Abstract
The genus Scrophularia is one of the largest genera belonging to the Scrophulariaceae family. Different members of the genus exhibit an interesting, wide spectrum of bioactivities. Accordingly, the current study aimed to investigate, for the first time, the chemical composition of the essential oil of Scrophularia peyronii Post. from Jordan. Additionally, extracts obtained from the aerial parts with solvents of different polarities were assayed for their phytochemical constituents and in vitro antioxidant activities. The major constituents detected in the essential oil, as revealed by GC/MS analysis, contained mainly Z,Z-farnesyl acetone (11.04%), β-elemene (6.36%), n-octanal (5.98%), and spathulenol (4.58%). Each of the aqueous methanol (Sp-M) and butanol (Sp-B) extracts contained flavonoids, saponins, anthraquinone, and glycosides. Both extracts were evaluated for their total phenolic content (TPC), total flavonoid content (TFC), and their in vitro antioxidant activity, which were assayed using the DPPH radical scavenging activity and ABTS radical scavenging methods. Additionally, the two extracts were then subjected to LC-ESI-MS/MS for the qualitative determination of their secondary metabolite content, especially in flavonoids and phenolic compounds. The results showed that the Sp-B extract of S. peyronii had the highest contents of both phenolic compounds and flavonoids and showed high radical scavenging activity, as determined by the two assay methods, when compared with the Sp-M extract. The LC-ESI-MS/MS analysis resulted in the detection of 21 compounds, including 8 flavonoids, 6 phenolic acids, 6 iridoids, and 2 acids. Although the majority of compounds were detected in both extracts, it was noticed that scropolioside B, 6′-O-cinnamoylharpagide, isoferulic acid, and 6-O-methylcatapol were only detected in the Sp-M fraction.
1. Introduction
Oxidative stress, also known as the imbalance between the production of reactive oxygen and nitrogen species (ROS/RNS) and the antioxidant defense, is a significant contributor to several pathophysiological disorders. Oxidative stress is characterized by the inability of endogenous antioxidants to prevent oxidative damage on biological targets. This condition can be brought on by either an increase in the generation of ROS/RNS or a decrease in the antioxidant network [1]. Therefore, considerable attention has been paid to the antioxidant potential of natural products, particularly the most consumable ones.
Scrophularia is one of the largest genera of the Scrophulariaceae family. Scrophularia is an annual or perennial herb distributed in the Euro-Siberian and Mediterranean regions [2]. In Jordan, there are many species of Scrophularia, including S. peyronii Post., S. deserti Delile., S. heterophylla Willd., S. lucida L., S. hierochuntina Boiss., S. macrophlla Boiss., S. nabataeoeorum Eig., S. sphaerocarpa Boiss., S. xanthoglossa Boiss., S. rubricaulis Boiss., and S. xylorrhiza Boiss. [3]. The use of several Scrophularia species in traditional medicine has been described in different cultures of the world. Plants belonging to this genus are utilized for their antioxidant, anti-tumor, anti-cancer, anti-protozoal, and anti-inflammatory effects [4]. Species such as S. striata and S. oxysepala have been reported for their wound-healing and anti-cancer activity, respectively. The ethanolic extract obtained from the aerial parts of S. hypericifolia growing wild in Saudi Arabia has shown hepatoprotective and nephroprotective effects [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. Flavonoids, phenylethanoids, glycoside esters, phenolic acids, iridoids, glycosides, fatty acid derivatives, triterpenes, triterpenoids glycosides, alkaloids, diterpenoids, and essential oils are among the various types of chemical compounds that have been identified in Scrophularia species [5,6,7,8,9,10,11,12,13,14,15,16].
The literature survey revealed that S. peyronii from Jordan has never been investigated before for its essential oil composition and has not been assayed for its possible antioxidant activity or secondary metabolite contents, especially those of flavonoids and phenolic acids. Therefore, we report in the current study the chemical composition of hydro-distilled essential oil obtained from the aerial parts of S. peyronii. Additionally, aqueous methanol and butanol (Sp-M, Sp-B, respectively) extracts of the aerial parts were evaluated for their total phenol content (TPC), total flavonoid content (TFC), and in vitro antioxidant activity. Moreover, the qualitative determination of the phenolic and flavonoid contents of both extracts is performed using LC-ESI-MS/MS.
2. Materials and Methods
2.1. Instrumentation
UV-vis spectra were measured on a Shimadzu UV-1800 UV/Visible Scanning Spectrophotometer (USA). The essential oil was extracted by the hydro-distillation of the plant’s aerial parts using Clevenger-style equipment. A Varian Chrompack CP-3800 GC/MS instrument was used for the GC/MS (Saturn, The Netherlands). HPLC was utilized to screen molecules of interest in both the positive (M+H) and negative (M-H) electrospray ionization modes using a Bruker Daltonik Impact II ESI-Q-TOF System connected to a Bruker Daltonik Elute UPLC system (Bremen, Germany).
2.2. Chemical Reagents
DPPH (2,2-diphenyl-1-picrylhydrazyl, purity N 99%), ascorbic acid (purity 99%), α-tocopherol (purity 99%), ABTS (2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt), Folin and Ciocaltea’s phenol reagent, Na2CO3, NaOH, anhydrous AlCl3, and NaNO2 were all products of Sigma-Aldrich (St. Louis, MO 68178, USA).
2.3. Plant Material and Fractionations
During the flowering season (April–June; 2019), aerial parts of S. peyronii (including stem, leaves, and flowers) were collected from the Al-Kourah region, located in North Jordan (N 32.437537; E 35.690143). The taxonomic identity of the plant was confirmed by the taxonomist Prof. Dr. Jamil N. Lahham (Department of Biology, Faculty of Science, Yarmouk University, Irbid, Jordan). A voucher specimen (No: YU/12/SS/1011) was kept at the Herbarium of Natural Products Research in the chemistry department of the Faculty of Science, Yarmouk University, Irbid, Jordan. The dried plant material was ground into a fine powder (0.50 Kg). Defatting of the dried plant material was performed by soaking it in petroleum ether (40–60 °C, 1 L, ambient temperature, and 10 days). Secondary metabolites were extracted from the defatted plant material by soaking in ethanol (5 times, 7 days each, and 1 L). After the evaporation of ethanol under reduced pressure, the obtained crude extract was partitioned between chloroform and water. Once the chloroform was evaporated, the obtained dried residue was partitioned between 10% aqueous methanol (Sp-M) (7.6 g) and hexane (5.9 g). Polar organic compounds were extracted from the water using n-butanol to obtain the butanol extract (Sp-B) (4.5 g).
2.4. Extraction of Essential Oils
In the current investigation, essential oil was extracted from the fresh aerial parts of S. peyronii, as described previously in the literature [22,23,24,25,26,27,28,29,30]. Aerial parts of S. peyronii (including stem, leaves, and flowers) were chopped into small pieces and then hydro-distillation was carried out in a Clevenger-type apparatus for 4 h. The oil was then dried over anhydrous Na2SO4 and kept in GC-grade n-hexane at 4 °C until the analysis was performed.
2.5. Phytochemical Analysis
Each of the Sp-M and Sp-B fractions were subjected to qualitative examination to characterize the major groups of secondary metabolites according to the procedure described by literatures [31,32,33,34].
2.6. GC/MS Analysis of Essential Oils
GC/MS analysis of the hydro-distilled essential oil was carried out on a Varian Chrompack CP-3800 instrument (Saturn, The Netherlands). The following was the chromatographic temperature program: 60 °C (isothermal, 1 min), then increased to 246 °C at 3 °C/min, then kept constant at 246 °C (3 min, isothermal); the injector and detector temperatures were 250 and 300 °C, respectively. The carrier gas was helium (0.90 mL/min flow rate). The capillary column used was an HP-5 MS (30 m 0.25 mm i.d., and 0.25 m film thickness). The MS source reached a temperature of nearly 180 °C. A potential of 70 eV was used for sample ionization. A hydrocarbon mixture of n-alkanes (C8-C20) was examined individually by GC/MS using the same HB-5 column under the same chromatographic conditions.
Identification of the Chemical Constituents
The identification of the separated essential oil components was accomplished by comparing their calculated retention indices (RIs) with the (C8-C20) n-alkanes values with a column of identical polarity and under the same chromatographic conditions, as well as matching their recorded mass spectra with those listed in the built-in libraries’ spectra (NIST, Gaithersburg, MD, USA and Wiley Co., Hoboken, NJ, USA). The principal components of the extracts were further identified by injecting authentic standard reference compounds under the same chromatographic conditions.
2.7. Determination of Antioxidant Activity
The antioxidant activities of the crude extracts and essential oils were determined by the DPPH and ABTS radical-scavenging methods according to the procedure described in the literature [22,23,24,25,26,27,28,29,30]. The scavenging activity was then measured according to the following equation:
where AC is the absorbance of the blank and AS is the absorbance in the presence of the extract.
2.7.1. DPPH Free Radical Scavenging Activity
Briefly, a stock solution from each extract and the obtained essential oil (EO) was prepared (Sp-B, Sp-M, and essential oil; concentration range: 0.005, 0.01, 0.05, 0.1, and 0.5 mg/mL). The absorbance of the solutions was measured using a UV–VIS spectrophotometer at 517 nm against blank samples after allowing them to stand at room temperature in the dark for 30 min. A linear regression approach of plotting the percent of antiradical activity against the concentration of the tested extracts/EO was used to calculate the IC50 values, which were defined as the concentration of the substrate that caused a 50% decrease in DPPH activity.
2.7.2. ABTS Radical Scavenging Assay
Briefly, the ABTS•+ cation radical solution was prepared by reacting equivalent quantities of 7 mM of ABTS•+ and 2.4 mM of potassium persulfate (K2S2O8) solution for 16 h at room temperature in the dark. Prior to use, this solution was diluted with methanol to produce absorbance of 0.75 ± 0.02 at 734 nm. The reaction mixture consisted of 2 mL of ABTS•+ solution and 1 mL of each extract (Sp-B, Sp-M)/EO at various concentrations (0.005–0.50 mg/mL). The absorbance of the combination at 734 nm was measured using a UV-Vis spectrophotometer. Each test included a blank and all measurements took a minimum of 5 min to complete. The IC50 values were determined by plotting the percent of antiradical activity against the concentration of the tested substances using the linear regression approach.
2.8. Statistical Analysis
The presented data were the means ± SD of the results from three independent experiments with similar patterns. Each concentration was tested in triplicate in each of the three independent experiments. Statistical analysis was performed using the one-way ANOVA of the GraphPad Prism 6 software. A p < 0.05 value was considered statistically significant.
3. Results and Discussion
3.1. Chemical Composition of S. peyronii EO
The hydro-distillation of the aerial flowering parts of S. peyronii produced a yellowish-colored EO (yielded 0.06% v/w). The obtained EO was subjected to GC/MS analysis to reveal its chemical constituents. The results are shown in Table 1—the compounds are listed in the table according to their elusion order. Figure 1 shows the GC chromatogram of the analyzed EO.

Table 1.
Chemical composition and percentages of essential oil components from aerial parts of S. peyronii Post. from Jordan.
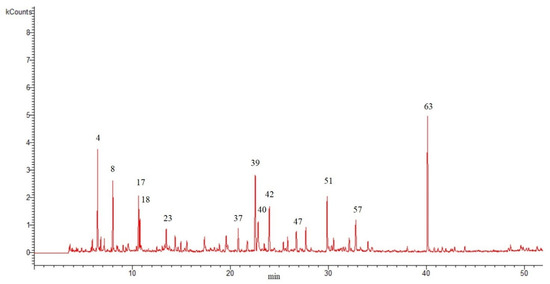
Figure 1.
Gas chromatogram for oil extracted from aerial parts of S. peyronii obtained from hydro-distillation of the fresh sample (the numbers above the peaks indicate the major chemicals that were identified and are listed by the same numbers in Table 1).
The results shown in the table grouped the different constituents of the EO into six classes based on their chemical structures that included monoterpene hydrocarbons (MH), oxygenate monoterpenes (OM), sesquiterpene hydrocarbons (SH), oxygenate sesquiterpenes (OS), aldehydes and ketones (AK), and other compounds (Figure 2). The GC/MS analysis resulted in the identification of a total of 66 constituents that represented 98.16% of the total composition (Table 1).

Figure 2.
Identified chemical groups from the essential oil of S. peyronii; monoterpenes hydrocarbon (MH), oxygenated monoterpenes (OM), sesquiterpenes hydrocarbon (SH), oxygenated sesquiterpenes (OS), aldehyde and ketone (AK), and others.
The EO obtained from the aerial flowering parts of S. peyronii was dominated by OS that accounted for 29.34% of the total composition. Z,Z-farnesyl acetone (11.04%) and spathulenol (4.58%) were the main components detected in this class. OM was detected at slightly lower concentrations (26.01%) and was represented mainly by dehydrosabinaketone (3.76%) and citronellyl acetate (2.07%). Other classes found to have appreciable concentration levels in the analyzed EO included SH (18.78%), in addition to carbonyl-containing compounds (AK), which amounted to 12.89% of the total composition.
To the best of our knowledge, this is the first report on the determination of the chemical constituents of the hydro-distilled essential oil extracted from fresh aerial parts of S. peyronii. There have been some studies reporting the essential oil composition of other different species of the same genus [35,36,37,38,39]. In these studies, volatile terpenoids, such as humulene, β-caryophyllene, caryophyllene oxide, phytol, linalool, 6α-acetoxymanoyl, and oxide, in addition to non-terpenoidal compounds, such as 1-octen-3-ol, 6,10,14-trimethyl-2-pentadecanone, and pentadecanone, are common in the Scrophularia genus [35,36,37,38,39]. In general, it was noticed that oxygenated monoterpenes were the main class of terpenoids detected in the essential oils of plants belonging to the Scrophulariaceae species. The major constituents detected in the essential oils extracted from three Scrophularia species growing wild in Iran, including S. amplexicaulis, S. frigida, and S. subaphylla, were eugenol and linalool [37,38,39]. The essential oil extracted from the aerial parts of Scrophularia deserti from Vietnam and Iran contained mainly α-pinene [40]. The main constituents of S. oxysepala were phytol, methyl benzyl alcohol, dehydrodieugenol, methyl benzaldehyde, and eugenol [35]. Hexahydrofarnesyl acetone, phytol, palmitic acid, β-damascenone, and copaene were the main components of the oil from S. umbrosa [41]. The compounds caryophyllene oxide, spathulenol, α-cadinol, and docosane were the main components of the oil from S. striata [42].
Our results are consistent with the previous studies conducted on other Scrophularia species. However, it was noticed that fatty acids were not detected in our current study. Factors such as those related to the plant’s genotypes, environmental factors, and experimental conditions could justify the differences in the chemical composition of the different plant species of the same genus.
3.2. Phytochemical Analysis
The main classes of secondary metabolites in the different fractions obtained from S. peyronii extract were determined by chemical methods. This phytochemical analysis revealed unique patterns (Table 2). The Sp-M fraction was found to be rich in alkaloids, flavonoids, saponins, anthraquinone, and glycosides. Fraction Sp-B, on the other hand, contained flavonoids, glycosides, saponins, and anthraquinone. Even though plants synthesize these secondary metabolites for unknown reasons, sometimes as part of their defense system, these different classes of secondary metabolites are important for their pharmacological effects [31,32,33,34].

Table 2.
Secondary metabolite classes detected in the extracts of S. peyronii from Jordan.
3.3. Phytochemical Profiling of Crude Extracts by Using LC-MS/MS
In the current investigation, the presence of a selected set of constituents in the Sp-M and Sp-B fractions was determined qualitatively by LC-MS using negative ionization modes. The total ion chromatograms (TICs) for the two fractions are shown in Figure 3. The two extracts were tested for the presence of a selected set of compounds (Table 3), including eight flavonoids, six phenolic acids, six iridoids, and one organic acid. These compounds in general were common to both the genus and the family [4,5,6,7,8,9,10,11,12,13,14,15,16].

Figure 3.
LC-MS chromatogram of Sp-M and Sp-B extracts obtained from S. peyronii from Jordan.
In general, the majority of compounds were detected in both investigated fractions (Table 3). It was noticed that, of the six investigated iridoids, three were not detected in the Sp-M fraction. These were scropolioside B, 6′-O-cinnamoylharpagide, and 6-O-methylcatapol. The phenolic compound, isoferulic acid, was also not detected in the Sp-M fraction.
Research on natural product chemistry is driven by the need to isolate and identify new compounds with interesting therapeutic effects. Plants belonging to the Scrophularia genus are well recognized for their use in traditional medicine for the treatment of many ailments, including fever, swelling, and constipation [4]. The presence of a wide spectrum of bioactive secondary metabolites, including glycoside esters, phenylpropanoid glycosides, saponins, and iridoids, is one of these species’ primary distinguishing characteristics. In particular, phenylpropanoids, glycosides, and iridoids are quite common in Scrophularia plants and have been found to have apparent therapeutic potential in numerous studies [43]. While phenylpropanoids are well known for their beneficial biological effects, including antioxidant, hepatoprotective, antitumor, and anti-inflammatory properties, Scrophularia plants are characterized by the detection of iridoids [43]. Iridoids are quite interesting in terms of their chemical and bioactivity properties. According to information gained from various studies, iridoids isolated from S. buergeriana roots were found to have interesting anti-inflammatory, anti-cancer, and antiprotozoal effects [20,44]. E-p-Methoxycinnamic acid, which was isolated from S. buergeriana, exhibited anti-amnesic properties and shielded cultured neuronal cells from glutamate-induced neurotoxicity [44]. The isolation of several phenylpropanoid esters, Buergeriside A1, Buergeriside B1, and (E)-p-methoxycinnamic acid from the roots of S. buergeriana showed better protection against glutamate-induced neurodegeneration [45,46]. The iridoids scropolioside B and scropolioside D isolated from S. dentata showed anti-inflammatory effects [4,45,46].

Table 3.
The compounds identified in the Sp-M and Sp-B fractions from S. peyronii growing wild in Jordan.
Table 3.
The compounds identified in the Sp-M and Sp-B fractions from S. peyronii growing wild in Jordan.
| No. | Rt (min) | m/z Meas. | MM Calculated | Name | Structure | Molecular Formula | Fractions | Classification | |
|---|---|---|---|---|---|---|---|---|---|
| Sp-M | Sp-B | ||||||||
| 1 | 0.99 | 117.0176 | 118.049 | Succinic acid | 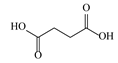 | C4H6O4 | + | + | Aliphatic acid |
| 2 | 2.14 | 375.1257 | 376.133 | 3,4-Dihydro-methyl catalpol | 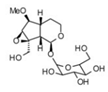 | C16H24O10 | + | + | Iridoid |
| 3 | 3.45 | 625.1359 | 626.1432 | Isorhamnetin-3-O-rutinoside | 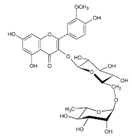 | C28H32O16 | + | + | Flavonoid |
| 4 | 3.81 | 811.2823 | 812.2896 | Scropolioside B | 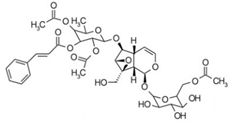 | C41H46O17 | − | + | Iridoid |
| 5 | 4.15 | 179.0325 | 180.0398 | Caffeic acid | 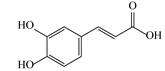 | C9H8O4 | + | + | Phenolic acid |
| 6 | 4.49 | 163.0379 | 164.0452 | p-Coumaric acid | 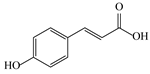 | C9H8O3 | + | + | Phenolic acid |
| 7 | 5.41 | 609.1411 | 610.1484 | Rutin | 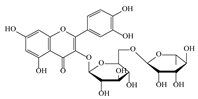 | C27H30O16 | + | + | Flavonoid |
| 8 | 5.61 | 609.1395 | 610.1467 | 3-Glu-7-Rha Quercetin | 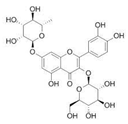 | C27H30O16 | + | + | Flavonoid |
| 9 | 5.76 | 495.203 | 496.2103 | 6′-O-cinnamoylharpagide | 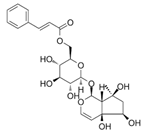 | C24H30O11 | − | + | Iridoid |
| 10 | 5.78 | 175.0377 | 194.0554 | Isoferulic acid | 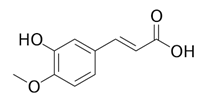 | C10H10O4 | − | + | Phenolic acid |
| 11 | 5.78 | 463.0837 | 464.0909 | Hyperoside | 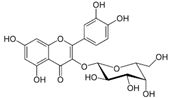 | C21H20O12 | + | + | Flavonoid |
| 12 | 6.35 | 593.1463 | 594.1535 | 3-O-Neohesperidoside Kaempferol | 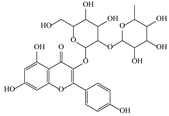 | C27H30O15 | + | + | Flavonoid |
| 13 | 6.56 | 447.0892 | 448.0965 | Kaempferol-3-O-glucoside | 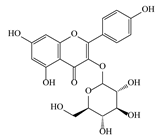 | C21H20O11 | + | + | Flavonoid |
| 14 | 6.57 | 495.3129 | 496.3202 | Nepitrin | 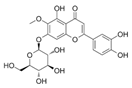 | C22H22O13 | + | + | Flavonoid |
| 15 | 7.96 | 377.0807 | 756.1759 | 6-O-Methylcatapol | 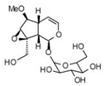 | C16H24O10 | − | + | Iridoid |
| 16 | 8.72 | 147.0427 | 148.05 | Cinnamic acid | 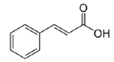 | C9H8O2 | + | + | Phenolic acid |
| 17 | 11.09 | 751.2377 | 752.2449 | Scropolioside A | 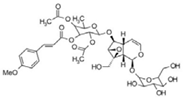 | C35H44O18 | + | + | Iridoid |
| 18 | 13.67 | 752.2377 | 753.245 | Scrovalentinoside | 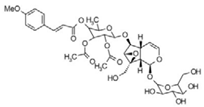 | C35H44O18 | + | + | Iridoid |
| 19 | 27.05 | 478.3855 | 479.3928 | Homoplantaginin | 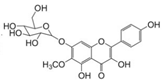 | C22H22O11 | + | + | Flavonoid |
| 20 | 29.97 | 351.2467 | 352.254 | Ningposide C | 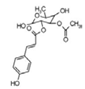 | C17H20O8 | + | + | Phenolic acid |
| 21 | 30.07 | 339.3222 | 340.3295 | 3-O-trans-Feruloylrhamnopyranose | 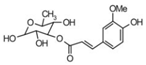 | C16H20O8 | + | + | Phenolic acid |
(+): Detected; (−): not detected; samples were verified against authentic samples isolated in our labs or purchased from Sigma-Aldrich. Rt refers to retention time in minutes, m/z refers to Mass to charge ratio.
3.4. Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity
The results of the TPC, TFC, and antioxidant activity determined for the two fractions (Sp-M and Sp-B) and the EO obtained from aerial parts of S. peyronii are shown in Table 4. As could be deduced from the obtained data, Sp-B had the highest TPC and TFC (51.90 ± 1.0 mg/g gallic acid equivalents; 148.54 ± 1.0 mg/g quercetin equivalents) as compared with the other fraction (Sp-M) or EO. The results of the IC50 values shown in Table 4 also revealed that, while the DPPH radical scavenging properties of both investigated fractions were comparable, Sp-B had much higher ABTS scavenging activity ((2.5 ± 0.02) × 10−2 mg/mL) when compared with the other investigated fraction (Sp-M: (26.0 ± 0.02) × 10−2 mg/mL). The observed activity for both fractions could be attributed to the high TPC and TFC of this extract [47,48]. This was also in total agreement with the observed LC-MS/MS results, which revealed the richness of the Sp-B fraction in phenolic acids, flavonoids, and iridoids.

Table 4.
Results of TPC (mg/g gallic acid equivalents), TFC (mg/g quercetin equivalents), and IC50 (mg/mL) for the antioxidant activity of the different fractions of S. peyronii from Jordan.
Based on the obtained IC50 values, the EO exhibited significant antiradical activity when compared with the employed positive controls (ascorbic acid and -tocopherol). This could be attributed to the significant content of monoterpene and oxygenated terpenoids, such as limonene, terpineol, (E)-ionone, and citronellol, which are known for their antioxidant properties, all of which were detected in the essential oil obtained from the aerial parts of S. peyronii essential oil [49,50]. Additionally, the high content of pulegone and spathulenol, known also for their antioxidant power, may account for the observed high antioxidant activity of the essential oil obtained from S. peyronii in our results as compared with the other EOs tested, as indicated in many mint species [51].
4. Conclusions
The current study is the first report on the phytochemical screening of S. peyronii that included the determination of TPC and TFC, and the evaluation of the antioxidant activity of the two main extracts and the essential oil obtained from the aerial parts of the plant material. Additionally, the essential oil composition and qualitative determination of the selected phenolic compounds and flavonoids were determined by GC/MS and LC-ESI-MS/MS techniques, respectively. The results revealed that the investigated S. peyronii extracts had relatively high TPC and TFC, and good antioxidant activity, as determined by the two assay methods (DDPH and ABTS), especially the butanol (Sp-B) extract. The observed antioxidant results were attributed to the presence of numerous phenolic and flavonoid chemicals.
The results of the current study revealed the detection of interesting compounds belonging to the phenolic acid, flavonoid, and iridoid classes. This urges further studies to undertake a detailed phytochemical investigation that is designed to isolate and characterize the active constituents.
Author Contributions
Conceptualization, Y.A.-D., S.A.J.A., N.A.-B., T.A.A., S.T.A.O. and M.A.A.-Q.; methodology, Y.A.-D., T.A.A. and M.A.A.-Q.; software, N.A.-B., T.T.B. and M.A.A.-Q.; validation, N.A.-B., T.T.B., T.A.A., S.T.A.O. and M.A.A.-Q.; formal analysis, Y.A.-D., H.I.A.-J., T.A.A., N.A.-B. and M.A.A.-Q.; investigation, Y.A.-D., S.A.J.A., T.A.A. and M.A.A.-Q.; resources, H.I.A.-J., S.A.J.A., A.A.A.-M. and M.A.A.-Q.; data curation, N.A.-B., T.T.B., T.A.A., S.T.A.O. and M.A.A.-Q.; writing—original draft preparation, Y.A.-D., S.A.J.A., T.A.A., H.I.A.-J., S.T.A.O. and M.A.A.-Q.; writing—review and editing, Y.A.-D., S.A.J.A., T.A.A., M.S.A.-S., A.A.A.-M., A.G.A., S.T.A.O. and M.A.A.-Q.; visualization, Y.A.-D., H.I.A.-J., T.A.A., N.A.-B., A.A.A.-M., A.G.A. and M.A.A.-Q.; supervision, S.T.A.O. and M.A.A.-Q.; project administration, Y.A.-D., S.T.A.O. and M.A.A.-Q.; funding acquisition, Y.A.-D., S.A.J.A., N.A.-B., M.S.A.-S., A.A.A.-M., A.G.A. and M.A.A.-Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research and Graduate Studies at Yarmouk University for funding this research. Additionally, we would like to thank the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University for funding this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bandyopadhyay, M.; Chakraborty, R.; Raychaudhuri, U. A process for preparing a natural antioxidant enriched dairy product (Sandesh). LWT Food Sci. Technol. 2007, 40, 842–851. [Google Scholar] [CrossRef]
- Zohary, M.; Feinbrun-Dothan, N. Flora Palastina; Israel Academy of Sciences and Humanitie: Jerusalem, Israel, 1966. [Google Scholar]
- Oran, S.A.S. Flora of Bader Al-Jadida County, western high mountains of Amman city/Jordan. Int. J. Herb. Med. 2015, 3, 49–59. [Google Scholar]
- Pasdaran, A.; Hamedi, A. The genus Scrophularia: A source of iridoids and terpenoids with a diverse biological activity. Pharm. Biol. 2017, 55, 2211–2233. [Google Scholar] [CrossRef] [PubMed]
- Giner, R.M.; Villalba, M.L.; Recio, M.C.; Máñez, S.; Cerdá-Nicolás, M.; Rίos, J.L. Anti-inflammatory glycoterpenoids from Scrophularia auriculata. Eur. J. Pharmacol. 2000, 389, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Calis, I.; Gross, G.A.; Sticher, O. Two phenylpropanoid glycosides from Scrophularia scopolii. Phytochemistry 1988, 27, 1465–1468. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Fontaine, J.; Malonne, H.; Claeys, M.; Luhmer, M.; Duez, P. A sugar ester and an iridoid glycoside from Scrophularia ningpoensis. Phytochemistry 2005, 66, 1186–1191. [Google Scholar] [CrossRef]
- Monsef-Esfahani, H.R.; Hajiaghaee, R.; Shahverdi, A.R.; Khorramizadeh, M.R.; Amini, M. Flavonoids, cinnamic acid and phenyl propanoid from aerial parts of Scrophularia striata. Pharm. Biol. 2010, 48, 333–336. [Google Scholar] [CrossRef]
- Bhandari, S.P.S.; Babu, U.V.; Garg, H.S. A triterpene glycoside from Scrophularia koelzii. Phytochemistry 1997, 45, 1717–1719. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Simmonds, M.S.; Sampson, J.; Houghton, P.J.; Grice, P. Wound healing activity of acylated iridoid glycosides from Scrophularia nodosa. Phytother. Res. 2002, 16, 33–35. [Google Scholar] [CrossRef]
- Mahmoud, B.; Hadavi, M.; Abbasi, N. Study of extraction and chemical compounds of Scrophularia striata Boiss. and Scrophularia deserti Delile using HS-SPME and GC-MS. Plant Biotechnol. Persa 2020, 2, 8–13. [Google Scholar] [CrossRef]
- Dıaz, A.M.; Abad, M.J.; Fernández, L.; Silván, A.M.; De Santos, J.; Bermejo, P. Phenylpropanoid glycosides from Scrophularia scorodonia: In vitro anti-inflammatory activity. Life Sci. 2004, 74, 2515–2526. [Google Scholar] [CrossRef]
- Panda, P.; Appalashetti, M.; MA Judeh, Z. Phenylpropanoid sucrose esters: Plant-derived natural products as potential leads for new therapeutics. Curr. Med. Chem. 2011, 18, 3234–3251. [Google Scholar] [CrossRef]
- Li, Y.M.; Jiang, S.H.; Gao, W.Y.; Zhu, D.Y. Phenylpropanoid glycosides from Scrophularia ningpoensis. Phytochemistry 2000, 54, 923–925. [Google Scholar] [CrossRef]
- Fernandez, M.A.; Saenz, M.T.; Garcia, M.D. Anti-inflammatory activity in rats and mice of phenolic acids isolated from Scrophularia frutescens. J. Pharm. Pharmacol. 1998, 50, 1183–1186. [Google Scholar] [CrossRef]
- Zhu, L.J.; Hou, Y.L.; Shen, X.Y.; Pan, X.D.; Zhang, X.; Yao, X.S. Monoterpene pyridine alkaloids and phenolics from Scrophularia ningpoensis and their cardioprotective effect. Fitoterapia 2013, 88, 44–49. [Google Scholar] [CrossRef]
- Kim, S.R.; Kim, Y.C. Neuroprotective phenylpropanoid esters of rhamnose isolated from roots of Scrophularia buergeriana. Phytochemistry 2000, 54, 503–509. [Google Scholar] [CrossRef]
- Baradaran, P.C.; Mohammadi, A.; Mansoori, B.; Baradaran, S.C.; Baradaran, B. Growth inhibitory effect of Scrophularia oxysepala extract on mouse mammary carcinoma 4T1 cells in vitro and in vivo systems. Biomed. Pharmacother. 2017, 85, 718–724. [Google Scholar] [CrossRef]
- Alqasoumi, S.I. Evaluation of the hepatroprotective and nephroprotective activities of Scrophularia hypericifolia growing in Saudi Arabia. Saudi Pharm. J. 2014, 22, 258–263. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, K.Y.; Koo, K.A.; Sung, S.H.; Lee, N.G.; Kim, J.; Kim, Y.C. Four new neuroprotective iridoid glycosides from Scrophularia buergeriana roots. J. Nat. Prod. 2002, 65, 1696–1699. [Google Scholar] [CrossRef]
- Sohn, S.H.; Ko, E.; Jeon, S.B.; Lee, B.J.; Kim, S.H.; Dong, M.S.; Lee, D.; Kwak, J.; Kim, Y.; Shin, M.; et al. The genome-wide expression profile of Scrophularia ningpoensis-treated thapsigargin-stimulated U-87MG cells. Neurotoxicology 2009, 30, 368–376. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Al-Jaber, H.I.; Zarga, M.H.A.; Orabi, S.T.A. Flavonoid and phenolic compounds from Salvia palaestina L. growing wild in Jordan and their antioxidant activities. Phytochemistry 2014, 99, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Al-Humaidi, J.Y.; Al-Qudah, M.A.; Al-Saleem, M.S.; Alotaibi, S.M. Antioxidant activity and chemical composition of essential oils of selected Cleome species growing in Saudi Arabia. Jordan J. Chem. 2019, 14, 29–37. Available online: https://jjc.yu.edu.jo/index.php/jjc/article/view/25 (accessed on 1 May 2023).
- Abu-Orabi, S.T.; Al-Qudah, M.A.; Saleh, N.R.; Bataineh, T.T.; Obeidat, S.M.; Al-Sheraideh, M.S.; Al-Jaber, H.I.; Tashtoush, H.I.; Lahham, J.N. Antioxidant activity of crude extracts and essential oils from flower buds and leaves of Cistus creticus and Cistus salviifolius. Arab. J. Chem. 2020, 13, 6256–6266. [Google Scholar] [CrossRef]
- Al-Dalahmeh, Y.; Al-Bataineh, N.; Al-Balawi, S.S.; Lahham, J.N.; Al-Momani, I.F.; Al-Sheraideh, M.S.; Mayyas, A.S.; Abu-Orabi, S.T.; Al-Qudah, M.A. LC-MS/MS screening, total phenolic, flavonoid and antioxidant contents of crude extracts from three Asclepiadaceae species growing in Jordan. Molecules 2022, 27, 859. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, R.Q.; Almasarweh, A.B.; Abdo, N.M.; Mayyas, A.S.; Al-Qudah, M.A.; Abu-Orabi, S.T. Chromatographic analysis (LC-MS and GC-MS), antioxidant activity, antibacterial activity, total phenol, and total flavonoid determination of Cleome arabica L. growing in Jordan. Int. J. Food Prop. 2022, 25, 1920–1933. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Migdadi, R.S.; Mayyas, A.S.; Al-Zereini, W.A.; Al-Dalahmeh, Y.; Abu Orabi, F.M.; Bataineh, T.T.; Abu-Orabi, S.T. Chemical Composition, Cytotoxicity and Antioxidant Activity of the Essential Oil from Flower Buds and Leaves of the Pulicaria incisa (Lam.) DC and Pulicaria crispa (Forskel) Oliver. J. Essent. Oil-Bear. Plants 2022, 25, 758–772. [Google Scholar] [CrossRef]
- Lo’ay, A.; Abu-Orabi, S.T.; Hlail, H.M.; Alkhatib, R.Q.; Al-Dalahmeh, Y.; Al-Qudah, M.A. Anthemis cotula L. from Jordan: Essential oil composition, LC-ESI-MS/MS profiling of phenolic acids-flavonoids and in vitro antioxidant activity. Arab. J. Chem. 2023, 16, 104470. [Google Scholar] [CrossRef]
- Al-Bataineh, N.; Algethami, F.K.; Al-Jaber, H.I.; Alhamzani, A.G.; Bataineh, R.M.; Al-Dalahmeh, Y.; Bataineh, T.T.; Abu-Orabi, S.T.; Al-Qudah, M.A. Ballota saxatilis from Jordan: Evaluation of Essential Oil Composition and Phytochemical Profiling of Crude Extracts and Their In-Vitro Antioxidant Activity. Separations 2023, 10, 114. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Al-Smadi, Z.M.; Al-Jaber, H.I.; Tashtoush, H.I.; Alkhatib, R.Q.; Bataineh, T.T.; Al-Dalahmeh, Y.; Orabi, S.T.A. GC/MS and LC-MS/MS phytochemical evaluation of the essential oil and selected secondary metabolites of Ajuga orientalis from Jordan and its antioxidant activity. Arab. J. Chem. 2023, 16, 104641. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Ali, M. Practical Pharmaceutical Chemistry; CBS Publishers & Distributors: New Delhi, India, 1997. [Google Scholar]
- Wadood, A.; Ghufran, M.; Jamal, S.B.; Naeem, M.; Khan, A.; Ghaffar, R. Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem. Anal Biochem. 2013, 2, 144. [Google Scholar] [CrossRef]
- Banu, K.S.; Cathrine, L. General techniques involved in phytochemical analysis. Int. J. Adv. Res. Chem. Sci. 2015, 2, 25–32. [Google Scholar]
- Ajayi, I.A.; Ajibade, O.; Oderinde, R.A. Preliminary phytochemical analysis of some plant seeds. Res. J. Chem. Sci. 2011, 1, 58–62. [Google Scholar]
- Pasdaran, A.; Nahar, L.; Asnaashari, S.; Sarker, S.D.; Delazar, A. Gc-ms analysis, free-radical-scavenging and insecticidal activities of essential oil of Scrophularia oxysepala boiss. Pharm. Sci. 2013, 19, 1–5. [Google Scholar]
- Miyazawa, M.; Okuno, Y. Volatile components from the roots of Scrophularia ningpoensis Hemsl. Flavour Fragr. J. 2003, 18, 398–400. [Google Scholar] [CrossRef]
- Asgharian, P.; Afshar, F.H.; Asnaashari, S.; Moghaddam, S.B.; Delazar, A. The seasonal variations of the chemical composition of essential oil obtained from Scrophularia frigida. Jundishapur J. Nat. Pharm. Prod. 2016, 11, 1–5. [Google Scholar] [CrossRef]
- Pasdaran, A.; Delazar, A.; Nazemiyeh, H.; Nahar, L.; Sarker, S.D. Chemical composition, and antibacterial (against Staphylococcus aureus) and free-radical-scavenging activities of the essential oil of Scrophularia amplexicaulis Benth. Rec. Nat. Prod. 2012, 6, 350–355. [Google Scholar]
- Asgharian, P.; Afshar, F.H.; Asnaashari, S.; Moghaddam, S.B.; Ebrahimi, A.; Delazar, A. Characterization of terpenoids in the essential oil extracted from the aerial parts of Scrophularia subaphylla growing in Iran. Adv. Pharm. Bull. 2015, 5, 557–561. [Google Scholar] [CrossRef]
- Mardani, M.; Rezapour, S.; Eftekhari, Z.; Majid, A.S.; Rashidipour, M.; Afsordeh, O.; Kazemzadeh, F.; Bahmani, F. Chemical composition of Scrophularia deserti. Int. J. Pharm. Tech Res. 2016, 9, 285–290. [Google Scholar] [CrossRef]
- Nikkhah, E.; Asnaashari, S.; Babaei, H.; Heshmati Afshar, F.; Delazar, A. Chemical composition and biological activities of essential oil and methanol extract of Scrophularia umbrosa. Res. J. Pharmacogn. 2017, 4, 41–50. [Google Scholar]
- Kerdar, T.; Moradkhani, S.; Dastan, D. Phytochemical and biological studies of Scrophularia striata from Ilam. Jundishapur J. Nat. Pharm. Prod. 2018, 13, e62705. [Google Scholar] [CrossRef]
- Ren, D.; Shen, Z.Y.; Qin, L.P.; Zhu, B. Pharmacology, phytochemistry, and traditional uses of Scrophularia ningpoensis Hemsl. J. Ethnopharmacol. 2021, 269, 113688. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.A.; Kim, H.L.; Lee, D.R.; Choi, B.K.; Yang, S.H. Anti-inflammatory effects of Scrophularia buergeriana extract mixture fermented with lactic acid bacteria. Biotechnol. Bioprocess Eng. 2022, 27, 370–378. [Google Scholar] [CrossRef]
- Lee, H.J.; Spandidos, D.A.; Tsatsakis, A.; Margina, D.; Izotov, B.N.; Yang, S.H. Neuroprotective effects of Scrophularia buergeriana extract against glutamate-induced toxicity in SH-SY5Y cells. Int. J. Mol. Med. 2019, 43, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, T.; Qian, F.; Xu, J.; Dorje, G.; Zhao, Z.; Li, Y. Iridoid glycosides isolated from Scrophularia dentata Royle ex Benth. and their anti-inflammatory activity. Fitoterapia 2014, 98, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lympho-cytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef]
- Guo, Y.; Baschieri, A.; Amorati, R.; Valgimigli, L. Synergic antioxidant activity of γ-terpinene with phenols and polyphe-nols enabled by hydroperoxyl radicals. Food Chem. 2021, 345, 128468. [Google Scholar] [CrossRef]
- Do Nascimento, K.F.; Moreira, F.M.F.; Santos, J.A.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Formagio, A.S.N. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guin-eense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).