Immobilization of Lipase B from Candida antarctica on Magnetic Nanoparticles Enhances Its Selectivity in Kinetic Resolutions of Chiral Amines with Several Acylating Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Enzyme and Materials
2.2. Methods
2.2.1. Analytical Methods and Calculations
2.2.2. Preparation of Isopropyl 2-Ethoxyacetate 2C
2-Ethoxyacetic Acid
Isopropyl 2-Ethoxyacetate 2C
2.2.3. Preparation of CaLB-MNP Biocatalysts
2.2.4. Kinetic Resolution of the Racemic Amines (±)-1a–d in Batch Mode with Different Acylating Agents 2A–C and CaLB on Different Supports
2.2.5. Kinetic Resolution of the Racemic Amines (±)-1a,b,d with Isopropyl 2-Cyanoacetate 2B in Batch Mode Using CaLB-MNPs for Isolation of the New Amides (R)-3(a,b,d)B
2-Cyano-N-(2-heptanyl)acetamide (R)-3aB
2-Cyano-N-(1-methoxy-2-propanyl)acetamide (R)-3bB
2-Cyano-N-(4-phenyl-2-butanyl)acetamide (R)-3dB
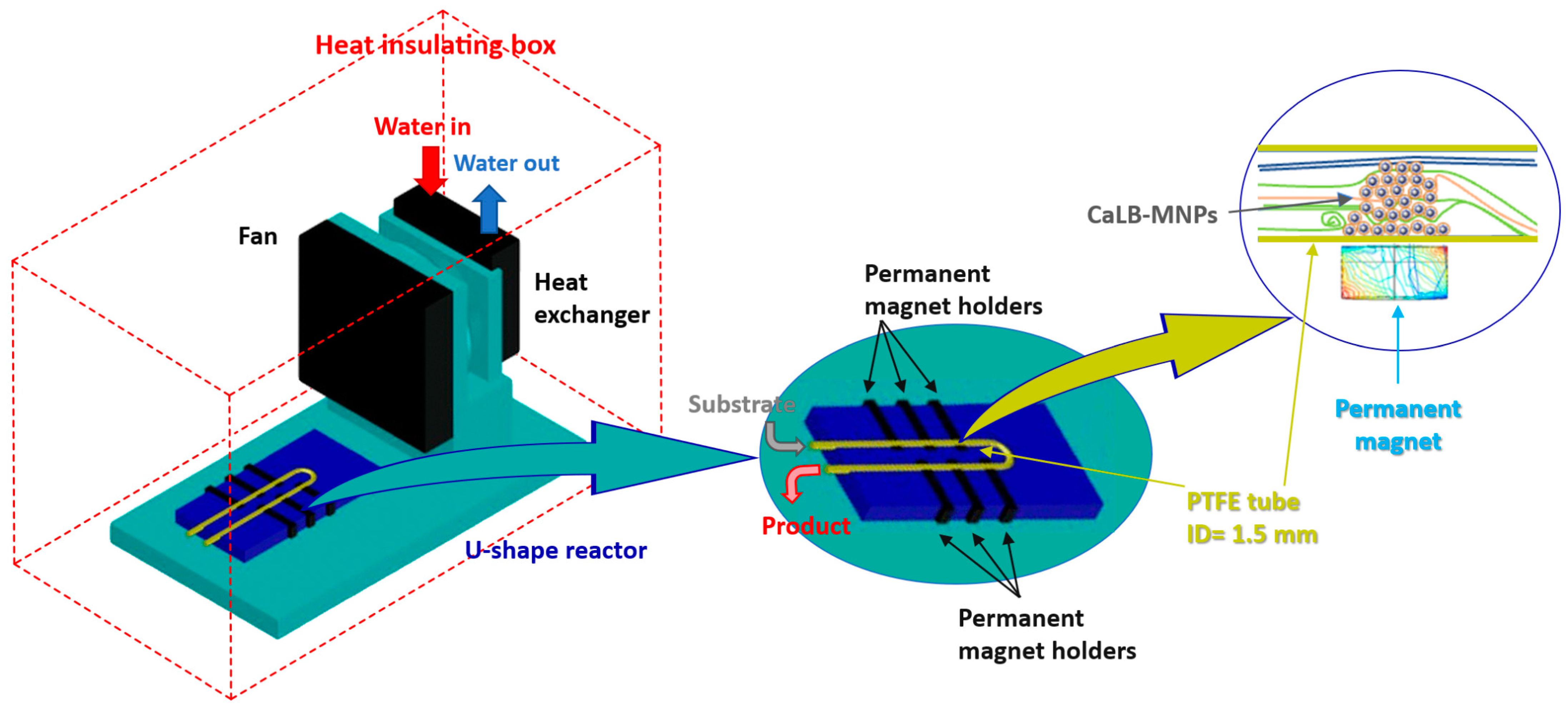
2.2.6. Design and Assembly of the Thermostatted U-Shape MNP Reactor
2.2.7. Kinetic Resolution of the Racemic Amines (±)-1b and (±)-1c with Isopropyl 2-Ethoxyacetate 2C Using CaLB-MNPs in Continuous-Flow U-Shape Reactor
3. Results and Discussion
3.1. Biocatalyst Characterization
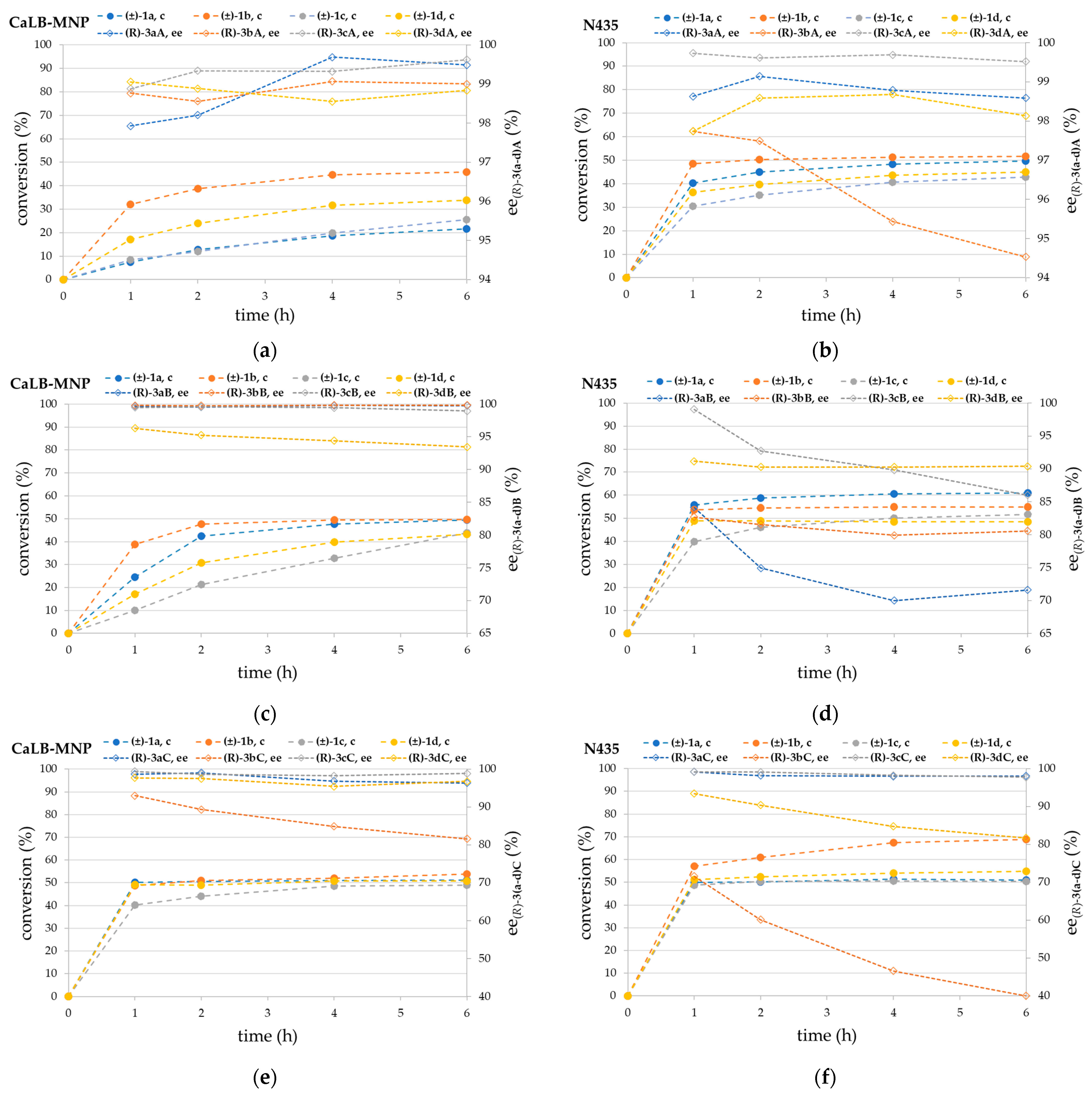
3.2. Kinetic Resolution of Chiral Amines (±)-1a–d with Different Acylating Agents (2A–C) in Batch Mode with CaLB-MNPs and N435
3.3. CaLB-MNP-Catalyzed Kinetic Resolution of Chiral Amines (±)-1b and (±)-1c with Isopropyl 2-Ethoxyacetate 2C in Thermostatted Continuous-Flow U-Shape Reactor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghislieri, D.; Turner, N.J. Biocatalytic Approaches to the Synthesis of Enantiomerically Pure Chiral Amines. Top. Catal. 2013, 57, 284–300. [Google Scholar] [CrossRef]
- Grogan, G. Synthesis of chiral amines using redox biocatalysis. Curr. Opin. Chem. Biol. 2018, 43, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.D.; Grogan, G.; Bommarius, A.; Yun, H. Recent Advances in ω-Transaminase-Mediated Biocatalysis for the Enantioselective Synthesis of Chiral Amines. Catalysts 2018, 8, 254. [Google Scholar] [CrossRef]
- Aguillón, A.R.; Miranda, A.S.; Junior, I.I.; Souza, R.O.M.A. Biocatalysis toward the Synthesis of Chiral Amines. In Synthetic Approaches to Nonaromatic Nitrogen Heterocycles; Faisca Philips, A.M.M.M., Ed.; Wiley: Hoboken, NJ, USA, 2020; Volume 2, pp. 667–697. [Google Scholar] [CrossRef]
- Musa, M.M. Enzymatic racemization of alcohols and amines: An approach for bi-enzymatic dynamic kinetic resolution. Chirality 2019, 32, 147–157. [Google Scholar] [CrossRef]
- Ismail, A.R.; Baek, K.H. Lipase immobilization with support materials, preparation techniques, and applications: Present and future aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef]
- Zhang, K.; Pan, Z.; Diao, Z.; Liang, S.; Han, S.; Zheng, S.; Lin, Y. Kinetic resolution of sec-alcohols catalysed by Candida antarctica lipase B displaying Pichia pastoris whole-cell biocatalyst. Enzym. Microb. Technol. 2018, 110, 8–13. [Google Scholar] [CrossRef]
- Imarah, A.O.; Silva, F.M.W.G.; Tuba, L.; Malta-Lakó, Á.; Szemes, J.; Sánta-Bell, E.; Poppe, L. A Convenient U-Shape Microreactor for Continuous Flow Biocatalysis with Enzyme-Coated Magnetic Nanoparticles-Lipase Catalyzed Enantiomer Selective Acylation of 4-(Morpholin-4-yl)butan-2-ol. Catalysts 2022, 12, 1065. [Google Scholar] [CrossRef]
- Silva, F.M.W.G.; Imarah, A.O.; Takács, O.; Tuba, L.; Poppe, L. Scalability of U-Shape Magnetic Nanoparticles-Based Microreactor–Lipase-Catalyzed Preparative Scale Kinetic Resolutions of Drug-like Fragments. Catalysts 2023, 13, 384. [Google Scholar] [CrossRef]
- Xing, X.; Jia, J.Q.; Zhang, J.F.; Zhou, Z.W.; Li, J.; Wang, N.; Yu, X.Q. CALB Immobilized onto Magnetic Nanoparticles for Efficient Kinetic Resolution of Racemic Secondary Alcohols: Long-Term Stability and Reusability. Molecules 2019, 24, 490. [Google Scholar] [CrossRef]
- Kuo, C.H.; Kou, B.S.; Tsai, S.W. CALB-catalyzed kinetic resolution of (RS)-3-benzoylthio-2-methylpropyl azolides: Kinetic and thermodynamic analysis. Biocatal. Biotransform. 2020, 38, 376–384. [Google Scholar] [CrossRef]
- Bäckvall, J.E.; Gustafson, K.; Görbe, T.; de Gonzalo, G.; Yang, N.; Schreiber, C.; Shchukarev, A.; Tai, C.-W.; Persson, I.; Zou, X. Chemoenzymatic Dynamic Kinetic Resolution of Primary Benzylic Amines using Pd(0)-CalB CLEA as a Biohybrid Catalyst. Chem. Eur. J. 2019, 25, 9174–9179. [Google Scholar] [CrossRef]
- El-Behairy, M.F.; Hassan, R.M.; Sundby, E. Enantioselective Chromatographic Separation and Lipase Catalyzed Asymmetric Resolution of Biologically Important Chiral Amines. Separations 2021, 8, 165. [Google Scholar] [CrossRef]
- Szemes, J.; Malta-Lakó, Á.; Tóth, R.E.; Poppe, L. Diisopropyl Malonate as Acylating Agent in Kinetic Resolution of Chiral Amines with Lipase B from Candida antarctica. Period. Polytech. Chem. Eng. 2022, 66, 458–464. [Google Scholar] [CrossRef]
- Csuka, P.; Boros, Z.; Őrfi, L.; Dobos, J.; Poppe, L.; Hornyánszky, G. Chemoenzymatic route to Tyrphostins involving lipase-catalyzed kinetic resolution of 1-phenylethanamine with alkyl cyanoacetates as novel acylating agents. Tetrahedron Asymmetry 2015, 26, 644–649. [Google Scholar] [CrossRef]
- Oláh, M.; Boros, Z.; Hornyánszky, G.; Poppe, L. Isopropyl 2-ethoxyacetate—An efficient acylating agent for lipase-catalyzed kinetic resolution of amines in batch and continuous-flow modes. Tetrahedron 2016, 72, 7249–7255. [Google Scholar] [CrossRef]
- Oláh, M.; Kovács, D.; Katona, G.; Hornyánszky, G.; Poppe, L. Optimization of 2-alkoxyacetates as acylating agent for enzymatic kinetic resolution of chiral amines. Tetrahedron 2018, 74, 3663–3670. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Overview of Immobilized Enzymes’ Applications in Pharmaceutical, Chemical, and Food Industry. In Immobilization of Enzymes and Cells. Methods in Molecular Biology; Guisan, J., Bolivar, J., López-Gallego, F., Rocha-Martín, J., Eds.; Humana: New York, NY, USA, 2020; Volume 2100. [Google Scholar] [CrossRef]
- Heckmann, C.M.; Paradisi, F. Looking Back: A Short History of the Discovery of Enzymes and How They Became Powerful Chemical Tools. ChemCatChem 2020, 12, 6082–6102. [Google Scholar] [CrossRef]
- Falus, P.; Boros, Z.; Hornyánszky, G.; Nagy, J.; Ürge, L.; Darvas, F.; Poppe, L. Synthesis and Lipase Catalysed Kinetic Resolution of Racemic Amines. Stud. Univ. Babes-Bolyai Ser. Chem. 2010, 4, 289–296. [Google Scholar]
- Boros, Z.; Falus, P.; Márkus, M.; Weiser, D.; Oláh, M.; Hornyánszky, G.; Nagy, J.; Poppe, L. How the mode of Candida antarctica lipase B immobilization affects the continuous-flow kinetic resolution of racemic amines at various temperatures. J. Mol. Catal. B Enzym. 2013, 85–86, 119–125. [Google Scholar] [CrossRef]
- Päiviö, M.; Perkiö, P.; Kanerva, L.T. Solvent-free kinetic resolution of primary amines catalyzed by Candida antarctica lipase B: Effect of immobilization and recycling stability. Tetrahedron Asymmetry 2012, 23, 230–236. [Google Scholar] [CrossRef]
- Joubioux, F.L.; Achour, O.; Bridiau, N.; Graber, M.; Maugard, T.J. Kinetic study of 2-butanol O-acylation and sec-butylamine N-acylation catalyzed by Candida antarctica lipase B. J. Mol. Catal. B Enzym. 2011, 70, 108–113. [Google Scholar] [CrossRef]
- Hietanen, A.; Saloranta, T.; Leino, R.; Kanerva, L.T. Lipase catalysis in the preparation of 3-(1-amino-3-butenyl)pyridine enantiomers. Tetrahedron Asymmetry 2012, 23, 1629–1632. [Google Scholar] [CrossRef]
- Cavicchioli, M.; Pevarello, P.; Salom, B.; Vulpetti, A. Aminoisoxazole Derivatives Active as Kinase Inhibitors. International Patent Application WO 03/013517 A1, 20 February 2003. [Google Scholar]
- Viñambres, M.; Filice, M.; Marciello, M. Modulation of the Catalytic Properties of Lipase B from Candida antarctica by Immobilization on Tailor-Made Magnetic Iron Oxide Nanoparticles: The Key Role of Nanocarrier Surface Engineering. Polymers 2018, 10, 615. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, K.; Johnston, E.V.; Verho, O.; Gustafson, K.P.J.; Shakeri, M.; Tai, C.W.; Backvall, J.E. Co-immobilization of an enzyme and a metal into the compartments of mesoporous silica for cooperative tandem catalysis: An artificial metalloenzyme. Angew. Chem. 2013, 52, 14006–14010. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.P.J.; Lihammar, R.; Verho, O.; Engström, K.; Bäckvall, J.E. Chemoenzymatic Dynamic Kinetic Resolution of Primary Amines Using a Recyclable Palladium Nanoparticle Catalyst Together with Lipases. J. Org. Chem. 2014, 79, 3747–3751. [Google Scholar] [CrossRef]
- Xu, S.; Wang, M.; Feng, B.; Han, X.; Lan, Z.; Gu, H.; Li, H.; Li, H. Dynamic kinetic resolution of amines by using palladium nanoparticles confined inside the cages of amine-modified MIL-101 and lipase. J. Catal. 2018, 363, 9–17. [Google Scholar] [CrossRef]
- Li, P.; Zhu, J.; Zhang, H.; Wang, L.; Wang, S.; Zhang, M.; Wu, J.; Yang, L.; Xu, G. Preparation of Coupling Catalyst HamZIF-90@Pd@CALB with Tunable Hollow Structure for Efficient Dynamic Kinetic Resolution of 1-Phenylethylamine. Molecules 2023, 28, 922. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 238–242. [Google Scholar] [CrossRef]
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Mohammadlou, M.; Berenjian, A.; Anarjan, N.; Jafari, N.; Nasiri, S. Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol. Lett. 2015, 38, 223–233. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef]
- Gupta, A.R.; Rathod, V.K. Biodiesel synthesis from palm fatty acid distillate using enzyme immobilized on magnetic nanoparticles. SN Appl. Sci. 2020, 2, 1778. [Google Scholar] [CrossRef]
- Alikhani, N.; Shahedi, M.; Habibi, Z.; Yousefi, M.; Ghasemi, S.; Mohammadi, M. A multi-component approach for co-immobilization of lipases on silica-coated magnetic nanoparticles: Improving biodiesel production from waste cooking oil. Bioprocess Biosyst. Eng. 2022, 45, 2043–2060. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Wang, M.; He, W.; Wu, J.; Liu, X.; Guan, Y. Ultra-small magnetic Candida antarctica lipase B nanoreactors for enzyme synthesis of bixin-maltitol ester. Food Chem. 2023, 421, 136132. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, X.; Zheng, L. Engineering actively magnetic crosslinked inclusion bodies of Candida antarctica lipase B: An efficient and stable biocatalyst for enzyme-catalyzed reactions. Mol. Catal. 2021, 504, 111467. [Google Scholar] [CrossRef]
- Ferraz, C.A.; do Nascimento, M.A.; Almeida, R.F.O.; Sergio, G.G.; Junior, A.A.T.; Dalmônico, G.; Caraballo, R.; Finotelli, P.V.; Leão, R.A.C.; Wojcieszak, R.; et al. Synthesis and characterization of a magnetic hybrid catalyst containing lipase and palladium and its application on the dynamic kinetic resolution of amines. Mol. Catal. 2020, 493, 111106. [Google Scholar] [CrossRef]
- Baumann, M.; Moody, T.S.; Smyth, M.; Wharry, S. A Perspective on Continuous Flow Chemistry in the Pharmaceutical Industry. Org. Process Res. Dev. 2020, 24, 1802–1813. [Google Scholar] [CrossRef]
- Rakels, J.L.L.; Straathof, A.J.J.; Heijnen, J.J. A simple method to determine the enantiomeric ratio in enantioselective biocatalysis. Enzym. Microb. Technol. 1993, 15, 1051–1056. [Google Scholar] [CrossRef]
- Chen, C.S.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 1982, 104, 7294–7299. [Google Scholar] [CrossRef]
- Abaházi, E.; Lestál, D.; Boros, Z.; Poppe, L. Tailoring the Spacer Arm for Covalent Immobilization of Candida antarctica Lipase B–Thermal Stabilization by Bisepoxide-activated Aminoalkyl Resins in Continuous-flow Reactors. Molecules 2016, 21, 767. [Google Scholar] [CrossRef]
- Csuka, P.; Molnár, Z.; Tóth, V.; Imarah, A.O.; Balogh-Weiser, D.; Vértessy, B.G.; Poppe, L. Immobilization of the Aspartate Ammonia-lyase from Pseudomonas fluorescens R124 on Magnetic Nanoparticles—Characterization and Kinetics. ChemBioChem 2022, 23, e202100708. [Google Scholar] [CrossRef]
- Hellner, G.; Boros, Z.; Tomin, A.; Poppe, L. Novel Sol-Gel Lipases by Designed Bioimprinting for Continuous-Flow Kinetic Resolutions. Adv. Synth. Catal. 2011, 353, 2481–2491. [Google Scholar] [CrossRef]
- Csajági, C.; Szatzker, G.; Tőke, E.R.; Ürge, L.; Darvas, F.; Poppe, L. Enantiomer selective acylation of racemic alcohols by lipases in continuous-flow bioreactors. Tetrahedron Asymmetry 2008, 19, 237–246. [Google Scholar] [CrossRef]
- Imarah, A.O.; Silva, F.M.W.G.; Bataa, N.; Decsi, B.; Balogh-Weiser, D.; Poppe, L. Magnetically Agitated Continuous Flow Tube Reactors with Aspartate Ammonia-Lyase Immobilized on Magnetic Nanoparticles. React. Chem. Eng. 2023, 8, 1250–1259. [Google Scholar] [CrossRef]


| Entry | Amine | AA a | Amount | CaLB | t | c | ee(R)-3(a–d)(A–C) | ee(S)-1a | E | UB | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine (mM) | AA (eq.) | Support | Amount (mg mL−1) | (h) | (%) | (%) | (%) | (U g−1) | |||||
| 1 | (±)-1a | 2A | 45 | 1 | MNPs | 50 | 6 | 21.6 | 99.5 | 29.0 | ≫200 | 0.5 | b |
| 2 | 2A | 45 | 1 | N435 | 50 | 1 | 40.4 | 98.6 | 63.7 | >200 | 6.1 | b | |
| 3 | 2A | 180 | 1 | N435 | 100 | 4 | 50.0 | 98.5 | 98.7 | ≫200 | - | [14] | |
| 4 | 2B | 45 | 1 | MNPs | 50 | 6 | 49.5 | 99.8 | 97.0 | ≫200 | 1.2 | b | |
| 5 | 2B | 45 | 1 | N435 | 50 | 1 | 55.7 | 84.2 | 99.0 | 59.7 | 8.4 | b | |
| 6 | 2C | 45 | 1 | MNPs | 50 | 1 | 50.5 | 99.1 | 97.7 | ≫200 | 7.6 | b | |
| 7 | 2C | 45 | 1 | N435 | 50 | 1 | 49.9 | 99.1 | 97.9 | ≫200 | 7.5 | b | |
| 8 | (±)-1b | 2A | 45 | 1 | MNPs | 50 | 6 | 45.9 | 99.0 | 83.9 | ≫200 | 1.2 | b |
| 9 | 2A | 45 | 1 | N435 | 50 | 1 | 48.6 | 97.7 | 93.0 | >200 | 7.3 | b | |
| 10 | 2A | 180 | 1 | N435 | 100 | 4 | 52.1 | 92.0 | 99.9 | >100 | - | [14] | |
| 11 | 2B | 45 | 1 | MNPs | 50 | 4 | 49.8 | 99.8 | 98.7 | ≫200 | 1.9 | b | |
| 12 | 2B | 45 | 1 | N435 | 50 | 1 | 53.7 | 82.7 | 96.1 | 41.2 | 8.1 | b | |
| 13 | 2C | 45 | 1 | MNPs | 50 | 1 | 48.7 | 93.0 | 89.1 | 83.0 | 7.3 | b | |
| 14 | 2C | 45 | 1 | N435 | 50 | 1 | 57.2 | 71.6 | 95.6 | 22.4 | 8.6 | b | |
| 15 | (±)-1c | 2A | 45 | 1 | MNPs | 50 | 6 | 25.6 | 99.6 | 34.4 | ≫200 | 0.6 | b |
| 16 | 2A | 45 | 1 | N435 | 50 | 1 | 30.5 | 99.7 | 43.8 | ≫200 | 4.6 | b | |
| 17 | 2A | 180 | 1 | N435 | 100 | 4 | 45.0 | 99.9 | 81.5 | ≫200 | - | [14] | |
| 18 | 2B | 45 | 1 | MNPs | 50 | 6 | 43.9 | 98.9 | 74.5 | >200 | 1.1 | b | |
| 19 | 2B | 45 | 1 | N435 | 50 | 1 | 39.9 | 99.0 | 65.6 | >200 | 6.0 | b | |
| 20 | 2B* c | 200 | 0.5 | N435 | 50 | 24 | 31.7 c | 99.9 | 46.4 | - | 0.9 | [15] | |
| 21 | 2B* c | 200 | 1 | N435 | 50 | 24 | 50.1 c | 98.2 | 98.5 | - | 1.4 | [15] | |
| 22 | 2B* c | 82.5 | 0.5 | T2-150 | 100 | 24 | 20.0 c | 91.0 | 22.7 | - | - | [15] | |
| 23 | 2B* c | 82.5 | 0.5 | G250P | 100 | 24 | 17.1 c | 99.3 | 20.7 | - | - | [15] | |
| 24 | 2C | 45 | 1 | MNPs | 50 | 1 | 40.2 | 99.4 | 63.1 | ≫200 | 6.0 | b | |
| 25 | 2C | 45 | 1 | N435 | 50 | 1 | 48.8 | 99.1 | 94.1 | ≫200 | 7.3 | b | |
| 26 | 2C | 385 | 0.6 | G250P | 25 | 1 | 33.8 | >99.9 | - | ≫200 | - | [16] | |
| 27 | (±)-1d | 2A | 45 | 1 | MNPs | 50 | 6 | 33.8 | 98.8 | 51.2 | >200 | 0.8 | b |
| 28 | 2A | 45 | 1 | N435 | 50 | 1 | 36.3 | 97.7 | 55.3 | >100 | 5.5 | b | |
| 29 | 2A | 180 | 1 | N435 | 100 | 4 | 47.0 | 98.5 | 87.5 | >200 | - | [14] | |
| 30 | 2B | 45 | 1 | MNPs | 50 | 6 | 43.3 | 93.5 | 70.6 | 62.6 | 1.1 | b | |
| 31 | 2B | 45 | 1 | N435 | 50 | 1 | 49.0 | 91.2 | 83.4 | 56.6 | 7.3 | b | |
| 32 | 2C | 45 | 1 | MNPs | 50 | 1 | 49.1 | 97.7 | 93.4 | ≫200 | 7.4 | b | |
| 33 | 2C | 45 | 1 | N435 | 50 | 1 | 51.2 | 93.4 | 99.3 | >100 | 7.7 | b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.M.W.G.; Szemes, J.; Mustashev, A.; Takács, O.; Imarah, A.O.; Poppe, L. Immobilization of Lipase B from Candida antarctica on Magnetic Nanoparticles Enhances Its Selectivity in Kinetic Resolutions of Chiral Amines with Several Acylating Agents. Life 2023, 13, 1560. https://doi.org/10.3390/life13071560
Silva FMWG, Szemes J, Mustashev A, Takács O, Imarah AO, Poppe L. Immobilization of Lipase B from Candida antarctica on Magnetic Nanoparticles Enhances Its Selectivity in Kinetic Resolutions of Chiral Amines with Several Acylating Agents. Life. 2023; 13(7):1560. https://doi.org/10.3390/life13071560
Chicago/Turabian StyleSilva, Fausto M. W. G., József Szemes, Akan Mustashev, Orsolya Takács, Ali O. Imarah, and László Poppe. 2023. "Immobilization of Lipase B from Candida antarctica on Magnetic Nanoparticles Enhances Its Selectivity in Kinetic Resolutions of Chiral Amines with Several Acylating Agents" Life 13, no. 7: 1560. https://doi.org/10.3390/life13071560
APA StyleSilva, F. M. W. G., Szemes, J., Mustashev, A., Takács, O., Imarah, A. O., & Poppe, L. (2023). Immobilization of Lipase B from Candida antarctica on Magnetic Nanoparticles Enhances Its Selectivity in Kinetic Resolutions of Chiral Amines with Several Acylating Agents. Life, 13(7), 1560. https://doi.org/10.3390/life13071560









