrossRef]
- Borah, A.; Das, R.; Mazumdar, R.; Thakur, D. Culturable endophytic bacteria of Camellia species endowed with plant growth promoting characteristics. J. Appl. Microbiol. 2019, 127, 825–844. [Google Scholar] [CrossRef]
- de Oliveira, A.A.; Ramalho, M.O.; Moreau, C.S.; Campos, A.E.C.; Harakava, R.; Bueno, O.C. Exploring the diversity and potential interactions of bacterial and fungal endophytes associated with different cultivars of olive (Olea europaea) in Brazil. Microbiol. Res. 2022, 263, 127128. [Google Scholar] [CrossRef]
- Kolytaite, A.; Vaitiekunaite, D.; Antanyniene, R.; Baniulis, D.; Frercks, B. Monilinia fructigena Suppressing and Plant Growth Promoting Endophytic Pseudomonas spp. Bacteria Isolated from Plum. Microorganisms 2022, 10, 2402. [Google Scholar] [CrossRef]
- Ogbe, A.A.; Gupta, S.; Stirk, W.A.; Finnie, J.F.; Van Staden, J. Growth-Promoting Characteristics of Fungal and Bacterial Endophytes Isolated from a Drought-Tolerant Mint Species Endostemon obtusifolius (E. Mey. ex Benth.) NE Br. Plants 2023, 12, 638. [Google Scholar] [CrossRef]
- Mahlangu, S.G.; Tai, S.L. Morphological and molecular characterization of bacterial endophytes from Centella asiatica leaves. J. Genet. Eng. Biotechnol. 2022, 20, 171. [Google Scholar] [CrossRef] [PubMed]
- Priyanto, J.A.; Prastya, M.E.; Astuti, R.I.; Kristiana, R. The Antibacterial and Antibiofilm Activities of the Endophytic Bacteria Associated with Archidendron pauciflorum against Multidrug-Resistant Strains. Appl. Biochem. Biotechnol. 2023, 1–22. [Google Scholar] [CrossRef]
- Shahane, K.; Kshirsagar, M.; Tambe, S.; Jain, D.; Rout, S.; Ferreira, M.K.M.; Mali, S.; Amin, P.; Srivastav, P.P.; Cruz, J.; et al. An Updated Review on the Multifaceted Therapeutic Potential of Calendula officinalis L. Pharmaceuticals 2023, 16, 611. [Google Scholar] [CrossRef]
- Brimson, J.M.; Prasanth, M.I.; Kumaree, K.K.; Thitilertdecha, P.; Malar, D.S.; Tencomnao, T.; Prasansuklab, A. Tea Plant (Camellia sinensis): A Current Update on Use in Diabetes, Obesity, and Cardiovascular Disease. Nutrients 2022, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Silva-Junior, O.B.; Grattapaglia, D.; Novaes, E.; Collevatti, R.G. Genome assembly of the Pink Ipe (Handroanthus impetiginosus, Bignoniaceae), a highly valued, ecologically keystone Neotropical timber forest tree. Gigascience 2018, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Igwe, E.O.; Charlton, K.E. A Systematic Review on the Health Effects of Plums (Prunus domestica and Prunus salicina). Phytother. Res. 2016, 30, 701–731. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint essential oil: Its phytochemistry, biological activity, pharmacological effect and application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Punia, A.; Joshi, R.; Kumar, R. Identification and quantification of eight alkaloids in Aconitum heterophyllum using UHPLC-DAD-QTOF-IMS: A valuable tool for quality control. Phytochem. Anal. 2022, 33, 1121–1134. [Google Scholar] [CrossRef]
- Hafeez, S.; Yaqoob, S.; Magray, A.R.; Kamili, A.N.; Ganai, B.A. Molecular characterization of fungal endophyte diversity isolated from Aconitum heterophyllum: A critically endangered medicinal plant of Kashmir Himalaya. Int. Microbiol. 2023, 26, 651–662. [Google Scholar] [CrossRef]
- Matraszek-Gawron, R.; Chwil, M.; Terlecki, K.; Skoczylas, M.M. Current Knowledge of the Antidepressant Activity of Chemical Compounds from Crocus sativus L. Pharmaceuticals 2022, 16, 58. [Google Scholar] [CrossRef]
- Maqbool, Z.; Arshad, M.S.; Ali, A.; Aziz, A.; Khalid, W.; Afzal, M.F.; Bangar, S.P.; Addi, M.; Hano, C.; Lorenzo, J.M. Potential Role of Phytochemical Extract from Saffron in Development of Functional Foods and Protection of Brain-Related Disorders. Oxid. Med. Cell Longev. 2022, 2022, 6480590. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, J.; Zhang, J.; Zhu, Y.; Qin, L.; Zhu, B. Diversity of Culturable Endophytic Fungi in Crocus sativus and Their Correlation with Crocin Content. Curr. Microbiol. 2023, 80, 73. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.K.; Yang, J.S.; Huang, Y.F.; Liu, J.S.; Tsai, C.W.; Bau, D.T.; Chang, W.S. Culture Separation, Identification and Unique Anti-pathogenic Fungi Capacity of Endophytic Fungi from Gucheng Salvia Miltiorrhiza. In Vivo 2021, 35, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Sarsaiya, S.; Jain, A.; Jia, Q.; Fan, X.; Shu, F.; Chen, Z.; Zhou, Q.; Shi, J.; Chen, J. Molecular Identification of Endophytic Fungi and Their Pathogenicity Evaluation Against Dendrobium nobile and Dendrobium officinale. Int. J. Mol. Sci. 2020, 21, 316. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Ji, Y.; Peng, L.; Liu, Q.; Cao, H.; Yang, Y.; He, X.; Zeng, N. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Codonopsis pilosula: A review. Carbohydr. Polym. 2021, 261, 117863. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, Y.; Wang, X.; Leng, F.; Li, S.; Zhu, N.; Chen, K.; Wang, Y. Culturable endophytic fungi community structure isolated from Codonopsis pilosula roots and effect of season and geographic location on their structures. BMC Microbiol. 2023, 23, 132. [Google Scholar] [CrossRef]
- Dogra, N.K.; Kumar, S.; Kumar, D. Vernonia anthelmintica (L.) Willd.: An ethnomedicinal, phytochemical, pharmacological and toxicological review. J. Ethnopharmacol. 2020, 256, 112777. [Google Scholar] [CrossRef]
- Niu, L.; Rustamova, N.; Ning, H.; Paerhati, P.; Lu, C.; Yili, A. Diversity and Biological Activities of Endophytic Fungi from the Flowers of the Medicinal Plant Vernonia anthelmintica. Int. J. Mol. Sci. 2022, 23, 1935. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, S.; Tao, S.; Hou, G.; Zhao, F.; Tan, S.; Meng, Q. Dioscorea spp.: Bioactive Compounds and Potential for the Treatment of Inflammatory and Metabolic Diseases. Molecules 2023, 28, 2878. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, Y.; Li, C.; Wu, W.; Xu, Y.; Xia, W.; Huang, D.; Huang, X. Identification and genomic analyses of a novel endophytic actinobacterium Streptomyces endophytica sp. nov. with potential for biocontrol of yam anthracnose. Front. Microbiol. 2023, 14, 1139456. [Google Scholar] [CrossRef]
- Huang, L.; Lyu, Q.; Zheng, W.; Yang, Q.; Cao, G. Traditional application and modern pharmacological research of Eucommia ulmoides Oliv. Chin. Med. 2021, 16, 73. [Google Scholar] [CrossRef]
- Mo, P.; Chen, Y.; Zou, F.; Zhou, J.; Zou, W.; Gao, J. Nocardiopsis eucommiae sp. nov., a novel endophytic actinomycete isolated from leaves of Eucommia ulmoides Oliv. Int. J. Syst. Evol. Microbiol. 2022, 72, 005654. [Google Scholar] [CrossRef] [PubMed]
- Mo, P.; Li, K.; Zhou, J.; Zhou, F.; He, J.; Zou, W.; Gao, J. Nocardiopsis changdeensis sp. nov., an endophytic actinomycete isolated from the roots of Eucommia ulmoides Oliv. J. Antibiot. 2023, 76, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Musa, Z.; Ma, J.; Egamberdieva, D.; Abdelshafy Mohamad, O.A.; Abaydulla, G.; Liu, Y.; Li, W.J.; Li, L. Diversity and Antimicrobial Potential of Cultivable Endophytic Actinobacteria Associated with the Medicinal Plant Thymus roseus. Front. Microbiol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Yousefnia, S.; Naseri, D.; Seyed Forootan, F.; Tabatabaeian, M.; Moattar, F.; Ghafghazi, T.; Nasr Esfahani, M.H.; Ghaedi, K. Suppressive role of Viola odorata extract on malignant characters of mammosphere-derived breast cancer stem cells. Clin. Transl. Oncol. 2020, 22, 1619–1634. [Google Scholar] [CrossRef] [PubMed]
- Salwan, R.; Rana, A.; Saini, R.; Sharma, A.; Sharma, M.; Sharma, V. Diversity analysis of endophytes with antimicrobial and antioxidant potential from Viola odorata: An endemic plant species of the Himalayas. Braz. J. Microbiol. 2023, 1–14. [Google Scholar] [CrossRef]
- Han, J.; Zhang, S.; Jiang, B.; Wang, J.; Ge, X.; Wu, B.; Zhang, S.; Wang, D. Sesquiterpene lactones from Xanthium sibiricum Patrin alleviate asthma by modulating the Th1/Th2 balance in a murine model. Phytomedicine 2022, 99, 154032. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Y.; Wang, J.; Liu, K.; Tang, X.; Gao, J. Streptomyces xanthii sp. nov. and Streptomyces roseirectus sp. nov. isolated from a Chinese medicinal plant. Int. J. Syst. Evol. Microbiol. 2021, 71, 004962. [Google Scholar] [CrossRef] [PubMed]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant Growth Promoting and Biocontrol Activity of Streptomyces spp. as Endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef]
- Chen, M.S.; Chen, F.; Chen, X.H.; Zheng, Z.Q.; Ma, X.; Tuo, L. Nocardioides mangrovi sp. nov., a novel endophytic actinobacterium isolated from root of Kandelia candel. Int. J. Syst. Evol. Microbiol. 2022, 72, 005295. [Google Scholar] [CrossRef]
- Yan, X.R.; Chen, M.S.; Yang, C.; An, M.B.; Li, H.Y.; Shi, H.C.; Tuo, L. Nakamurella flava sp. nov., a novel endophytic actinobacterium isolated from Mentha haplocalyx Briq. Int. J. Syst. Evol. Microbiol. 2020, 70, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, D.; Lv, X.; Cheng, C.L.; Li, J.; Liang, W.; Xing, J.; Chen, W. A multilevel investigation to discover why Kandelia candel thrives in high salinity. Plant Cell Environ. 2016, 39, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Rout, P. Bioprospecting of underutilized mangrove fruits used by coastal communities in the Odisha coast, India: A review. Food Sci. Biotechnol. 2022, 31, 139–153. [Google Scholar] [CrossRef]
- He, X.F.; Geng, C.A.; Huang, X.Y.; Ma, Y.B.; Zhang, X.M.; Chen, J.J. Chemical Constituents from Mentha haplocalyx Briq. (Mentha canadensis L.) and Their α-Glucosidase Inhibitory Activities. Nat. Prod. Bioprospect. 2019, 9, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Gao, J.; Munir, I.; Zhang, M.; Liu, Y.; Moe, T.S.; Xue, J.; Zhang, X. Characterization of Endophytic Fungi, Acremonium sp., from Lilium davidii and Analysis of Its Antifungal and Plant Growth-Promoting Effects. BioMed Res. Int. 2021, 2021, 9930210. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, Q.; Wu, R.; Zhang, Y.; Wu, Q.; Li, M.; Ye, K.; Dai, W.; Huang, J. Biocontrol and plant growth promotion potential of endophytic Bacillus subtilis JY-7-2L on Aconitum carmichaelii Debx. Front. Microbiol. 2022, 13, 1059549. [Google Scholar] [CrossRef]
- Tao, L.; Qiuhong, L.; Fuqiang, Y.; Shuhui, Z.; Suohui, T.; Linyuan, F. Plant growth-promoting activities of bacterial endophytes isolated from the medicinal plant Pairs polyphylla var. yunnanensis. World J. Microbiol. Biotechnol. 2021, 38, 15. [Google Scholar] [CrossRef]
- Mathur, P.; Chaturvedi, P.; Sharma, C.; Bhatnagar, P. Improved seed germination and plant growth mediated by compounds synthesized by endophytic Aspergillus niger (isolate 29) isolated from Albizia lebbeck (L.) Benth. 3 Biotech. 2022, 12, 271. [Google Scholar] [CrossRef]
- Purushotham, N.; Jones, E.; Monk, J.; Ridgway, H. Community Structure of Endophytic Actinobacteria in a New Zealand Native Medicinal Plant Pseudowintera colorata (Horopito) and Their Influence on Plant Growth. Microb. Ecol. 2018, 76, 729–740. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted Interactions Between Endophytes and Plant: Developments and Prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Jia, M.; Chen, L.; Xin, H.L.; Zheng, C.J.; Rahman, K.; Han, T.; Qin, L.P. A Friendly Relationship between Endophytic Fungi and Medicinal Plants: A Systematic Review. Front. Microbiol. 2016, 7, 906. [Google Scholar] [CrossRef] [PubMed]
- Mili, C. Bioprospecting of endophytes associated with Solanum species: A mini review. Arch. Microbiol. 2023, 205, 254. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lang, D.; Wang, J.; Zhang, W.; Zhang, X. Plant-beneficial Streptomyces dioscori SF1 potential biocontrol and plant growth promotion in saline soil within the arid and semi-arid areas. Environ. Sci. Pollut. Res. Int. 2023, 30, 70194–70212. [Google Scholar] [CrossRef] [PubMed]
- Boonmahome, P.; Namwongsa, J.; Vorasoot, N.; Jogloy, S.; Riddech, N.; Boonlue, S.; Mongkolthanaruk, W. Single and co-inoculum of endophytic bacteria promote growth and yield of Jerusalem artichoke through upregulation of plant genes under drought stress. PLoS ONE 2023, 18, e0286625. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Pu, Q.; Zhang, C.; Chen, Y.; Lin, Z.; Hu, X.; Li, O. Complete genome of Sphingomonas paucimobilis ZJSH1, an endophytic bacterium from Dendrobium officinale with stress resistance and growth promotion potential. Arch. Microbiol. 2023, 205, 132. [Google Scholar] [CrossRef]
- Li, M.; Ren, Y.; He, C.; Yao, J.; Wei, M.; He, X. Complementary Effects of Dark Septate Endophytes and Trichoderma Strains on Growth and Active Ingredient Accumulation of Astragalus mongholicus under Drought Stress. J. Fungi 2022, 8, 920. [Google Scholar] [CrossRef]
- Wang, J.F.; Hou, W.P.; Christensen, M.J.; Li, X.Z.; Xia, C.; Li, C.J.; Nan, Z.B. Role of Epichloe Endophytes in Improving Host Grass Resistance Ability and Soil Properties. J. Agr. Food Chem. 2020, 68, 6944–6955. [Google Scholar] [CrossRef]
- Wu, W.; Chen, W.H.; Liu, S.Y.; Wu, J.J.; Zhu, Y.T.; Qin, L.P.; Zhu, B. Beneficial Relationships between Endophytic Bacteria and Medicinal Plants. Front. Plant Sci. 2021, 12, 646146. [Google Scholar] [CrossRef]
- Godara, H.; Ramakrishna, W. Endophytes as nature’s gift to plants to combat abiotic stresses. Lett. Appl. Microbiol. 2023, 76, ovac067. [Google Scholar] [CrossRef]
- Chen, H.; Chen, J.; Qi, Y.; Chu, S.; Ma, Y.; Xu, L.; Lv, S.; Zhang, H.; Yang, D.; Zhu, Y.; et al. Endophytic fungus Cladosporium tenuissimum DF11, an efficient inducer of tanshinone biosynthesis in Salvia miltiorrhiza roots. Phytochemistry 2022, 194, 113021. [Google Scholar] [CrossRef]
- Xu, W.; Jin, X.; Yang, M.; Xue, S.; Luo, L.; Cao, X.; Zhang, C.; Qiao, S.; Zhang, C.; Li, J.; et al. Primary and secondary metabolites produced in Salvia miltiorrhiza hairy roots by an endophytic fungal elicitor from Mucor fragilis. Plant Physiol. Biochem. 2021, 160, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Masoudi Khorasani, F.; Ganjeali, A.; Asili, J.; Cheniany, M. Beneficial effects of endophytic fungi inoculation on tanshinones and phenolic compounds of Salvia abrotanoides. Iran. J. Basic Med. Sci. 2023, 26, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.T.; Luo, S.Q.; Yang, Z.N.; Wang, Y.S.; Ding, Q.; Wang, K.F.; Yang, S.X.; Wang, Y. Endophytic fungi stimulate the concentration of medicinal secondary metabolites in houttuynia cordata thunb. Plant Signal Behav. 2021, 16, 1929731. [Google Scholar] [CrossRef]
- Xie, X.G.; Zhang, Z.Z.; Chen, L.; Ming, Q.L.; Sheng, K.X.; Chen, X.; Rahman, K.; Feng, K.M.; Su, J.; Han, T. An endophytic fungus Schizophyllum commune isolated from Panax ginseng enhances hairy roots growth and ginsenoside biosynthesis. Can. J. Microbiol. 2023, 69, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Palanichamy, P.; Krishnamoorthy, G.; Kannan, S.; Marudhamuthu, M. Bioactive potential of secondary metabolites derived from medicinal plant endophytes. Egypt. J. Basic Appl. Sci. 2019, 5, 303–312. [Google Scholar] [CrossRef]
- Santra, H.K.; Banerjee, D. Antifungal activity of volatile and non-volatile metabolites of endophytes of Chloranthus elatior Sw. Front. Plant Sci. 2023, 14, 1156323. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Bashir, A.; Farooq, S.; Riyaz-Ul-Hassan, S. Burkholderia gladioli E39CS3, an endophyte of Crocus sativus Linn., induces host resistance against corm-rot caused by Fusarium oxysporum. J. Appl. Microbiol. 2022, 132, 495–508. [Google Scholar] [CrossRef]
- Anand, U.; Pal, T.; Yadav, N.; Singh, V.K.; Tripathi, V.; Choudhary, K.K.; Shukla, A.K.; Sunita, K.; Kumar, A.; Bontempi, E.; et al. Current Scenario and Future Prospects of Endophytic Microbes: Promising Candidates for Abiotic and Biotic Stress Management for Agricultural and Environmental Sustainability. Microb. Ecol. 2023, 1–32. [Google Scholar] [CrossRef]
- Rajendran, S.; Robertson, L.P.; Kosgahakumbura, L.; Fernando, C.; Goransson, U.; Wang, H.; Hettiarachchi, C.; Gunasekera, S. Antibacterial eremophilane sesquiterpenoids from Xylaria feejeensis, an endophytic fungi of the medicinal plant Geophila repens. Fitoterapia 2023, 167, 105496. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, G.; Li, Z.; Zhou, Y.; Gao, N. Screening saikosaponin d (SSd)-producing endophytic fungi from Bupleurum scorzonerifolium Willd. World J. Microbiol. Biotechnol. 2022, 38, 242. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.P.; Liu, S.F.; Yin, C.Y.; Tang, D.Y.; Li, Y.H.; Zhang, L.X. 7-Methoxy-13-dehydroxypaxilline: New indole diterpenoid from an endophytic fungus Penicillium sp. Nb 19. Nat. Prod. Res. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Zhang, S.; Liu, L.; Yang, Z.; Zhao, F.; Tian, Y. Antimicrobial Potential of Endophytic Fungi from Artemisia argyi and Bioactive Metabolites from Diaporthe sp. AC1. Front. Microbiol. 2022, 13, 908836. [Google Scholar] [CrossRef] [PubMed]
- Santra, H.K.; Maity, S.; Banerjee, D. Production of Bioactive Compounds with Broad Spectrum Bactericidal Action, Bio-Film Inhibition and Antilarval Potential by the Secondary Metabolites of the Endophytic Fungus Cochliobolus sp. APS1 Isolated from the Indian Medicinal Herb Andrographis paniculata. Molecules 2022, 27, 1459. [Google Scholar] [CrossRef] [PubMed]
- Yehia, R.S. Multi-Function of a New Bioactive Secondary Metabolite Derived from Endophytic Fungus Colletotrichum acutatum of Angelica sinensis. J. Microbiol. Biotechnol. 2023, 33, 806–822. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, Z.; Song, L.; Fu, W.; Liu, L. Anti-Alzheimer’s Natural Products Derived from Plant Endophytic Fungi. Molecules 2023, 28, 2259. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.; Mahmoud, B.K.; Millan-Aguinaga, N.; Abdelmohsen, U.R.; Fouad, M.A. The endophytic Fusarium strains: A treasure trove of natural products. RSC Adv. 2023, 13, 1339–1369. [Google Scholar] [CrossRef]
- Wang, Z.C.; Wang, L.; Pan, Y.P.; Zheng, X.X.; Liang, X.N.; Sheng, L.L.; Zhang, D.; Sun, Q.; Wang, Q. Research advances on endophytic fungi and their bioactive metabolites. Bioproc. Biosyst. Eng. 2023, 46, 165–170. [Google Scholar] [CrossRef]
- Nzimande, B.; Makhwitine, J.P.; Mkhwanazi, N.P.; Ndlovu, S.I. Developments in Exploring Fungal Secondary Metabolites as Antiviral Compounds and Advances in HIV-1 Inhibitor Screening Assays. Viruses 2023, 15, 1039. [Google Scholar] [CrossRef]
- Xu, F.; Wang, S.; Li, Y.; Zheng, M.; Xi, X.; Cao, H.; Cui, X.; Guo, H.; Han, C. Yield enhancement strategies of rare pharmaceutical metabolites from endophytes. Biotechnol. Lett. 2018, 40, 797–807. [Google Scholar] [CrossRef]
- Mursyidah, A.K.; Hafizzudin-Fedeli, M.; Muhammad, N.A.N.; Latiff, A.; Firdaus-Raih, M.; Wan, K.L. Dissecting the Biology of Rafflesia Species: Current Progress and Future Directions Made Possible with High-Throughput Sequencing Data. Plant Cell Physiol. 2023, 64, 368–377. [Google Scholar] [CrossRef]
- Riva, V.; Mapelli, F.; Bagnasco, A.; Mengoni, A.; Borin, S. A Meta-Analysis Approach to Defining the Culturable Core of Plant Endophytic Bacterial Communities. Appl. Environ. Microbiol. 2022, 88, e0253721. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, C.M.; Ju, X.Y.; Xiong, Y.W.; Xing, K.; Qin, S. Community Composition and Metabolic Potential of Endophytic Actinobacteria from Coastal Salt Marsh Plants in Jiangsu, China. Front. Microbiol. 2019, 10, 1063. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.J.; Miao, L.Y.; Fan, S.P.; Lv, P.W.; Lin, A.H.; Geng, H.; Song, F.J.; Zhang, P. New insights into the composition and diversity of endophytic bacteria in cultivated Huperzia serrata. Can. J. Microbiol. 2023, 69, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, J.B.A.; Lorenzi, A.S.; do Vale, H.M.M. Methods used for the study of endophytic fungi: A review on methodologies and challenges, and associated tips. Arch. Microbiol. 2022, 204, 675. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Exploring the potentialities of beneficial endophytes for improved plant growth. Saudi J. Biol. Sci. 2020, 27, 3622–3633. [Google Scholar] [CrossRef]
- Eevers, N.; Gielen, M.; Sanchez-Lopez, A.; Jaspers, S.; White, J.C.; Vangronsveld, J.; Weyens, N. Optimization of isolation and cultivation of bacterial endophytes through addition of plant extract to nutrient media. Microb. Biotechnol. 2015, 8, 707–715. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front. Pharmacol. 2020, 10, 1480. [Google Scholar] [CrossRef]
- Applequist, W.L.; Brinckmann, J.A.; Cunningham, A.B.; Hart, R.E.; Heinrich, M.; Katerere, D.R.; van Andel, T. Scientists & apos; Warning on Climate Change and Medicinal Plants. Planta Med. 2020, 86, 10–18. [Google Scholar] [CrossRef]
- Cheng, Q.Q.; Ouyang, Y.; Tang, Z.Y.; Lao, C.C.; Zhang, Y.Y.; Cheng, C.S.; Zhou, H. Review on the Development and Applications of Medicinal Plant Genomes. Front. Plant Sci. 2021, 12, 791219. [Google Scholar] [CrossRef]
- Hilario, S.; Goncalves, M.F.M. Endophytic Diaporthe as Promising Leads for the Development of Biopesticides and Biofertilizers for a Sustainable Agriculture. Microorganisms 2022, 10, 2453. [Google Scholar] [CrossRef]
- Dwibedi, V.; Rath, S.K.; Joshi, M.; Kaur, R.; Kaur, G.; Singh, D.; Kaur, G.; Kaur, S. Microbial endophytes: Application towards sustainable agriculture and food security. Appl. Microbiol. Biotechnol. 2022, 106, 5359–5384. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Thapa, S.; Mahawar, H.; Kumar, D.; Geat, N.; Singh, S.K. Prospecting potential of endophytes for modulation of biosynthesis of therapeutic bioactive secondary metabolites and plant growth promotion of medicinal and aromatic plants. Antonie Van Leeuwenhoek 2022, 115, 699–730. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.D.; Liu, J.N.; Chen, C.; Mo, X.L.; Tan, Q.; He, Y.; Wang, Z.K.; Yin, J.; Zhou, G.Y. The Multifunctions and Future Prospects of Endophytes and Their Metabolites in Plant Disease Management. Microorganisms 2022, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhu, H.; Liu, L.; Lin, J.; Tang, K. A review: Recent advances and future prospects of taxol-producing endophytic fungi. Appl. Microbiol. Biotechnol. 2010, 86, 1707–1717. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; El-Sayed, M.T.; Rady, A.; Zein, N.; Enan, G.; Shindia, A.; El-Hefnawy, S.; Sitohy, M.; Sitohy, B. Exploiting the Biosynthetic Potency of Taxol from Fungal Endophytes of Conifers Plants; Genome Mining and Metabolic Manipulation. Molecules 2020, 25, 3000. [Google Scholar] [CrossRef]
- Cao, X.; Xu, L.; Wang, J.; Dong, M.; Xu, C.; Kai, G.; Wan, W.; Jiang, J. Endophytic fungus Pseudodidymocyrtis lobariellae KL27 promotes taxol biosynthesis and accumulation in Taxus chinensis. BMC Plant Biol. 2022, 22, 12. [Google Scholar] [CrossRef]
- Gill, H.; Vasundhara, M. Isolation of taxol producing endophytic fungus Alternaria brassicicola from non-Taxus medicinal plant Terminalia arjuna. World J. Microbiol. Biotechnol. 2019, 35, 74. [Google Scholar] [CrossRef]
- Subramanian, M.; Marudhamuthu, M. Hitherto Unknown Terpene Synthase Organization in Taxol-Producing Endophytic Bacteria Isolated from Marine Macroalgae. Curr. Microbiol. 2020, 77, 918–923. [Google Scholar] [CrossRef]
- Liu, J.; Liu, G. Analysis of Secondary Metabolites from Plant Endophytic Fungi. Methods Mol. Biol. 2018, 1848, 25–38. [Google Scholar] [CrossRef]
- Sharma, H.; Rai, A.K.; Dahiya, D.; Chettri, R.; Nigam, P.S. Exploring endophytes for in vitro synthesis of bioactive compounds similar to metabolites produced in vivo by host plants. AIMS Microbiol. 2021, 7, 175–199. [Google Scholar] [CrossRef] [PubMed]
- Sabzehzari, M.; Zeinali, M.; Naghavi, M.R. Alternative sources and metabolic engineering of Taxol: Advances and future perspectives. Biotechnol. Adv. 2020, 43, 107569. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Anand, U.; Lopez-Bucio, J.; Radha; Kumar, M.; Lal, M.K.; Tiwari, R.K.; Dey, A. Biostimulants and environmental stress mitigation in crops: A novel and emerging approach for agricultural sustainability under climate change. Environ. Res. 2023, 233, 116357. [Google Scholar] [CrossRef] [PubMed]
- Fontana, D.C.; de Paula, S.; Torres, A.G.; de Souza, V.H.M.; Pascholati, S.F.; Schmidt, D.; Neto, D.D. Endophytic Fungi: Biological Control and Induced Resistance to Phytopathogens and Abiotic Stresses. Pathogens 2021, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Kumar, V.; Prasher, I.B.; Sethi, M.; Raj, H.; Ranjan, H.; Chand, S.; Pandey, G.K. Bioactive molecules from fungal endophytes and their applications in pharmaceutical industries: Challenges and future scope. J. Basic Microb. 2023, 63, 690–708. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).