Warfarin and Antibiotics: Drug Interactions and Clinical Considerations
Abstract
1. Introduction
2. Methods
3. Results
3.1. Penicillins
3.2. Fluoroquinolones
3.3. Cephalosporins
3.4. TMP-SMX
3.5. Rifampin
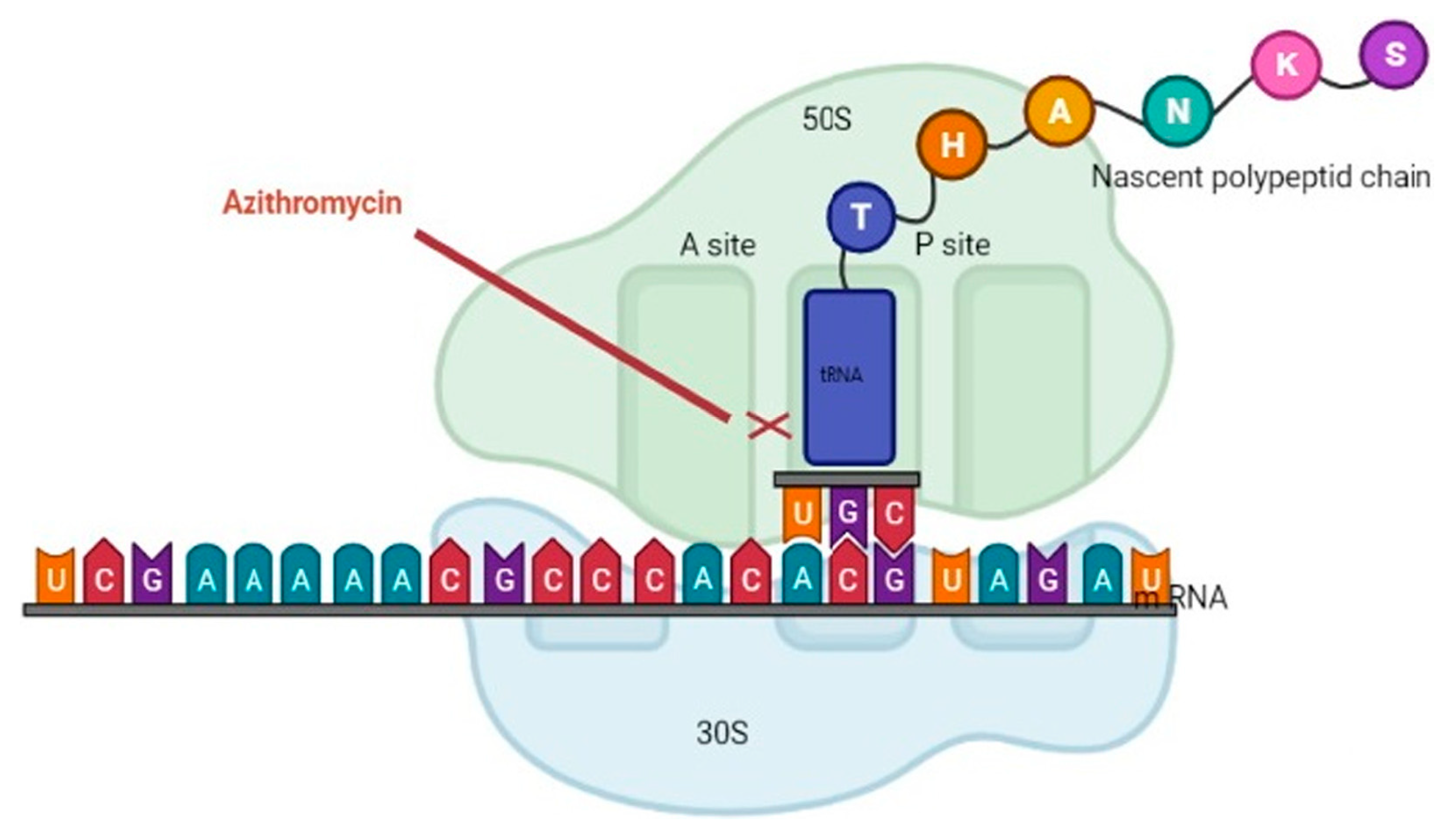
3.6. Macrolides
3.7. Metronidazole
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holbrook, A.M.; Pereira, J.A.; Labiris, R.; McDonald, H.; Douketis, J.D.; Crowther, M.; Wells, P.S. Systematic Overview of Warfarin and Its Drug and Food Interactions. Arch. Intern. Med. 2005, 165, 1095–1106. [Google Scholar] [CrossRef]
- Pirmohamed, M. Warfarin: Almost 60 years old and still causing problems. Br. J. Clin. Pharmacol. 2006, 62, 509–511. [Google Scholar] [CrossRef]
- Antithrombotic Therapy in Atrial Fibrillation—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0012369215607875?via%3Dihub (accessed on 23 July 2023).
- Coffey, S.; Roberts-Thomson, R.; Brown, A.; Carapetis, J.; Chen, M.; Enriquez-Sarano, M.; Zühlke, L.; Prendergast, B.D. Global epidemiology of valvular heart disease. Nat. Rev. Cardiol. 2021, 18, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Canestaro, W.J.; Patrick, A.R.; Avorn, J.; Ito, K.; Matlin, O.S.; Brennan, T.A.; Shrank, W.H.; Choudhry, N.K. Cost-Effectiveness of Oral Anticoagulants for Treatment of Atrial Fibrillation. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Heart Disease and Stroke Statistics—2023 Update: A Report from the American Heart Association | Circulation. Available online: https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001123 (accessed on 23 July 2023).
- Navar, A.M.; Kolkailah, A.A.; Overton, R.; Shah, N.P.; Rousseau, J.F.; Flaker, G.C.; Pignone, M.P.; Peterson, E.D. Trends in Oral Anticoagulant Use Among 436 864 Patients with Atrial Fibrillation in Community Practice, 2011 to 2020. J. Am. Heart Assoc. 2022, 11, e026723. Available online: https://www.ahajournals.org/doi/10.1161/JAHA.122.026723 (accessed on 23 July 2023). [CrossRef]
- Mannarino, M.G. Cost of warfarin versus direct oral anticoagulants. Can. Fam. Physician 2020, 66, 9. [Google Scholar]
- Patel, S.; Singh, R.; Preuss, C.V.; Patel, N. Warfarin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK470313/ (accessed on 27 March 2023).
- Baillargeon, J.; Holmes, H.M.; Lin, Y.L.; Raji, M.A.; Sharma, G.; Kuo, Y.F. Concurrent Use of Warfarin and Antibiotics and the Risk of Bleeding in Older Adults. Am. J. Med. 2012, 125, 183–189. [Google Scholar] [CrossRef]
- Lane, M.A.; Zeringue, A.; McDonald, J.R. Serious Bleeding Events due to Warfarin and Antibiotic Co-prescription in a Cohort of Veterans. Am. J. Med. 2014, 127, 657–663.e2. [Google Scholar] [CrossRef]
- Yip, D.W.; Gerriets, V. Penicillin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK554560/ (accessed on 27 March 2023).
- Kim, K.Y.; Frey, R.J.; Epplen, K.; Foruhari, F. Interaction between warfarin and nafcillin: Case report and review of the literature. Pharmacotherapy 2007, 27, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Davydov, L.; Yermolnik, M.; Cuni, L.J. Warfarin and Amoxicillin/Clavulanate Drug Interaction. Ann. Pharmacother. 2003, 37, 367–370. Available online: https://journals.sagepub.com/doi/10.1345/aph.1C243?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 15 July 2023). [CrossRef] [PubMed]
- Bandrowsky, T.; Vorono, A.A.; Borris, T.J.; Marcantoni, H.W. Amoxicillin-related postextraction bleeding in an anticoagulated patient with tranexamic acid rinses. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 82, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.D.; Deeble, T. Warfarin: Dangers with antibiotics. Dent. Update 1993, 350, 352–353. [Google Scholar]
- Abdel-Aziz, M.I.; Ali, M.A.S.; Hassan, A.K.M.; Elfaham, T.H. Warfarin-drug interactions: An emphasis on influence of polypharmacy and high doses of amoxicillin/clavulanate. J. Clin. Pharmacol. 2016, 56, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Baggio, D.; Ananda-Rajah, M.R. Fluoroquinolone antibiotics and adverse events. Aust. Prescr. 2021, 44, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.B.; Fugate, S.E. Levofloxacin and Warfarin Interaction. Ann. Pharmacother. 2002, 36, 1554–1557. [Google Scholar] [CrossRef]
- Mercadal Orfila, G.; Gracia García, B.; Leiva Badosa, E.; Perayre Badía, M.; Reynaldo Martínez, C.; Jodar Masanés, R. Retrospective assessment of potential interaction between levofloxacin and warfarin. Pharm. World Sci. 2009, 31, 224–229. [Google Scholar] [CrossRef]
- Bui, T.; Preuss, C.V. Cephalosporins. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK551517/ (accessed on 27 March 2023).
- Bohm, N.M.; Crosby, B. Hemarthrosis in a Patient on Warfarin Receiving Ceftaroline: A Case Report and Brief Review of Cephalosporin Interactions with Warfarin. Ann. Pharmacother. 2012, 46, e19. [Google Scholar] [CrossRef]
- Saum, L.M.; Balmat, R.P. Ceftriaxone Potentiates Warfarin Activity Greater Than Other Antibiotics in the Treatment of Urinary Tract Infections. J. Pharm. Pract. 2016, 29, 121–124. [Google Scholar] [CrossRef]
- Schelleman, H.; Bilker, W.B.; Brensinger, C.M.; Han, X.; Kimmel, S.E.; Hennessy, S. Warfarin with fluoroquinolones, sulfonamides, or azole antifungals: Interactions and the risk of hospitalization for gastrointestinal bleeding. Clin. Pharmacol. Ther. 2008, 84, 581–588. [Google Scholar] [CrossRef]
- Hemorrhage during Warfarin Therapy Associated with Cotrimoxazole and Other Urinary Tract Anti-Infective Agents: A Population-Based Study. Available online: https://reference.medscape.com/medline/abstract/20386005 (accessed on 12 April 2023).
- Vitry, A.I.; Roughead, E.E.; Ramsay, E.N.; Preiss, A.K.; Ryan, P.; Gilbert, A.L.; Caughey, G.E.; Shakib, S.; Esterman, A.; Zhang, Y.; et al. Major bleeding risk associated with warfarin and co-medications in the elderly population. Pharmacoepidemiol. Drug Saf. 2011, 20, 1057–1063. [Google Scholar] [CrossRef]
- Yang, C.S.; Boswell, R.; Bungard, T.J. A case series of the rifampin-warfarin drug interaction: Focus on practical warfarin management. Eur. J. Clin. Pharmacol. 2021, 77, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.H.; Hashmi, M.F. Macrolides. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK551495/ (accessed on 28 March 2023).
- Vázquez-Laslop, N.; Mankin, A.S. How macrolide antibiotics work. Trends Biochem. Sci. 2018, 43, 668–684. [Google Scholar] [CrossRef] [PubMed]
- BPJ 44: The Appropriate Use of Macrolides. Available online: https://bpac.org.nz/BPJ/2012/May/macrolides.aspx (accessed on 28 March 2023).
- Johnson, M.C.; Wood, M.; Vaughn, V.; Cowan, L.; Sharkey, A.M. Interaction of Antibiotics and Warfarin in Pediatric Cardiology Patients. Pediatr. Cardiol. 2005, 26, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Heidary, M.; Samangani, A.E.; Kargari, A.; Nejad, A.K.; Yashmi, I.; Motahar, M.; Taki, E.; Khoshnood, S. Mechanism of action, resistance, synergism, and clinical implications of azithromycin. J. Clin. Lab. Anal. 2022, 36, e24427. [Google Scholar] [CrossRef]
- Dhand, A.; Snydman, D. Mechanism of Resistance in Metronidazole. In Antimicrobial Drug Resistance; Humana Press: Totowa, NJ, USA, 2009; pp. 223–227. [Google Scholar]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Wells, P.S.; Holbrook, A.M.; Crowther, N.R.; Hirsh, J. Interactions of Warfarin with Drugs and Food. Ann. Intern. Med. 1994, 121, 676–683. [Google Scholar] [CrossRef]
- Martín-Pérez, M.; Gaist, D.; Abajo FJ de Rodríguez, L.A.G. Population Impact of Drug Interactions with Warfarin: A Real-World Data Approach. Thromb. Haemost. 2018, 118, 461–470. [Google Scholar] [CrossRef]
- Gurwitz, J.H.; Avorn, J.; Ross-Degnan, D.; Choodnovskiy, I.; Ansell, J. Aging and the Anticoagulant Response to Warfarin Therapy. Ann. Intern. Med. 1992, 116, 901–904. [Google Scholar] [CrossRef]
- Froom, P.; Hermoni, D.; Barak, M. Oral anticoagulation treatment—control, risks, benefits, and informed consent. Harefuah 2003, 142, 56–60. [Google Scholar]
- Torn, M.; Rosendaal, F.R. Oral anticoagulation in surgical procedures: Risks and recommendations. Br. J. Haematol. 2003, 123, 676–682. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.I.; Ali, M.A.S.; Hassan, A.K.M.; Elfaham, T.H. Factors influencing warfarin response in hospitalized patients. Saudi. Pharm. J. SPJ 2015, 23, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Wade, S.L. Flucloxacillin-warfarin interaction: An under-appreciated phenomenon. Intern. Med. J. 2018, 48, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Mannheimer, B.; Stage, T.B.; Pottegård, A.; Lindh, J.D. The Effect of Flucloxacillin on Warfarin Anticoagulation: A Swedish Register-Based Nationwide Cohort Study. Thromb. Haemost. 2019, 119, 1617–1623, Erratum in Thromb. Haemost. 2019, 119, e1. [Google Scholar] [CrossRef] [PubMed]
- Hellfritzsch, M.; Lund, L.C.; Ennis, Z.; Stage, T.; Damkier, P.; Bliddal, M.; Jensen, P.B.; Henriksen, D.; Ernst, M.T.; Olesen, M.; et al. Ischemic Stroke and Systemic Embolism in Warfarin Users With Atrial Fibrillation or Heart Valve Replacement Exposed to Dicloxacillin or Flucloxacillin. Clin. Pharmacol. Ther. 2020, 107, 607–616. [Google Scholar] [CrossRef]
| Study Name | Year | Type of Study | Participants | Methods | Results | Conclusions |
|---|---|---|---|---|---|---|
| Chaudhuri A, et al. [41] | 2018 | Retrospective study | Patients who were receiving flucloxacillin/other antibiotics for the minimum of 14 days | Variation in warfarin dose was measured | Patients treated with flucloxacillin had a remarkable enhancement in warfarin dose in the last 7 days of antibiotic therapy. No notable change in warfarin dose for cases on other antibiotics was reported. | International normalized ratio (INR) monitoring is necessary for cases on a continued flucloxacillin treatment. |
| Mannheimer B, et al. [42] | 2019 | Retrospective study | Patients who were treated with warfarin | INR values and warfarin doses were measured daily. | For patients with 10 days of treatment, the proportion of cases with a subtherapeutic INR of <2 increased from 22% in the week preceding. In patients with 30 days of treatment, the proportion enhanced from 34 to 63% by 42 days. | INR monitoring is required in patients taking flucloxacillin. |
| Hellfritzsch M, et al. [43] | 2020 | Cohort study | 212,182 warfarin users | Hazard ratios by evaluating 21-day risks of stroke/ embolism were assessed. | In comparison with phenoxymethylpenicillin, dicloxacillin/flucloxacillin was correlated with hazard ratios of stroke/embolism. | Dicloxacillin needs to be prescribed with caution in cases getting warfarin. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega, A.J.; Smith, C.; Matejowsky, H.G.; Thornhill, K.J.; Borne, G.E.; Mosieri, C.N.; Shekoohi, S.; Cornett, E.M.; Kaye, A.D. Warfarin and Antibiotics: Drug Interactions and Clinical Considerations. Life 2023, 13, 1661. https://doi.org/10.3390/life13081661
Vega AJ, Smith C, Matejowsky HG, Thornhill KJ, Borne GE, Mosieri CN, Shekoohi S, Cornett EM, Kaye AD. Warfarin and Antibiotics: Drug Interactions and Clinical Considerations. Life. 2023; 13(8):1661. https://doi.org/10.3390/life13081661
Chicago/Turabian StyleVega, Alexis J., Caitlin Smith, Hannah Grace Matejowsky, Katherine J. Thornhill, Grant E. Borne, Chizoba N. Mosieri, Sahar Shekoohi, Elyse M. Cornett, and Alan D. Kaye. 2023. "Warfarin and Antibiotics: Drug Interactions and Clinical Considerations" Life 13, no. 8: 1661. https://doi.org/10.3390/life13081661
APA StyleVega, A. J., Smith, C., Matejowsky, H. G., Thornhill, K. J., Borne, G. E., Mosieri, C. N., Shekoohi, S., Cornett, E. M., & Kaye, A. D. (2023). Warfarin and Antibiotics: Drug Interactions and Clinical Considerations. Life, 13(8), 1661. https://doi.org/10.3390/life13081661








