Hemostatic Effects of Raloxifene in Ovariectomized Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Ovariectomy
2.4. Experimental Design
2.5. Blood and Plasma Collection
2.6. Determination of Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT)
2.7. Standard Curve of Fibrinogen
2.8. Effects of Raloxifene and Estradiol on Blood Coagulation Factors VII, X, and XI
2.9. Determination of the Effects of Raloxifene and Estradiol on Fibrinogen Concentrations
2.10. Statistical Methods
3. Results
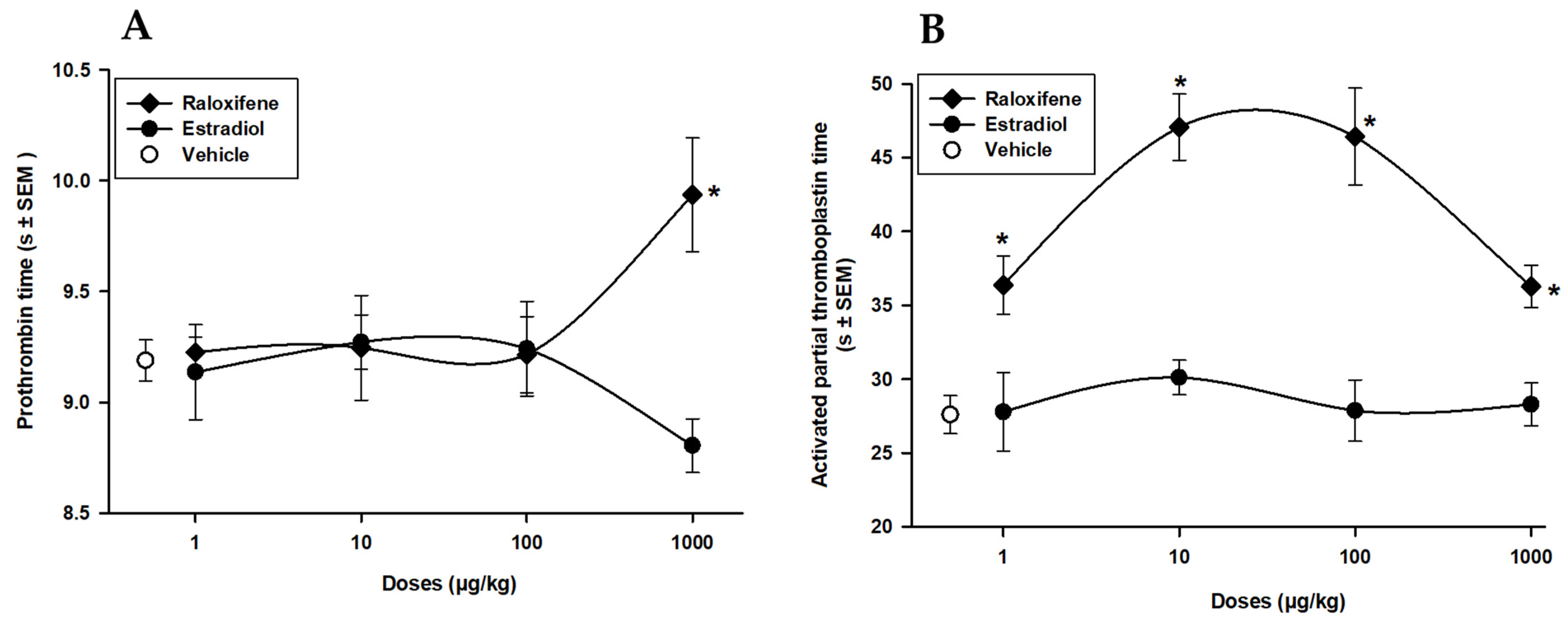
3.1. Raloxifene and Estradiol Effects on PT and APTT Screening Tests
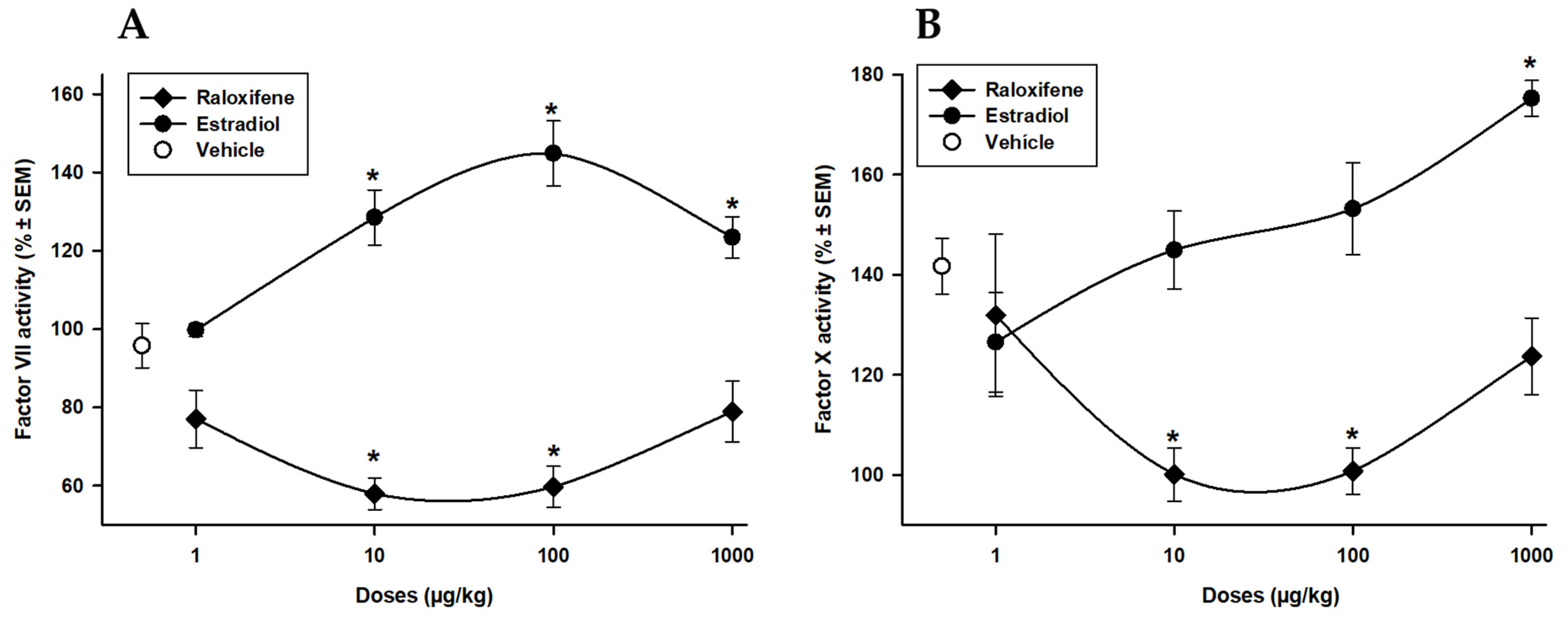
3.2. Raloxifene and Estradiol Effects on Extrinsic Factor VII and Common Pathway Factor X
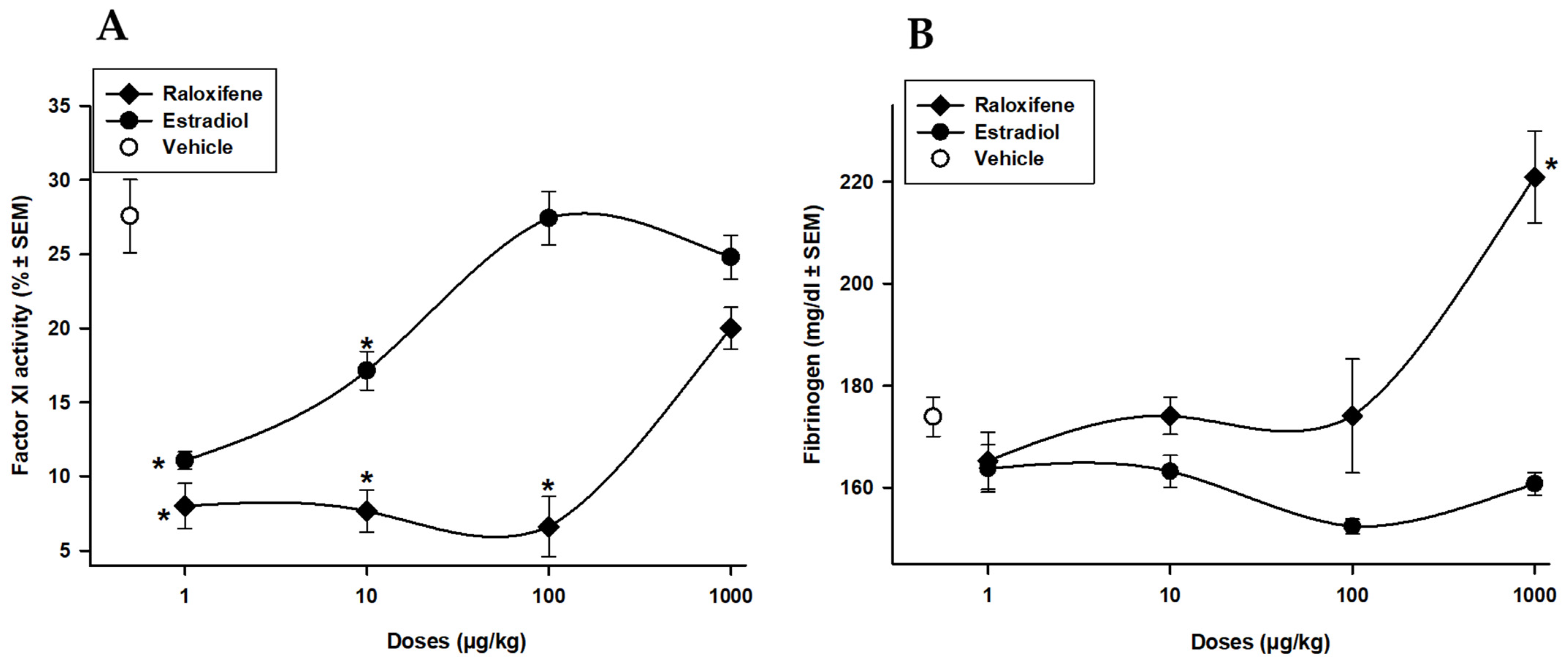
3.3. Raloxifene and Estradiol Effects on Intrinsic Factor XI and Fibrinogen Concentration
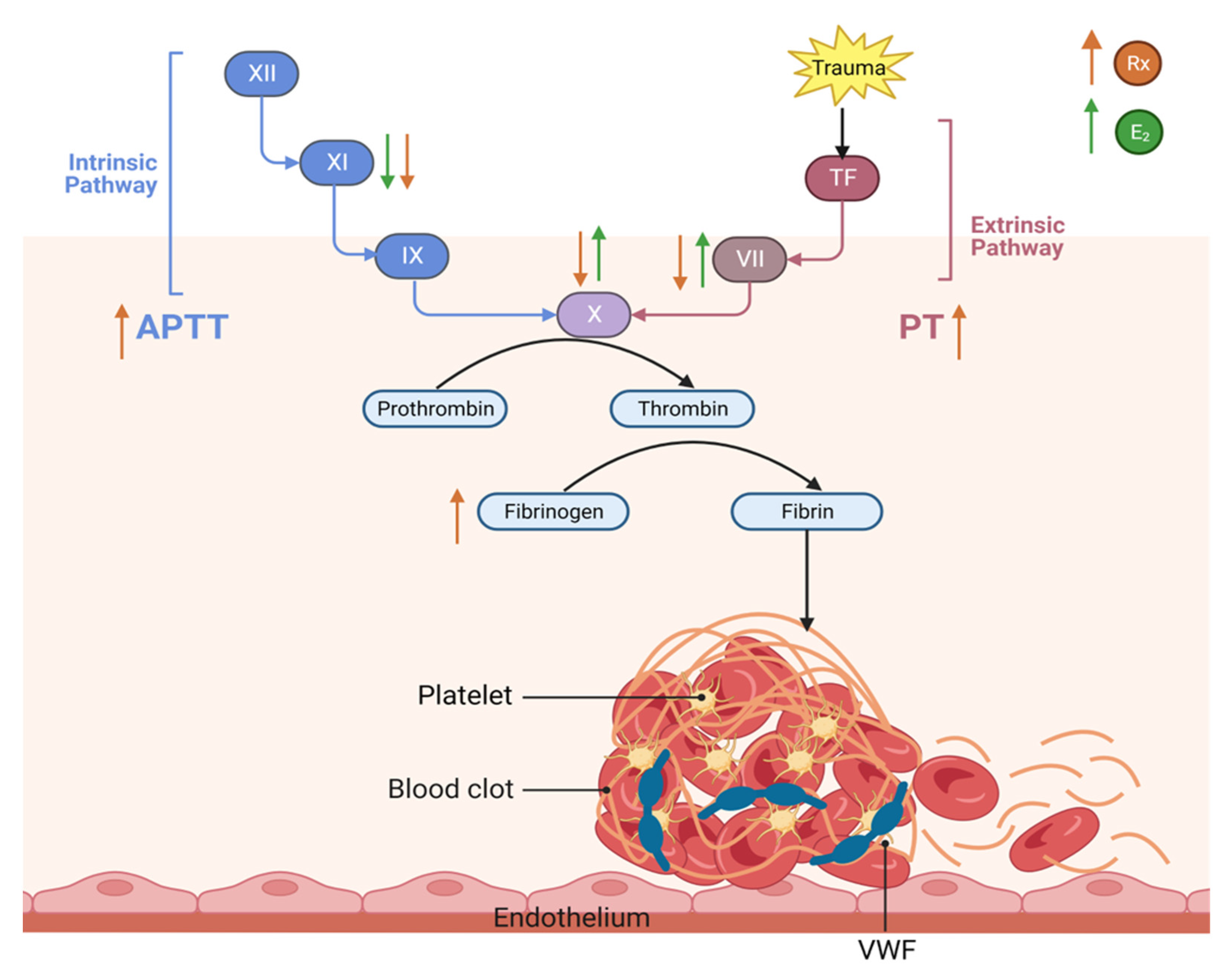
3.4. This Diagram Illustrates Our Current Understanding of the Effect of Raloxifene and Estrogen on Hemostasis and Thrombosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Biol. 2003, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Hernández, E.; Valera, R.; Alonzo, E.; Bajares-Lilue, M.; Carlini, R.; Capriles, F.; Martinis, R.; Bellorin-Font, E.; Weisinger, J.R. Effects of raloxifene on bone metabolism and serum lipids in postmenopausal women on chronic hemodialysis. Kidney Int. 2003, 63, 2269–2274. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S.; Jordan, V.C. Selective estrogen receptor modulators (SERMs): Mechanisms of anticarcinogenesis and drug resistance. Mutat. Res. 2005, 591, 247–263. [Google Scholar] [CrossRef]
- Ang, Z.; He, X.; Zhang, Y. The determination of raloxifene in rat tissue using HPLC. Biomed. Chromatogr. 2007, 21, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Abu-Fanne, R.; Brzezinski, A.; Golomb, M.; Grad, E.; Foldes, A.J.; Shufaro, Y.; Varon, D.; Brill, A.; Lotan, C.; Danenberg, H.D. Effects of estradiol and raloxifene on arterial thrombosis in ovariectomized mice. Menopause 2008, 15, 98–104. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Simental-Mendía, L.E.; Reiner, Z.; Banach, M.; Sahebkar, A. Raloxifene lowers plasma lipoprotein(a) concentrations: A systemic review and meta-analysis of randomized placebo-controlled trials. Cardiovasc. Drugs Ther. 2017, 31, 197–208. [Google Scholar] [CrossRef]
- Meyer, M.R.; Barton, M. Estrogens and coronary artery disease: New clinical perspectives. Adv. Pharmacol. 2016, 77, 307–360. [Google Scholar]
- Barrett-Connor, E.; Mosca, L.; Collins, P.; Geiger, M.J.; Grady, D.; Kornitzer, M.; McNabb, M.A.; Wenger, N.K. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N. Engl. J. Med. 2006, 355, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Gizzo, S.; Saccardi, C.; Patrelli, T.S.; Berretta, R.; Capobianco, G.; Di Gangi, S.; Vacilotto, A.; Bertocco, A.; Noventa, M.; Ancona, E. Update on raloxifene: Mechanism of action, clinical efficacy, adverse effects, and contraindications. Obstet. Gynecol. Surv. 2013, 68, 467–481. [Google Scholar] [CrossRef]
- Azevedo, G.D.; Franco, R.F.; Baggio, M.S.; Maranhao, T.M.; Ferriani, M.F.; Silva de Sa, M.F. Effects of raloxifene therapy on the anticoagulant system in postmenopausal women. Climacteric 2003, 6, 140–145. [Google Scholar] [CrossRef]
- Sato, M.; Rippy, M.K.; Bryant, H.U. Raloxifene, tamoxifen, nafoxidine, or estrogen effects on reproductive and nonreproductive tissues in ovariectomized rats. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1996, 10, 905–912. [Google Scholar] [CrossRef]
- Gao, H.; Xi, S.; Xu, L. Effects of raloxifene combined with conjugated equine estrogen on prothrombotic state in ovariectomized rats. Zhonghua Fu Chan Ke Za Zhi 2013, 48, 935–938. (In Chinese) [Google Scholar]
- Polini, N.; Rauschemberger, M.B.; Mendiberri, J.; Selles, J.; Massheimer, V. Effect of genistein and raloxifene on vascular dependent platelet aggregation. Mol. Cell. Endocrinol. 2007, 267, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Rosendaal, F.R.; Helmerhorst, F.M.; Vandenbroucke, J.P. Female Hormones and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Eckert, S.; Krueger, K.A.; Grady, D.; Powles, T.J.; Cauley, J.A.; Norton, L.; Nickelsen, T.; Bjarnason, N.H.; Morrow, M.; et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: Results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 1999, 281, 2189–2197. [Google Scholar] [CrossRef]
- Meier, C.R.; Jick, H. Tamoxifen and risk of idiopathic venous thromboembolism. Br. J. Clin. Pharmacol. 1998, 45, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Avsar, M.; Labreche, P.; Bracamonte, M.; Jayachandran, M.; Miller, V.M. Treatment with raloxifene and 17beta-estradiol differentially modulates nitric oxide and prostanoids in venous endothelium and platelets of ovariectomized pigs. Journal. Cardiovasc. Pharmacol. 2006, 48, 231–238. [Google Scholar] [CrossRef]
- Visvanathan, K.; Fabian, C.J.; Bantug, E.; Brewster, A.M.; Davidson, N.E.; Decensi, A.; Floyd, J.D.; Garber, J.E.; Hofstatter, E.W.; Khan, S.A.; et al. Use of Endocrine Therapy for Breast Cancer Risk Reduction: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2019, 37, 3152–3165. [Google Scholar] [CrossRef]
- Qaseem, A.; Hicks, L.A.; Etxeandia-Ikobaltzeta, I.; Shamliyan, T.; Cooney, T.G.; Clinical Guidelines Committee of the American College of Physicians; Cross, J.T., Jr.; Fitterman, N.; Lin, J.S.; Maroto, M.; et al. Pharmacologic Treatment of Primary Osteoporosis or Low Bone Mass to Prevent Fractures in Adults: A Living Clinical Guideline from the American College of Physicians. Ann. Intern. Med. 2023, 176, 224–238. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0, a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Felgel-Farnholz, V.; Hlusicka, E.B.; Edemann-Callesen, H.; Garthe, A.; Winter, C.; Hadar, R. Adolescent raloxifene treatment in females prevents cognitive deficits in a neurodevelopmental rodent model of schizophrenia. Behav. Brain Res. 2023, 441, 114276. [Google Scholar] [CrossRef]
- Quick, A.J. Normal values for coagulation test. N. Engl. J. Med. 1954, 290, 751. [Google Scholar]
- Proctor, R.R.; Rapaport, S. The partial thromboplastin time with kaolin. A simple screening test for first stage plasma clotting factor deficiencies. Am. J. Clin. Pathol. 1961, 36, 212–219. [Google Scholar] [CrossRef] [PubMed]
- García-Manzano, A.; González, L.J.; Lemini, C.; Rubio-Póo, C. Standardization of rat blood clotting tests with reagents used for humans. Proc. West. Pharmacol. Soc. 2001, 44, 153–155. [Google Scholar]
- Clauss, A. Coagulation fast method for the determination of fibrinogen. Acta Hematol. 1957, 17, 237–240. [Google Scholar] [CrossRef]
- Abou-Ismail, M.Y.; Citla Sridhar, D.; Nayak, L. Estrogen and thrombosis: A bench to bedside review. Thromb. Res. 2020, 192, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Roqué, M.; Sitges, M.; Sala, J.; Delgado, V.; Morales, M.; Marrugat, J.; Vila, J.; Subirana, I.; Tàssies, D.; Reverter, J.C. Effects of raloxifene on endotelial function and hemostasis in women with ischemic heart disease. Rev. Esp. Cardiol. 2011, 64, 572–578. [Google Scholar] [CrossRef]
- Ando, H.; Otoda, T.; Ookami, H.; Nagai, Y.; Inano, A.; Takamura, T.; Ushijima, K.; Hosohata, K.; Matsushita, E.; Saito, T.; et al. Dosing time-dependent effect of raloxifene on plasma plasminogen activator inhibitor-1 concentrations in post-menopausal women with osteoporosis. Clin. Exp. Pharmacol. Physiol. 2013, 40, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Eilertsen, A.L.; Dahm, A.E.A.; Høibraaten, E.; Lofthus, C.M.; Mowinckel, M.C.; Sandset, P.M. Relationship between sex hormone binding globulin and blood coagulation in women on postmenopausal hormone treatment. Blood Coagul. Fibrinolysis Int. J. Haemost. Thrombosis. 2019, 30, 17–23. [Google Scholar] [CrossRef]
- Cosman, F.; Baz-Hecht, M.; Cushman, M.; Vardy, M.D.; Cruz, J.D.; Nieves, J.W.; Zion, M.; Lindsay, R. Short-term effects of estrogen, tamoxifen and raloxifene on hemostasis: A randomized-controlled study and review of the literature. Thromb. Res. 2005, 116, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W. Influence of fibrinogen on cardiovascular disease. Drugs 1997, 54, 32–40. [Google Scholar] [CrossRef] [PubMed]
- De la Serna, G. Fibrinogen: A new major risk factor for cardiovascular disease. A review of the literature. J. Fam. Pract. 1994, 39, 468–477. [Google Scholar]
- Montaño, L.M.; Calixto, E.; Figueroa, A.; Flores-Soto, E.; Carbajal, V.; Perusquía, M. Relaxation of androgens on rat thoracic aorta: Testosterone concentration dependent agonist/antagonist L-type Ca2+ channel activity, and 5beta-dihydrotestosterone restricted to L-type Ca2+ channel blockade. Endocrinology 2008, 149, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Almstrup, K.; Fernández, M.F.; Petersen, J.H.; Olea, N.; Skakkebaek, N.E.; Leffers, H. Dual effects of phytoestrogens result in u-shaped dose-response curves. Environ. Health Perspect. 2002, 110, 743–748. [Google Scholar] [CrossRef]
- Townsend, E.A.; Thompson, M.A.; Pabelick, C.M.; Prakash, Y.S. Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. American journal of physiology. Lung Cell. Mol. Physiol. 2010, 298, L521–L530. [Google Scholar] [CrossRef]
- Cushman, M. Effects of estrogen and selective estrogen receptor modulators on hemostasis and inflammation. Ann. N. Y Acad. Sci. 2001, 949, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Tsuruoka, S.; Hasegawa, G.; Kaneda, T.; Maeda, A.; Fujimura, A. Dosing time-dependent effect of raloxifene on plasma fibrinogen concentration in ovariectomized rats. Chronobiol. Int. 2008, 25, 808–818. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alva-Chavarría, D.; Soto-Núñez, M.; Flores-Soto, E.; Jaimez, R. Hemostatic Effects of Raloxifene in Ovariectomized Rats. Life 2023, 13, 1612. https://doi.org/10.3390/life13071612
Alva-Chavarría D, Soto-Núñez M, Flores-Soto E, Jaimez R. Hemostatic Effects of Raloxifene in Ovariectomized Rats. Life. 2023; 13(7):1612. https://doi.org/10.3390/life13071612
Chicago/Turabian StyleAlva-Chavarría, Denys, Maribel Soto-Núñez, Edgar Flores-Soto, and Ruth Jaimez. 2023. "Hemostatic Effects of Raloxifene in Ovariectomized Rats" Life 13, no. 7: 1612. https://doi.org/10.3390/life13071612
APA StyleAlva-Chavarría, D., Soto-Núñez, M., Flores-Soto, E., & Jaimez, R. (2023). Hemostatic Effects of Raloxifene in Ovariectomized Rats. Life, 13(7), 1612. https://doi.org/10.3390/life13071612






