iTRAQ-Based Quantitative Proteomics Unveils Protein Dynamics in the Root of Solanum melongena L. under Waterlogging Stress Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sources, Cultivation Conditions, and Waterlogging Stress Interventions
2.2. Protein Isolation
2.3. Protein Quantification and Proteolysis
2.4. iTRAQ Tagging and Separation
2.5. Capillary Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
2.6. Statistical Analysis
2.7. Bioinformatics and Annotations
3. Results
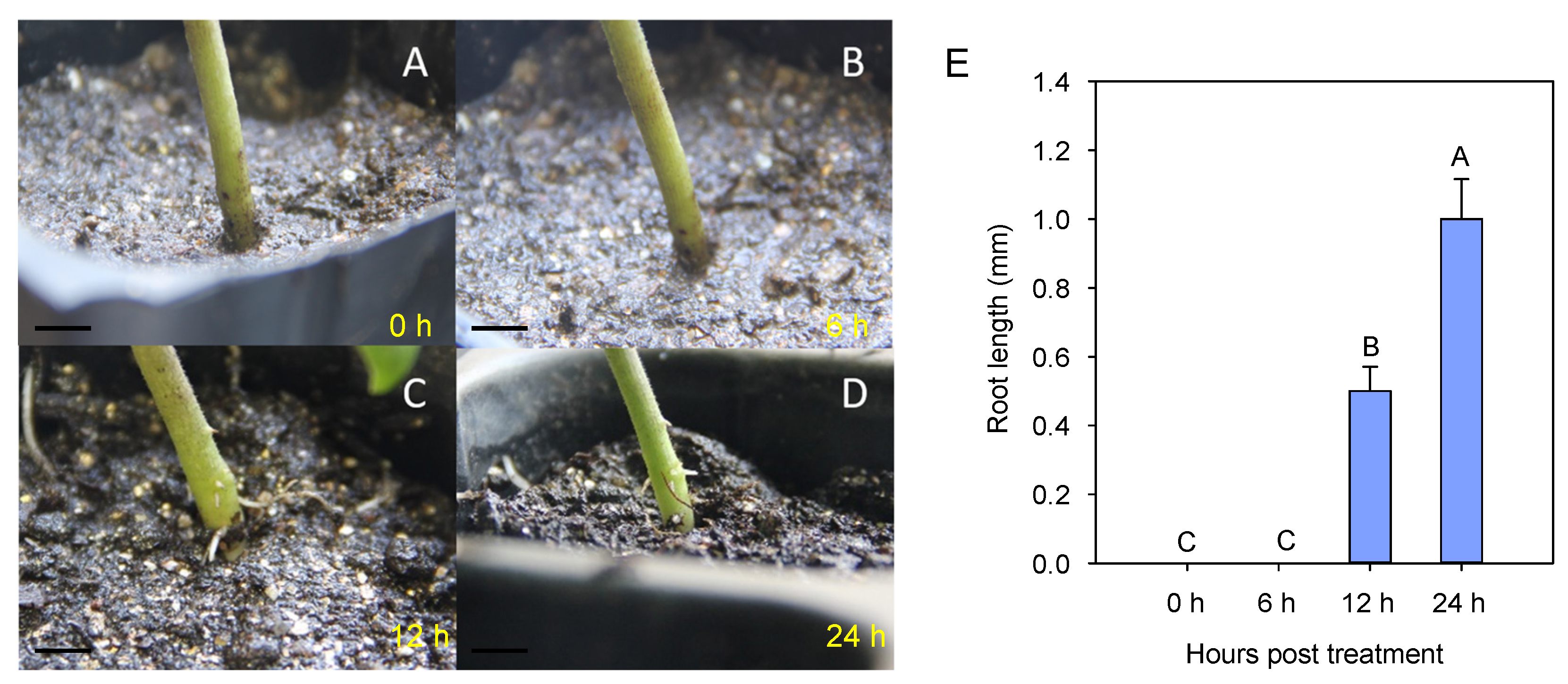
3.1. Stress Symptoms in Eggplant Seedlings during Waterlogging
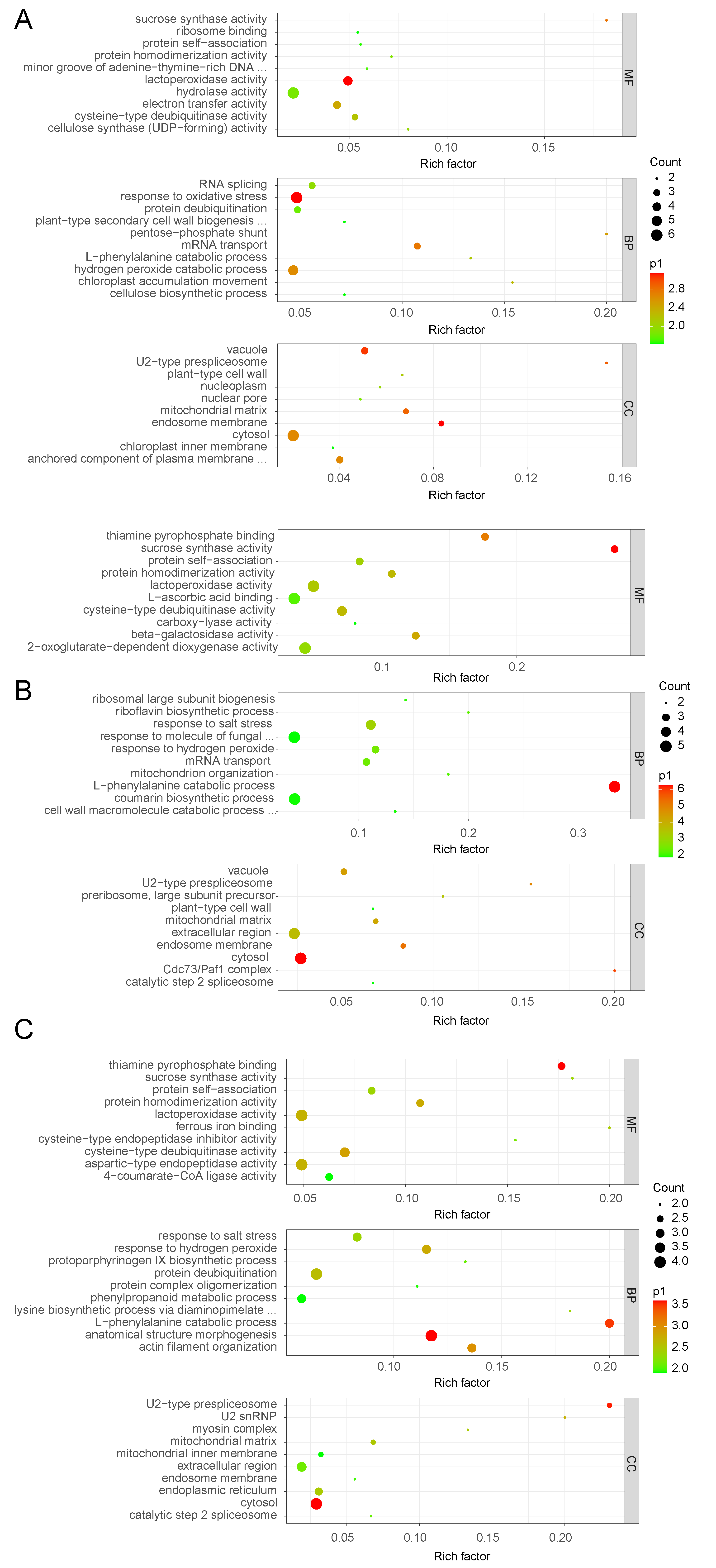
3.2. Protein Response to Waterlogging Stress in the Root of Solanum Melongena Identified with GO Analysis
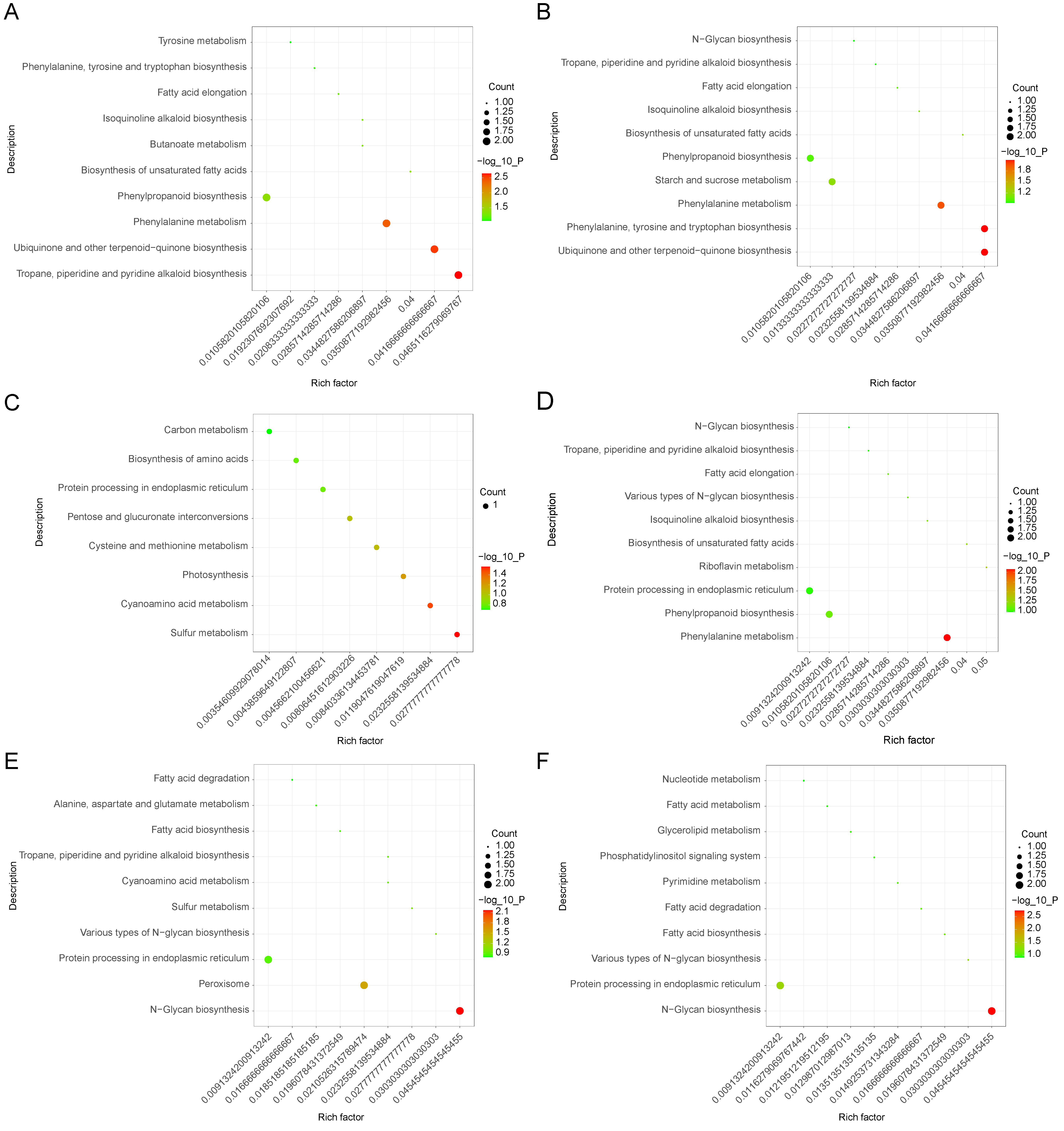
3.3. Analysis of KEGG Pathway Enrichment of Eggplant Treated with Waterlogging Stress
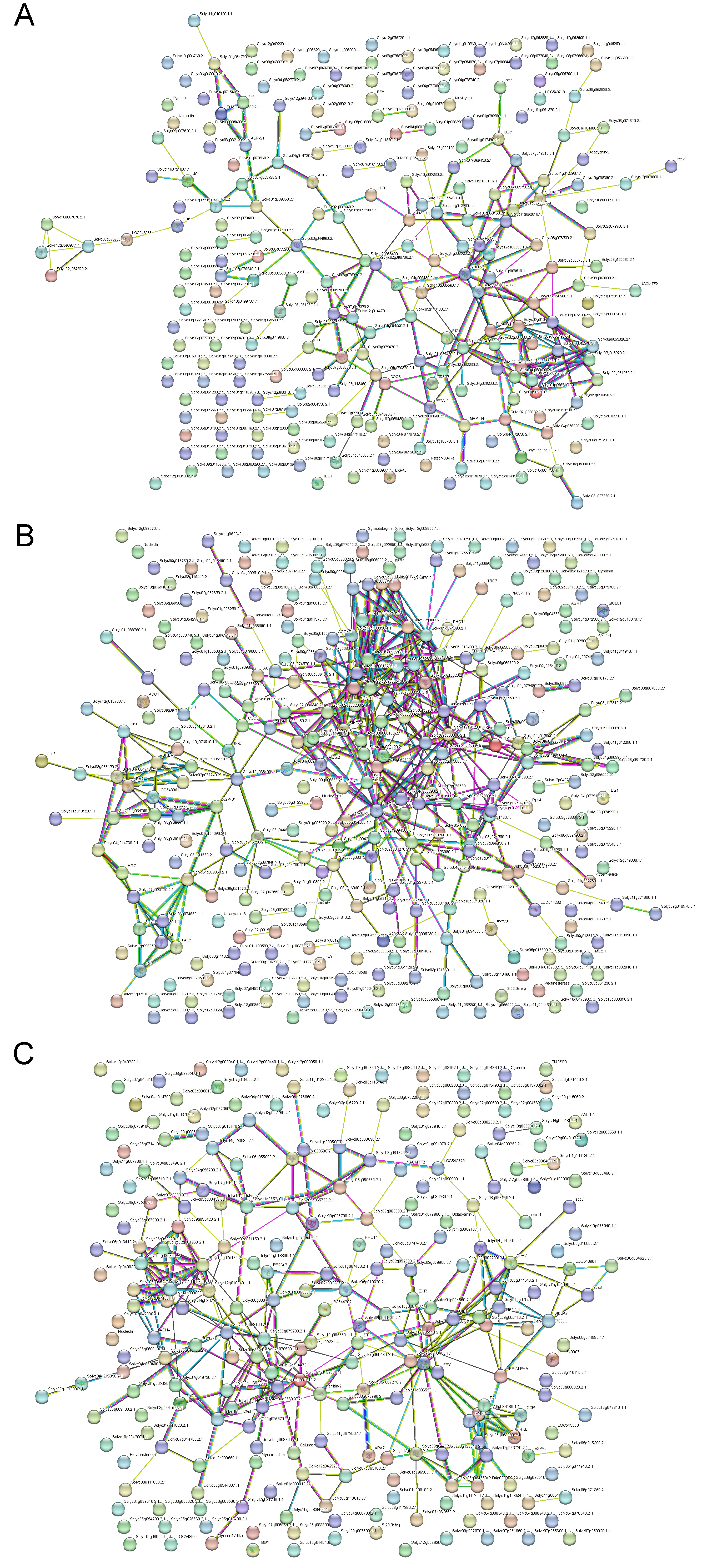
3.4. Protein–Protein Interaction Analysis
3.5. Analysis of Waterlogging-Related Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langan, P.; Bernad, V.; Walsh, J.; Henchy, J.; Khodaeiaminjan, M.; Mangina, E.; Negrao, S. Phenotyping for waterlogging tolerance in crops: Current trends and future prospects. J. Exp. Bot. 2022, 73, 5149–5169. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing crops: Effective flooding survival strategies. Plant. Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, O.; Perata, P.; Voesenek, L. Flooding and low oxygen responses in plants. Funct. Plant. Biol. 2017, 44, iii–vi. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.B.; Ishizawa, K.; Ito, O. Evolution and mechanisms of plant tolerance to flooding stress. Ann. Bot. 2009, 103, 137–142. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant. Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Gimeno, V.; Syvertsen, J.P.; Simon, I.; Martinez, V.; Garcia-Sanchez, F. Interstock of ‘Valencia’ orange affects the flooding tolerance in ‘Verna’ lemon trees. HortScience 2012, 47, 403–409. [Google Scholar] [CrossRef]
- Else, M.A.; Coupland, D.; Dutton, L.; Jackson, M.B. Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiol. Plant. 2001, 111, 46–54. [Google Scholar] [CrossRef]
- Kumar, P.; Pal, M.; Joshi, R.; Sairam, R.K. Yield, growth and physiological responses of mung bean [Vigna radiata (L.) Wilczek] genotypes to waterlogging at vegetative stage. Physiol. Mol. Biol. Plants 2013, 19, 209–220. [Google Scholar] [CrossRef]
- Liu, Z.B.; Cheng, R.M.; Xiao, W.F.; Guo, Q.S.; Wang, N. Effect of off-season flooding on growth, photosynthesis, carbohydrate partitioning, and nutrient uptake in Distylium chinense. PLoS ONE 2014, 9, e107636. [Google Scholar] [CrossRef]
- Lopes, N.G.M.; Kloss, R.B.; Dos Santos, I.C.; Souza, V.L.; Prasad, M.N.V.; Mangabeira, P.A.O.; Franca, M.G.C. Soil flooding and its outcome on cadmium and nutrient uptake affect photosynthetic activity in Inga laurina plants. Ecotoxicology 2023, 32, 73–81. [Google Scholar] [CrossRef]
- Shabala, S. Physiological and cellular aspects of phytotoxicity tolerance in plants: The role of membrane transporters and implications for crop breeding for waterlogging tolerance. New Phytol. 2011, 190, 289–298. [Google Scholar] [CrossRef]
- Li, C.W.; Su, J.S.; Zhao, N.; Lou, L.; Ou, X.L.; Yan, Y.J.; Wang, L.K.; Jiang, J.F.; Chen, S.M.; Chen, F.D. CmERF5-CmRAP2.3 transcriptional cascade positively regulates waterlogging tolerance in Chrysanthemum morifolium. Plant. Biotechnol. J. 2023, 21, 270–282. [Google Scholar] [CrossRef]
- Raineri, J.; Caraballo, L.; Rigalli, N.; Portapila, M.; Otegui, M.E.; Chan, R.L. Expressing the sunflower transcription factor HaHB11 in maize improves waterlogging and defoliation tolerance. Plant. Physiol. 2022, 189, 230–247. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Huang, S.N.; Wang, G.; Xuan, J.P.; Guo, Z.R. Overexpression of Actinidia deliciosa pyruvate decarboxylase 1 gene enhances waterlogging stress in transgenic Arabidopsis thaliana. Plant. Physiol. Biochem. 2016, 106, 244–252. [Google Scholar] [CrossRef]
- Phukan, U.J.; Jeena, G.S.; Tripathi, V.; Shukla, R.K. MaRAP2-4, a waterlogging-responsive ERF from Mentha, regulates bidirectional sugar transporter AtSWEET10 to modulate stress response in Arabidopsis. Plant. Biotechnol. J. 2018, 16, 221–233. [Google Scholar] [CrossRef]
- Kosova, K.; Vitamvas, P.; Prasil, I.T.; Renaut, J. Plant proteome changes under abiotic stress--contribution of proteomics studies to understanding plant stress response. J. Proteom. 2011, 74, 1301–1322. [Google Scholar] [CrossRef]
- Chen, S.; Harmon, A.C. Advances in plant proteomics. Proteomics 2006, 6, 5504–5516. [Google Scholar] [CrossRef]
- Wright, N.C.; Pandhal, J.; Biggs, C.A.; Ow, S.Y. Comparative proteomics study of salt tolerance between a nonsequenced extremely halotolerant cyanobacterium and its mildly halotolerant relative using in vivo metabolic labeling and in vitro isobaric labeling. J. Proteome Res. 2009, 8, 818. [Google Scholar]
- Ali, A.E.E.; Husselmann, L.H.; Tabb, D.L.; Ludidi, N. Comparative proteomics analysis between maize and sorghum uncovers important proteins and metabolic pathways mediating drought tolerance. Life 2023, 13, 170. [Google Scholar] [CrossRef]
- Guo, J.; Qu, L.L.; Hu, Y.F.; Lu, W.P.; Lu, D.L. Proteomics reveals the effects of drought stress on the kernel development and starch formation of waxy maize. BMC Plant. Biol. 2021, 21, 434. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.R.; Song, X.Q.; Zhang, F.L.; Liu, Y. TMT-based proteomic analysis of liquorice root in response to drought stress. BMC Genom. 2022, 23, 524. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.K.; Zheng, L.; Li, B.J.; Shen, R.F.; Lan, P. Comparative proteomics reveals new insights into the endosperm responses to drought, salinity and submergence in germinating wheat seeds. Plant. Mol. Biol. 2021, 105, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Singh, K.; Ahuja, S.; Tasleem, M.; Singh, I.; Kumar, S.; Grover, M.; Mishra, D.; Rai, G.K.; Goswami, S.; et al. Quantitative proteomic analysis reveals novel stress-associated active proteins (SAAPs) and pathways involved in modulating tolerance of wheat under terminal heat. Funct. Integr. Genomics 2019, 19, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Yu, J.J.; Li, Y.; Zhang, H.; Bao, X.S.; Bian, J.Y.; Xu, C.X.; Wang, X.L.; Cai, X.F.; Wang, Q.H.; et al. Heat-responsive proteomics of a heat-sensitive spinach variety. Int. J. Mol. Sci. 2019, 20, 3872. [Google Scholar] [CrossRef]
- Fei, L.W.; Chu, J.P.; Zhang, X.; Dong, S.X.; Dai, X.L.; He, M.R. Physiological and proteomic analyses indicate delayed sowing improves photosynthetic capacity in wheat flag leaves under heat stress. Front. Plant. Sci. 2022, 13, 848464. [Google Scholar] [CrossRef]
- Xiong, E.H.; Zhang, C.; Ye, C.; Jiang, Y.H.; Zhang, Y.L.; Chen, F.; Dong, G.J.; Zeng, D.L.; Yu, Y.C.; Wu, L.M. iTRAQ-based proteomic analysis provides insights into the molecular mechanisms of rice formyl tetrahydrofolate deformylase in salt response. Planta 2021, 254, 76. [Google Scholar] [CrossRef]
- Ji, W.; Cong, R.; Li, S.; Li, R.; Qin, Z.W.; Li, Y.J.; Zhou, X.L.; Chen, S.X.; Li, J. Comparative proteomic analysis of soybean leaves and roots by iTRAQ provides insights into response mechanisms to short-term salt stress. Front. Plant Sci. 2016, 7, 573. [Google Scholar] [CrossRef]
- Pottiez, G.; Wiederin, J.; Fox, H.S.; Ciborowski, P. Comparison of 4-plex to 8-plex iTRAQ quantitative measurements of proteins in human plasma samples. J. Proteome Res. 2012, 11, 3774–3781. [Google Scholar] [CrossRef]
- Cui, K.D.; He, L.M.; Zhao, Y.H.; Mu, W.; Lin, J.; Liu, F. Comparative analysis of Botrytis cinerea in response to the microbial secondary metabolite benzothiazole using iTRAQ-based quantitative proteomics. Phytopathology 2021, 111, 1313–1326. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhang, Y.; Wang, M.; Fang, X.A.; Cai, X. Comparative proteomic analysis of latex from Euphorbia kansui laticifers at different development stages with and without UV-B treatment via iTRAQ-coupled two-dimensional liquid chromatography-MS/MS. Funct. Plant Biol. 2019, 47, 67–79. [Google Scholar] [CrossRef]
- Shen, L.; Zhao, E.P.; Liu, R.E.; Yang, X. Transcriptome analysis of eggplant under salt stress: AP2/ERF transcription factor SmERF1 acts as a positive regulator of salt stress. Plants 2022, 11, 2205. [Google Scholar] [CrossRef]
- Wei, Q.Z.; Wang, J.L.; Wang, W.H.; Hu, T.H.; Hu, H.J.; Bao, C.L. A high-quality chromosome-level genome assembly reveals genetics for important traits in eggplant. Hortic. Res. 2020, 7, 153. [Google Scholar] [CrossRef]
- Hammond, J.B.; Kruger, N.J. The bradford method for protein quantitation. Methods Mol. Biol. 1988, 3, 25–32. [Google Scholar]
- Li, S.L.; Zan, H.T.; Zhu, Z.; Lu, D.D.; Krall, L. Plant phosphopeptideidentification and label-free quantification by MaxQuant and Proteome Discoverer Software. Methods Mol. Biol. 2021, 2358, 179–187. [Google Scholar]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Garcia-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Bandeira, N.; Perez-Riverol, Y.; Sharma, V.; Carver, J.J.; Mendoza, L.; Kundu, D.J.; Wang, S.; Bandla, C.; Kamatchinathan, S.; et al. The ProteomeXchange consortium at 10 years: 2023 update. Nucleic Acids Res. 2023, 51, D1539–D1548. [Google Scholar] [CrossRef]
- Khan, D.; Millar, J.L.; Girard, I.J.; Belmonte, M.F. Transcriptional circuitry underlying seed coat development in Arabidopsis. Plant. Sci. 2014, 219–220, 51–60. [Google Scholar] [CrossRef]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Urbanowicz, B.R.; Bharadwaj, V.S.; Alahuhta, M.; Pena, M.J.; Lunin, V.V.; Bomble, Y.J.; Wang, S.; Yang, J.Y.; Tuomivaara, S.T.; Himmel, M.E.; et al. Structural, mutagenic and in silico studies of xyloglucan fucosylation in Arabidopsis thaliana suggest a water-mediated mechanism. Plant. J. 2017, 91, 931–949. [Google Scholar] [CrossRef]
- Li, X.; Rehman, S.U.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Yamaguchi, T.; Sunohara, Y.; Matsumoto, H.; Komatsu, S. Proteomic analysis of the effect of plant-derived smoke on soybean during recovery from flooding stress. J. Proteom. 2018, 181, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.C.; Kim, D.W.; You, Y.N.; Seok, M.S.; Park, J.M.; Hwang, H.; Kim, B.G.; Luan, S.; Park, H.S.; Cho, H.S. Classification of rice (Oryza sativa L. Japonica nipponbare) immunophilins (FKBPs, CYPs) and expression patterns under water stress. BMC Plant Biol. 2010, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Yadav, N.S.; Tiwari, V.; Agarwal, P.K.; Jha, B. A SNARE-like superfamily protein SbSLSP from the halophyte Salicornia brachiata confers salt and drought tolerance by maintaining membrane sStability, K(+)/Na(+) ratio, and antioxidant machinery. Front. Plant. Sci. 2016, 7, 737. [Google Scholar] [CrossRef] [PubMed]
- Leshem, Y.; Golani, Y.; Kaye, Y.; Levine, A. Reduced expression of the v-SNAREs AtVAMP71/AtVAMP7C gene family in Arabidopsis reduces drought tolerance by suppression of abscisic acid-dependent stomatal closure. J. Exp. Bot. 2010, 61, 2615–2622. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Fang, L.; Zheng, H.K.; Zhang, Y.; Chen, J.; Zhang, Z.J.; Wang, J.; Li, S.T.; Li, R.Q.; Bolund, L.; et al. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2006, 34, W293–W297. [Google Scholar] [CrossRef] [PubMed]
- Lahrmann, U.; Strehmel, N.; Langen, G.; Frerigmann, H.; Leson, L.; Ding, Y.; Scheel, D.; Herklotz, S.; Hibert, M.; Zuccaro, A. Mutualistic root endophytism is not associated with the reduction of saprotrophic traits and requires a noncompromised plant innate immunity. New Phytol. 2015, 207, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.G.; Yang, M.; Zhang, M.; Wang, P.; Kang, Y.X.; Liu, J.J. Genome-wide transcriptome analysis of genes involved in flavonoid biosynthesis between red and white strains of Magnolia sprengeri pamp. BMC Genom. 2014, 25, 706. [Google Scholar] [CrossRef]
- Chung, H.J.; Ferl, R.J. Arabidopsis alcohol dehydrogenase expression in both shoots and roots is conditioned by root growth environment. Plant Physiol. 1999, 121, 429–436. [Google Scholar] [CrossRef]
- Thompson, C.E.; Fernandes, C.L.; de Souza, O.N.; de Freitas, L.B.; Salzano, F.M. Evaluation of the impact of functional diversification on Poaceae, Brassicaceae, Fabaceae, and Pinaceae alcohol dehydrogenase enzymes. J. Mol. Model. 2010, 16, 919–928. [Google Scholar] [CrossRef]
- Rocha, M.; Licausi, F.; Araujo, W.L.; Nunes-Nesi, A.; Sodek, L.; Fernie, A.R.; van Dongen, J.T. Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol. 2010, 152, 1501–1513. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Xu, X.Y.; Gong, Q.Q.; Chen, X.; Fan, W.; Yang, J.L.; Lin, Q.S.; Zheng, S.J. Root proteome of rice studied by iTRAQ provides integrated insight into aluminum stress tolerance mechanisms in plants. J. Proteom. 2014, 98, 189–205. [Google Scholar] [CrossRef]
- Yu, F.; Han, X.S.; Geng, C.J.; Zhao, Y.X.; Zhang, Z.X.; Qiu, F.Z. Comparative proteomic analysis revealing the complex network associated with waterlogging stress in maize (Zea mays L.) seedling root cells. Proteomics 2015, 15, 135–147. [Google Scholar] [CrossRef]
- Shao, G.C.; Lan, J.J.; Yu, S.E.; Liu, N.; Guo, R.Q.; She, D.L. Photosynthesis and growth of winter wheat in response to waterlogging at different growth stages. Photosynthetica 2013, 51, 429–437. [Google Scholar] [CrossRef]
- Zou, X.L.; Jiang, Y.Y.; Lei, L.; Zhang, Z.X.; Zheng, Y.L. Identification of transcriptome induced in roots of maize seedlings at the late stage of waterlogging. BMC Plant Biol. 2010, 10, 189. [Google Scholar] [CrossRef]
- Bhantana, P.; Lazarovitch, N. Evapotranspiration, crop coefficient and growth of two young pomegranate (Punica granatum L.) varieties under salt stress. Agric. Water Manag. 2010, 97, 715–722. [Google Scholar] [CrossRef]
- Lin, K.; Weng, C.C.; Lo, H.F.; Chen, J.T. Study of the root antioxidative system of tomatoes and eggplants under waterlogged conditions. Plant. Sci. 2004, 167, 355–365. [Google Scholar] [CrossRef]
- An, Y.Y.; Qi, L.; Wang, L.J. ALA pretreatment improves waterlogging tolerance of fig plants. PLoS ONE 2016, 11, e0147202. [Google Scholar] [CrossRef]
- Xu, B.B.; Cheng, Y.; Zou, X.L.; Zhang, X.K. Ethanol content in plants of Brassica napus L. correlated with waterlogging tolerance index and regulated by lactate dehydrogenase and citrate synthase. Acta Physiol. Plant. 2016, 38, 81. [Google Scholar] [CrossRef]
- Huang, S.; Greenway, H.; Colmer, T.D.; Millar, A.H. Protein synthesis by rice coleoptiles during prolonged anoxia: Implications for glycolysis, growth and energy utilization. Ann. Bot. 2005, 96, 703–715. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Chavan, P.D. Study of some aspects of anaerobic metabolism in roots of finger millet and rice plants subjected to waterlogging stress. Int. J. Bot. 2013, 9, 80–85. [Google Scholar] [CrossRef]
- Wang, L.H.; Li, D.H.; Zhang, Y.X.; Gao, Y.; Yu, J.Y.; Wei, X.; Zhang, X.R. Tolerant and susceptible sesame genotypes reveal waterlogging stress response patterns. PLoS ONE 2016, 11, e0149912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Toai, T.V.; Huynh, L.; Preiszner, J. Development of flooding-tolerant Arabidopsis thaliana by autoregulated cytokinin production. Mol. Breed. 2000, 6, 135–144. [Google Scholar] [CrossRef]
- Bertholdsson, N.O. Screening for barley waterlogging tolerance in nordic barley cultivars (Hordeum vulgare L.) using chlorophyll fluorescence on hydroponically-grown Plants. Agronomy 2013, 3, 376–390. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hwang, S.J.; Waqas, M.; Khan, A.L.; Lee, J.H.; Lee, J.D.; Nguyen, H.T.; Lee, I.J. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front. Plant. Sci. 2015, 6, 714. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Lin, H.W.; Chern, R.H.; Lo, H.F.; Li, L. Reduced susceptibility to waterlogging together with high-light stress is related to increases in superoxide dismutase and catalase activities in sweet potato. Plant. Growth Regul. 1999, 27, 167–172. [Google Scholar] [CrossRef]
- Ma, A.S.; Moran-Jones, K.; Shan, J.; Munro, T.P.; Snee, M.J.; Hoek, K.S.; Smith, R. Heterogeneous nuclear ribonucleoprotein A3, a novel RNA trafficking response element-binding protein. J. Biol. Chem. 2002, 277, 18010–18020. [Google Scholar] [CrossRef]
- Hristova, E.; Fal, K.; Klemme, L.; Windels, D.; Bucher, E. HISTONE DEACETYLASE6 controls gene expression patterning and DNA methylation-independent euchromatic silencing. Plant Physiol. 2015, 168, 1298–1308. [Google Scholar] [CrossRef]
- Ristic, Z.; Bukovnik, U.; Momcilovic, I.; Fu, J.; Vara Prasad, P.V. Heat-induced accumulation of chloroplast protein synthesis elongation factor, EF-Tu, in winter wheat. J. Plant Physiol. 2008, 165, 192–202. [Google Scholar] [CrossRef]
- Lin, H.H.; Lin, K.H.; Chen, S.C.; Shen, Y.H.; Lo, H.F. Proteomic analysis of broccoli (Brassica oleracea) under high temperature and waterlogging stresses. Bot. Stud. 2015, 56, 18. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wu, B.; Yin, H.F.; Fan, Z.Q.; Li, X.L.; Ni, S.; He, L.B.; Li, J.Y. Overexpression of CaAPX induces orchestrated reactive oxygen scavenging and enhances cold and heat tolerances in tobacco. Biomed. Res. Int. 2017, 2017, 4049534. [Google Scholar]
- Jia, X.M.; Zhu, Y.F.; Hu, Y.; Zhang, R.; Cheng, L.; Zhu, Z.L.; Zhao, T.; Zhang, X.; Wang, Y.X. Integrated physiologic, proteomic, and metabolomic analyses of Malus halliana adaptation to saline-alkali stress. Hortic. Res. 2019, 6, 91. [Google Scholar] [CrossRef]
- Huang, B.L.; Li, X.; Liu, P.; Ma, L.; Wu, W.; Zhang, X.; Li, Z.; Huang, B. Transcriptomic analysis of Eruca vesicaria subs. sativa lines with contrasting tolerance to polyethylene glycol-simulated drought stress. BMC Plant Biol. 2019, 19, 419. [Google Scholar] [CrossRef]
- Chiang, C.M.; Chen, L.F.O.; Shih, S.W.; Lin, K.H. Expression of eggplant ascorbate peroxidase increases the tolerance of transgenic rice plants to flooding stress. J. Plant Biochem. Biotechnol. 2015, 24, 257–267. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Jiang, Z.; He, J.; Shen, L. iTRAQ-Based Quantitative Proteomics Unveils Protein Dynamics in the Root of Solanum melongena L. under Waterlogging Stress Conditions. Life 2023, 13, 1399. https://doi.org/10.3390/life13061399
Yang X, Jiang Z, He J, Shen L. iTRAQ-Based Quantitative Proteomics Unveils Protein Dynamics in the Root of Solanum melongena L. under Waterlogging Stress Conditions. Life. 2023; 13(6):1399. https://doi.org/10.3390/life13061399
Chicago/Turabian StyleYang, Xu, Zheng Jiang, Jie He, and Lei Shen. 2023. "iTRAQ-Based Quantitative Proteomics Unveils Protein Dynamics in the Root of Solanum melongena L. under Waterlogging Stress Conditions" Life 13, no. 6: 1399. https://doi.org/10.3390/life13061399
APA StyleYang, X., Jiang, Z., He, J., & Shen, L. (2023). iTRAQ-Based Quantitative Proteomics Unveils Protein Dynamics in the Root of Solanum melongena L. under Waterlogging Stress Conditions. Life, 13(6), 1399. https://doi.org/10.3390/life13061399





