The Role of Magnetic Resonance Imaging and Computed Tomography in Spinal Cord Injury
Abstract
1. Introduction
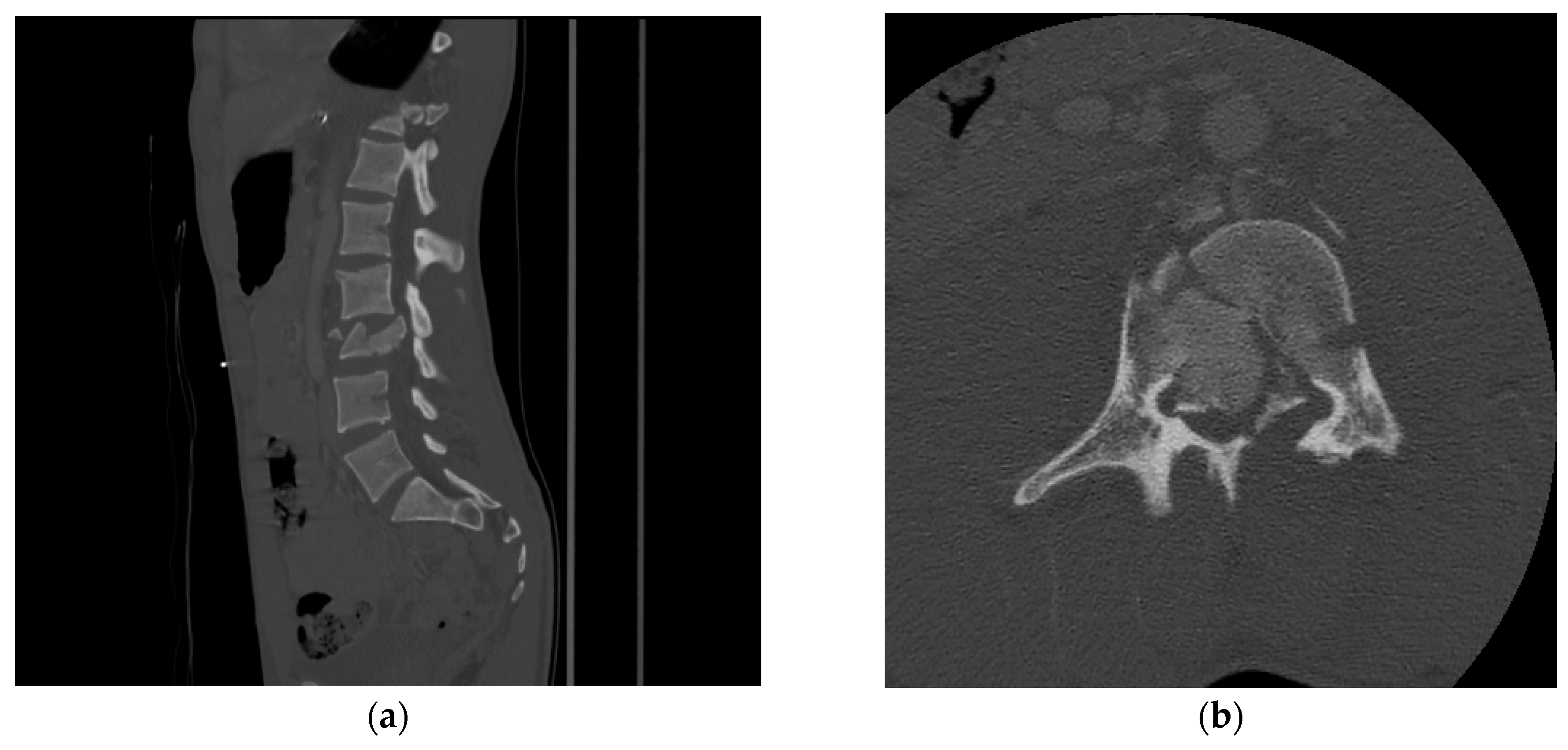
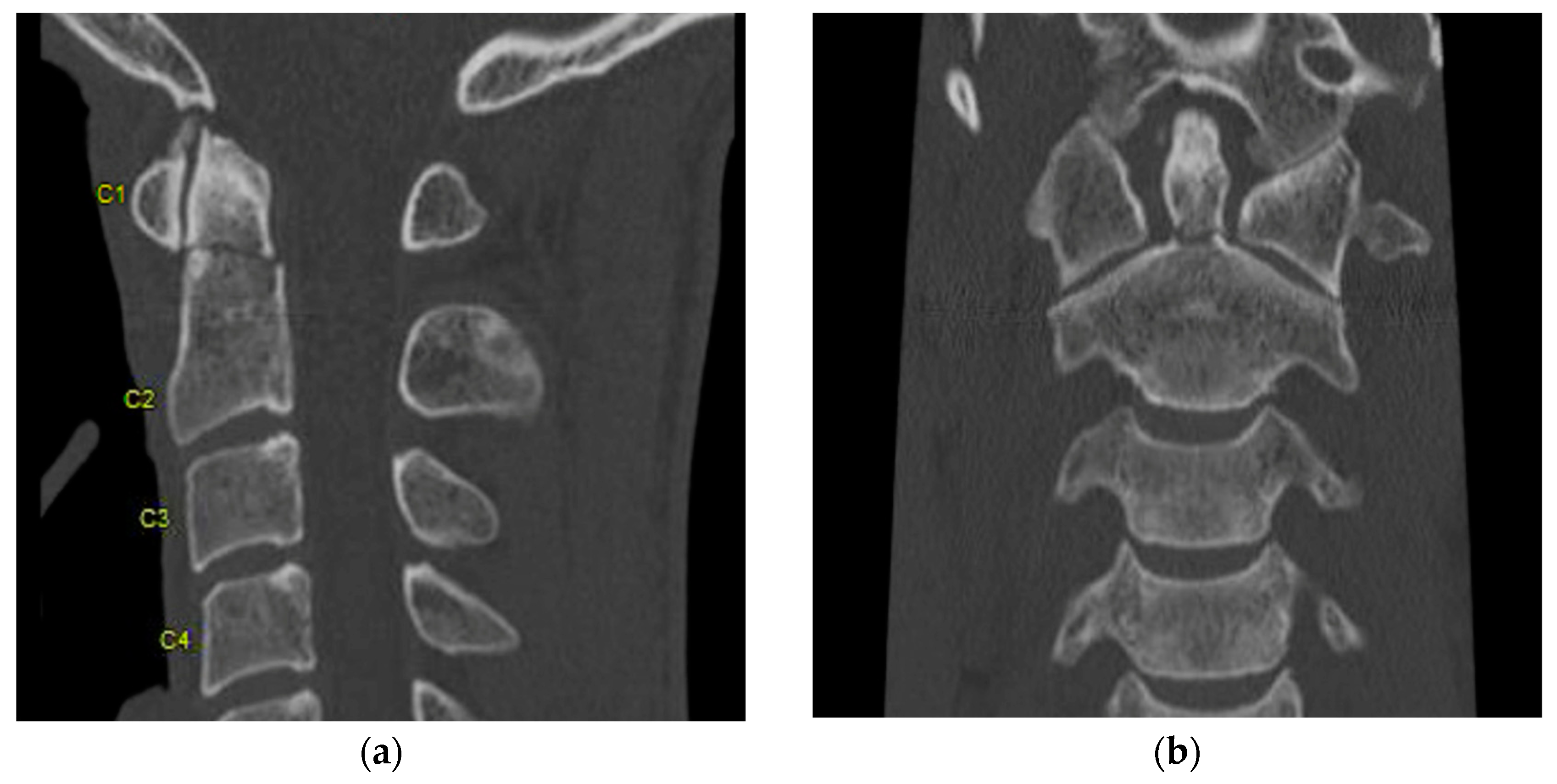
2. Computed Tomography (CT)
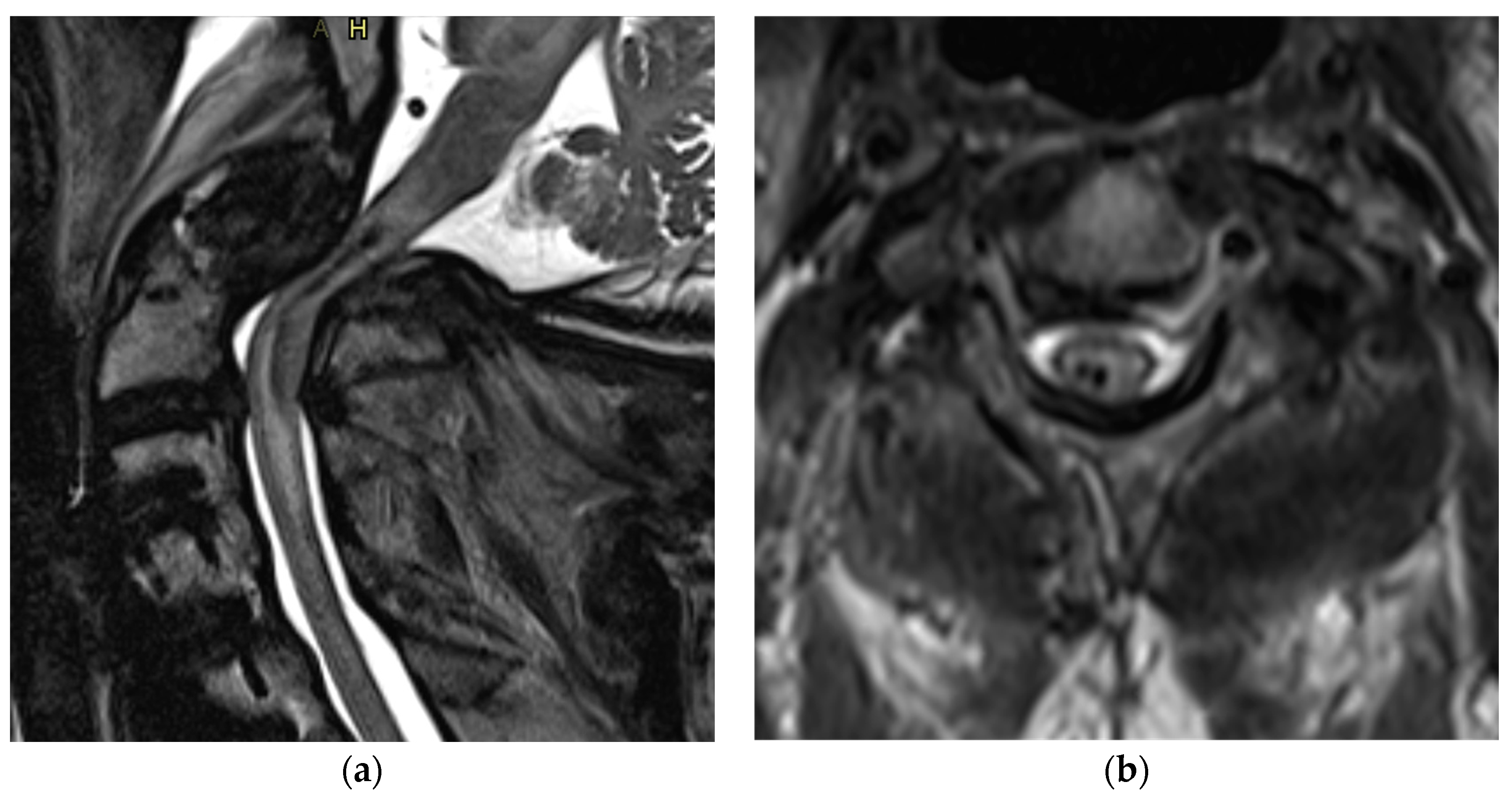
3. Magnetic Resonance Imaging (MRI)
4. Diffusion Weighted Imaging (DWI)
5. Functional MRI (fMRI)
6. Perfusion MRI
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Den Hauwe, L.S.P.; Flanders, A.E. Spinal Trauma and Spinal Cord Injury (SCI). In Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging [Internet]; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; Springer: Cham, Germany, 2020; Chapter 19; Available online: https://www.ncbi.nlm.nih.gov/books/NBK554330/ (accessed on 15 February 2020).
- Shabani, S.; Kaushal, M.; Soliman, H.M.; Nguyen, H.S.; Aarabi, B.; Fehlings, M.G.; Kotter, M.; Kwon, B.K.; Harrop, J.S.; Kurpad, S.N. AOSpine Global Survey: International Trends in Utilization of Magnetic Resonance Imaging/Computed Tomography for Spinal Trauma and Spinal Cord Injury across AO Regions. J. Neurotrauma 2019, 36, 3323–3331. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.W. Principles of CT and CT Technology. J. Nucl. Med. Technol. 2007, 35, 115–128, quiz 129–130. [Google Scholar] [CrossRef]
- Goldberg, A.L.; Kershah, S.M. Advances in Imaging of Vertebral and Spinal Cord Injury. J. Spinal Cord Med. 2010, 33, 105–116. [Google Scholar] [CrossRef]
- Riedel, C.H.; Zoubie, J.; Ulmer, S.; Gierthmuehlen, J.; Jansen, O. Thin-slice reconstructions of nonenhanced CT images allow for detection of thrombus in acute stroke. Stroke 2012, 43, 2319–2323. [Google Scholar] [CrossRef] [PubMed]
- Daffner, R.H.; Hackney, D.B. ACR Appropriateness Criteria® on Suspected Spine Trauma. J. Am. Coll. Radiol. 2007, 4, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Platzer, P.; Jaindl, M.; Thalhammer, G.; Dittrich, S.; Wieland, T.; Vecsei, V.; Gaebler, C. Clearing the cervical spine in critically injured patients: A comprehensive C-spine protocol to avoid unnecessary delays in diagnosis. Eur. Spine J. 2006, 15, 1801–1810. [Google Scholar] [CrossRef]
- Wintermark, M.; Mouhsine, E.; Theumann, N.; Mordasini, P.; van Melle, G.; Leyvraz, P.F.; Schnyder, P. Thoracolumbar Spine Fractures in Patients Who Have Sustained Severe Trauma: Depiction with Multi–Detector Row CT. Radiology 2003, 227, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Glover, G.H. Overview of Functional Magnetic Resonance Imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef]
- Shah, N.G.; Keraliya, A.; Nunez, D.B.; Schoenfeld, A.; Harris, M.B.; Bono, C.M.; Khurana, B. Injuries to the Rigid Spine: What the Spine Surgeon Wants to Know. RadioGraphics 2019, 39, 449–466. [Google Scholar] [CrossRef]
- Girão, M.M.V.; Miyahara, L.K.; Dwan, V.S.Y.; Baptista, E.; Taneja, A.K.; Gotfryd, A.; Castro, A.D.A. Imaging features of the postoperative spine: A guide to basic understanding of spine surgical procedures. Insights Imaging 2023, 14, 103. [Google Scholar] [CrossRef]
- Pomerantz, S.R. Myelography: Modern technique and indications. Handb. Clin. Neurol. 2016, 135, 193–208. [Google Scholar] [PubMed]
- Esquivel, A.; Ferrero, A.; Mileto, A.; Baffour, F.; Horst, K.; Rajiah, P.S.; Inoue, A.; Leng, S.; McCollough, C.; Fletcher, J.G. Photon-Counting Detector CT: Key Points Radiologists Should Know. Korean J. Radiol. 2022, 23, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Sundaram, M.; Subhas, N. Dual-Energy CT in Musculoskeletal Imaging: What Is the Role Beyond Gout? Am. J. Roentgenol. 2019, 213, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Cicero, G.; Ascenti, G.; Albrecht, M.H.; Blandino, A.; Cavallaro, M.; D’angelo, T.; Carerj, M.L.; Vogl, T.J.; Mazziotti, S. Extra-abdominal dual-energy CT applications: A comprehensive overview. La Radiol. Medica 2020, 125, 384–397. [Google Scholar]
- Foti, G.; Serra, G.; Iacono, V.; Zorzi, C. Identification of Traumatic Bone Marrow Oedema: The Pearls and Pitfalls of Dual-Energy CT (DECT). Tomography 2021, 7, 387–396. [Google Scholar] [CrossRef]
- Bäcker, H.C.; Wu, C.H.; Perka, C.; Panics, G. Dual-Energy Computed Tomography in Spine Fractures: A Systematic Review and Meta-Analysis. Int. J. Spine Surg. 2021, 15, 525–535. [Google Scholar] [CrossRef]
- Cavallaro, M.; D’Angelo, T.; Albrecht, M.H.; Yel, I.; Martin, S.S.; Wichmann, J.L.; Lenga, L.; Mazziotti, S.; Blandino, A.; Ascenti, G.; et al. Comprehensive comparison of dual-energy computed tomography and magnetic resonance imaging for the assessment of bone marrow edema and fracture lines in acute vertebral fractures. Eur. Radiol. 2022, 32, 561–571. [Google Scholar] [CrossRef]
- Edward Boas, D.F. CT artifacts: Cases and reduction techniques. Imaging Med. 2012, 4, 229–240. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Rao, S.C.; Tator, C.H.; Skaf, G.; Arnold, P.; Benzel, E.; Dickman, C.; Cuddy, B.; Green, B.; Hitchon, P.; et al. The optimal radiologic method for assessing spinal canal compromise and cord compression in patients with cervi-cal spinal cord injury. Part II: Results of a multicenter study. Spine 1999, 24, 605–613. [Google Scholar] [CrossRef]
- Magu, S.; Singh, D.; Yadav, R.K.; Bala, M. Evaluation of Traumatic Spine by Magnetic Resonance Imaging and Correlation with Neurological Recovery. Asian Spine J. 2015, 9, 748–756. [Google Scholar] [CrossRef]
- Maynard, F.M., Jr.; Bracken, M.B.; Creasey, G.; Ditunno, J.F., Jr.; Donovan, W.H.; Ducker, T.B.; Garber, S.L.; Marino, R.J.; Stover, S.L.; Tator, C.H.; et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. Spinal Cord 1997, 35, 266–274. [Google Scholar] [CrossRef]
- Bondurant, F.J.; Cotler, H.B.; Kulkarni, M.V.; McArdle, C.B.; Harris, J.H. Acute spinal cord injury. A study using physical examination and magnetic resonance imaging. Spine 1990, 15, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Marciello, M.A.; Flanders, A.E.; Herbison, G.J.; Schaefer, D.M.; Friedman, D.P.; Lane, J.I. Magnetic resonance imaging related to neurologic outcome in cervical spinal cord injury. Arch. Phys. Med. Rehabil. 1993, 74, 940–946. [Google Scholar] [PubMed]
- Yamashita, Y.; Takahashi, M.; Matsuno, Y.; Kojima, R.; Sakamoto, Y.; Oguni, T.; Sakae, T.; Kim, E.E. Acute spinal cord injury: Magnetic resonance imaging correlated with myelopathy. Br. J. Radiol. 1991, 64, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Sliker, C.W.; Mirvis, S.E.; Shanmuganathan, K. Assessing Cervical Spine Stability in Obtunded Blunt Trauma Patients: Review of Medical Literature. Radiology 2005, 234, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari-Rafi, A.; Peterson, C.; Leon-Rojas, J.E.; Tadokoro, N.; Lange, S.F.; Kaushal, M.; Tetreault, L.; Fehlings, M.G.; Martin, A.R. The Role of Magnetic Resonance Imaging to Inform Clinical Decision-Making in Acute Spinal Cord Injury: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 4948. [Google Scholar] [CrossRef]
- Bozzo, A.; Marcoux, J.; Radhakrishna, M.; Pelletier, J.; Goulet, B.; Malomo, T.; Brown, A.A.; Bale, K.; Yung, A.; Kozlowski, P.; et al. The Role of Magnetic Resonance Imaging in the Management of Acute Spinal Cord Injury. J. Neurotrauma 2011, 28, 1401–1411. [Google Scholar] [CrossRef]
- Haefeli, J.; Mabray, M.; Whetstone, W.; Dhall, S.; Pan, J.; Upadhyayula, P.; Manley, G.; Bresnahan, J.; Beattie, M.; Ferguson, A.; et al. Multivariate Analysis of MRI Biomarkers for Predicting Neurologic Impairment in Cervical Spinal Cord Injury. Am. J. Neuroradiol. 2017, 38, 648–655. [Google Scholar] [CrossRef]
- Kumar, Y.; Hayashi, D. Role of magnetic resonance imaging in acute spinal trauma: A pictorial review. BMC Musculoskelet. Disord. 2016, 17, 310. [Google Scholar] [CrossRef]
- Flanders, A.E.; Spettell, C.M.; Tartaglino, L.M.; Friedman, D.P.; Herbison, G.J. Forecasting motor recovery after cervical spinal cord injury: Value of MR imaging. Radiology 1996, 201, 649–655. [Google Scholar] [CrossRef]
- Hackney, D.B.; Asato, R.; Joseph, P.M.; Carvlin, M.J.; McGrath, J.T.; Grossman, R.I.; Kassab, E.A.; DeSimone, D. Hemorrhage and edema in acute spinal cord compression: Demonstration by MR imaging. Radiology 1986, 161, 387–390. [Google Scholar] [CrossRef]
- Wang, M.; Dai, Y.; Han, Y.; Haacke, E.M.; Dai, J.; Shi, D. Susceptibility weighted imaging in detecting hemorrhage in acute cervical spinal cord injury. Magn. Reson. Imaging 2011, 29, 365–373. [Google Scholar] [CrossRef]
- Kulkarni, M.V.; McArdle, C.B.; Kopanicky, D.; Miner, M.; Cotler, H.B.; Lee, K.F.; Harris, J.H. Acute spinal cord injury: MR imaging at 1.5 T. Radiology 1987, 164, 837–843. [Google Scholar] [CrossRef]
- Mabray, M.C.; Talbott, J.F.; Whetstone, W.D.; Dhall, S.S.; Phillips, D.B.; Pan, J.Z.; Manley, G.T.; Bresnahan, J.C.; Beattie, M.S.; Haefeli, J.; et al. Multidimensional Analysis of Magnetic Resonance Imaging Predicts Early Impairment in Thoracic and Thoracol-umbar Spinal Cord Injury. J. Neurotrauma. 2016, 33, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Talbott, J.F.; Whetstone, W.D.; Readdy, W.J.; Ferguson, A.R.; Bresnahan, J.C.; Saigal, R.; Hawryluk, G.W.J.; Beattie, M.S.; Mabray, M.C.; Pan, J.Z.; et al. The Brain and Spinal Injury Center score: A novel, simple, and reproducible method for assessing the severity of acute cervical spinal cord injury with axial T2-weighted MRI findings. J. Neurosurg. Spine 2015, 23, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, D.M.; Flanders, A.E.; Osterholm, J.L.; Northrup, B.E. Prognostic significance of magnetic resonance imaging in the acute phase of cervical spine injury. J. Neurosurg. 1992, 76, 218–223. [Google Scholar] [CrossRef]
- Flanders, A.E.; Spettell, C.M.; Friedman, D.P.; Marino, R.J.; Herbison, G.J. The Relationship between the Functional Abilities of Patients with Cervical Spinal Cord Injury and the Severity of Damage Revealed by MR Imaging. Am. J. Neuroradiol. 1999, 20, 926–934. [Google Scholar]
- Boldin, C.; Raith, J.; Fankhauser, F.; Haunschmid, C.; Schwantzer, G.; Schweighofer, F. Predicting Neurologic Recovery in Cervical Spinal Cord Injury with Postoperative MR Imaging. Spine 2006, 31, 554–559. [Google Scholar] [CrossRef]
- Ramón, S.; Domínguez, R.; Ramírez, L.; Paraira, M.; Olona, M.; Castelló, T.; Fernández, L.G. Clinical and magnetic resonance imaging correlation in acute spinal cord injury. Spinal Cord 1997, 35, 664–673. [Google Scholar] [CrossRef]
- Kaushal, M.; Shabani, S.; Budde, M.D.; Kurpad, S.N. Diffusion Tensor Imaging in Acute Spinal Cord Injury: A Review of Animal and Human Studies. J. Neurotrauma 2019, 36, 2279–2286. [Google Scholar] [CrossRef]
- Cheran, S.; Shanmuganathan, K.; Zhuo, J.; Mirvis, S.E.; Aarabi, B.; Alexander, M.T.; Gullapalli, R.P. Correlation of MR Diffusion Tensor Imaging Parameters with ASIA Motor Scores in Hemorrhagic and Nonhemorrhagic Acute Spinal Cord Injury. J. Neurotrauma 2011, 28, 1881–1892. [Google Scholar] [CrossRef]
- Shanmuganathan, K.; Zhuo, J.; Chen, H.H.; Aarabi, B.; Adams, J.; Miller, C.; Menakar, J.; Gullapalli, R.P.; Mirvis, S.E. Diffusion Tensor Imaging Parameter Obtained during Acute Blunt Cervical Spinal Cord Injury in Pre-dicting Long-Term Outcome. J. Neurotrauma. 2017, 34, 2964–2971. [Google Scholar] [CrossRef] [PubMed]
- Shabani, S.; Kaushal, M.; Budde, M.; Kurpad, S.N. Correlation of magnetic resonance diffusion tensor imaging parameters with American Spinal Injury Association score for prognostication and long-term outcomes. Neurosurg. Focus 2019, 46, E2. [Google Scholar] [CrossRef] [PubMed]
- Poplawski, M.M.; Alizadeh, M.; Oleson, C.V.; Fisher, J.; Marino, R.J.; Gorniak, R.J.; Leiby, B.E.; Flanders, A.E. Application of Diffusion Tensor Imaging in Forecasting Neurological Injury and Recovery after Human Cervi-cal Spinal Cord Injury. J. Neurotrauma. 2019, 36, 3051–3061. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, F.; Riederer, S.J.; Pelc, N.J. Analysis of T2 limitations and off-resonance effects on spatial resolution and artifacts in echo-planar imaging. Magn. Reson. Med. 1990, 14, 123–139. [Google Scholar] [CrossRef]
- Jezzard, P.; Barnett, A.S.; Pierpaoli, C. Characterization of and correction for eddy current artifacts in echo planar diffusion imaging. Magn. Reason. Med. 1998, 39, 801–812. [Google Scholar] [CrossRef]
- Skinner, N.P.; Kurpad, S.N.; Schmit, B.D.; Muftuler, L.T.; Budde, M.D. Rapid in vivo detection of rat spinal cord injury with double-diffusion-encoded magnetic resonance spectroscopy. Magn. Reson. Med. 2017, 77, 1639–1649. [Google Scholar] [CrossRef]
- Skinner, N.P.; Lee, S.-Y.; Kurpad, S.N.; Schmit, B.D.; Muftuler, L.T.; Budde, M.D. Filter-probe diffusion imaging improves spinal cord injury outcome prediction. Ann. Neurol. 2018, 84, 37–50. [Google Scholar] [CrossRef]
- Krzyżak, A.T.; Olejniczak, Z. Improving the accuracy of PGSE DTI experiments using the spatial distribution of b matrix. Magn. Reson. Imaging 2015, 33, 286–295. [Google Scholar] [CrossRef]
- Freund, P.; Seif, M.; Weiskopf, N.; Friston, K.; Fehlings, M.G.; Thompson, A.J.; Curt, A. MRI in traumatic spinal cord injury: From clinical assessment to neuroimaging biomarkers. Lancet Neurol. 2019, 18, 1123–1135. [Google Scholar] [CrossRef]
- Landelle, C.; Lungu, O.; Vahdat, S.; Kavounoudias, A.; Marchand-Pauvert, V.; De Leener, B.; Doyon, J. Investigating the human spinal sensorimotor pathways through functional magnetic resonance imaging. Neuroimage 2021, 245, 118684. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Aleksanderek, I.; Cohen-Adad, J.; Tarmohamed, Z.; Tetreault, L.; Smith, N.; Cadotte, D.W.; Crawley, A.; Ginsberg, H.; Mikulis, D.J.; et al. Translating state-of-the-art spinal cord MRI techniques to clinical use: A systematic review of clinical studies uti-lizing DTI, MT, MWF, MRS, and fMRI. NeuroImage: Clin. 2016, 10, 192–238. [Google Scholar] [CrossRef] [PubMed]
- Petrella, J.R.; Provenzale, J.M. MR Perfusion Imaging of the Brain. Am. J. Roentgenol. 2000, 175, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.P.; Hirschler, L.; Lee, S.; Kurpad, S.N.; Warnking, J.M.; Barbier, E.L.; Budde, M.D. Optimized cervical spinal cord perfusion MRI after traumatic injury in the rat. J. Cereb. Blood Flow Metab. 2021, 41, 2010–2025. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.P.; Lee, S.-Y.; Kurpad, S.N.; Budde, M.D. Differential Trajectory of Diffusion and Perfusion Magnetic Resonance Imaging of Rat Spinal Cord Injury. J. Neurotrauma. 2022, 40, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Wilkins, N.; Schmit, B.D.; Kurpad, S.N.; Budde, M.D. Relationships between spinal cord blood flow measured with flow-sensitive alternating inversion recovery (FAIR) and neurobehavioral outcomes in rat spinal cord injury. Magn. Reson. Imaging 2021, 78, 42–51. [Google Scholar] [CrossRef] [PubMed]


| Classification Scheme | Type | Name |
|---|---|---|
| Kulkarni et al. (1987) [34] MRI patterns for prognosticating acute spinal cord injury | Pattern 1 | Cord hemorrhage |
| Pattern 2 | Cord edema | |
| Pattern 3 | Mixed | |
| Bondurant et al. (1990) [23] classification scheme for prognostication of acute spinal cord injury | Pattern 1 | Normal MRI signal |
| Pattern 2 | Single level edema | |
| Pattern 3 | Multi-level edema | |
| Pattern 4 | Mixed hemorrhage and edema | |
| Schaefer et al. (1992) [37] types of MRI findings associated with acute spinal cord injury | Type 1 | Central intramedullary cord hemorrhage |
| Type 2 | T2 hyperintense contusion extending longitudinally greater than 1 vertebral body | |
| Type 3 | T2 hyperintense contusion, confined to a single vertebral body | |
| Type 4 | No evidence of SCI on MRI | |
| The Brain and Spinal Injury Center Score (BASIC) for classifying acute SCIs on the basis of axial T2-weighted imaging | Basic 0 | No intramedullary cord signal abnormality |
| Basic 1 | Intramedullary T2 hyperintensity confined to central gray matter | |
| Basic 2 | Intramedullary T2 hyperintensity involves both gray and white matter but does not cover the entire transverse extent of the spinal cord | |
| Basic 3 | Intramedullary T2 hyperintensity covers the entire transverse extent of the spinal cord | |
| Basic 4 | Intramedullary T2 hyperintensity covers the entire transverse extent of the spinal cord plus T2 hypointense foci |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, O.; Kaushal, M.; Agarwal, N.; Kurpad, S.; Shabani, S. The Role of Magnetic Resonance Imaging and Computed Tomography in Spinal Cord Injury. Life 2023, 13, 1680. https://doi.org/10.3390/life13081680
Hussain O, Kaushal M, Agarwal N, Kurpad S, Shabani S. The Role of Magnetic Resonance Imaging and Computed Tomography in Spinal Cord Injury. Life. 2023; 13(8):1680. https://doi.org/10.3390/life13081680
Chicago/Turabian StyleHussain, Omar, Mayank Kaushal, Nitin Agarwal, Shekar Kurpad, and Saman Shabani. 2023. "The Role of Magnetic Resonance Imaging and Computed Tomography in Spinal Cord Injury" Life 13, no. 8: 1680. https://doi.org/10.3390/life13081680
APA StyleHussain, O., Kaushal, M., Agarwal, N., Kurpad, S., & Shabani, S. (2023). The Role of Magnetic Resonance Imaging and Computed Tomography in Spinal Cord Injury. Life, 13(8), 1680. https://doi.org/10.3390/life13081680





