A Cross-Sectional Study of Bone Nanomechanics in Hip Fracture and Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Donor Information
2.2. Sample Preparation
2.3. Tensile Testing
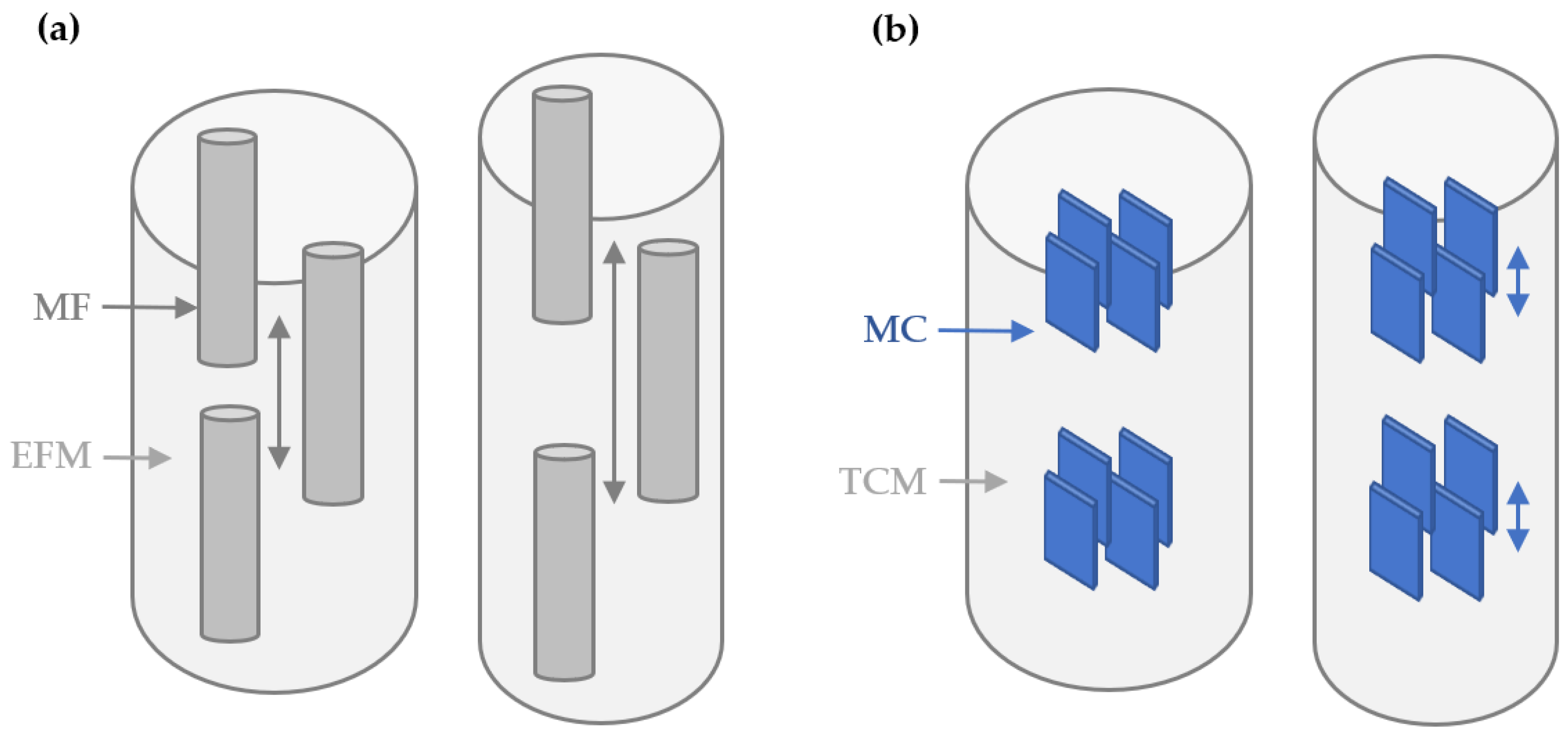
2.4. I22 Beam Setup and Specifications
2.5. Data Analysis and Statistics
3. Results
4. Discussion
4.1. Hip-Fractures
4.2. Aging
4.3. Crystal Size and Composition Might Affect Nanomechanics
4.4. Limitations of Synchrotron Experiments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abel, R.; Stavri, R.; Marena, G.; Hansen, U. Clinical importance of bone matrix damage mechanisms for ageing and fracture prevention. Curr. Osteoporos. Rep. 2021, 19, 318–326. [Google Scholar] [CrossRef]
- Burr, D.B. Changes in bone matrix properties with aging. Bone 2019, 120, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.A.; Fiedler, I.A.K.; Busse, B. Breaking new ground in mineralized tissue: Assessing tissue quality in clinical and laboratory studies. J. Mech. Behav. Biomed. Mater. 2021, 113, 104138. [Google Scholar] [CrossRef]
- Boskey, A.L.; Imbert, L. Bone quality changes associated with aging and disease: A review. Ann. N. Y. Acad. Sci. 2017, 1410, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.J.; van der Meulen, M.C.H. Understanding bone strength is not enough. J. Bone Miner. Res. 2017, 32, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Unal, M.; Creecy, A.; Nyman, J.S. The Role of Matrix Composition in the Mechanical Behavior of Bone. Curr. Osteoporos. Rep. 2018, 16, 205–215. [Google Scholar] [CrossRef]
- Ma, S.; Goh, E.L.; Jin, A.; Bhattacharya, R.; Boughton, O.R.; Patel, B.; Karunaratne, A.; Vo, N.T.; Atwood, R.; Cobb, J.P.; et al. Long-term effects of bisphosphonate therapy: Perforations, microcracks and mechanical properties. Sci. Rep. 2017, 7, 43399. [Google Scholar] [CrossRef]
- Wittig, N.K.; Birkedal, H. Bone hierarchical structure: Spatial variation across length scales. Acta Crystallogr. Sect. B 2022, 78, 305–311. [Google Scholar] [CrossRef]
- Gustafsson, A.; Mathavan, N.; Turunen, M.J.; Engqvist, J.; Khayyeri, H.; Hall, S.A.; Isaksson, H. Linking multiscale deformation to microstructure in cortical bone using in situ loading, digital image correlation and synchrotron X-ray scattering. Acta Biomater. 2018, 69, 323–331. [Google Scholar] [CrossRef]
- Statnik, E.S.; Salimon, A.I.; Besnard, C.; Chen, J.; Wang, Z.; Moxham, T.; Dolbnya, I.P.; Korsunsky, A.M. Ovine Bone Morphology and Deformation Analysis Using Synchrotron X-ray Imaging and Scattering. Quantum. Beam. Sci. 2020, 4, 29. [Google Scholar] [CrossRef]
- Zimmermann, E.A.; Schaible, E.; Bale, H.; Barth, H.D.; Tang, S.Y.; Reichert, P.; Busse, B.; Alliston, T.; Ager, J.W.; Ritchie, R.O. Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc. Natl. Acad. Sci. USA 2011, 108, 14416–14421. [Google Scholar] [CrossRef]
- Gupta, H.S.; Seto, J.; Wagermaier, W.; Zaslansky, P.; Boesecke, P.; Fratzl, P. Cooperative deformation of mineral and collagen in bone at the nanoscale. Proc. Natl. Acad. Sci. USA 2006, 103, 17741–17746. [Google Scholar] [CrossRef]
- Ma, S.; Goh, E.L.; Tay, T.; Wiles, C.C.; Boughton, O.; Churchwell, J.H.; Wu, Y.; Karunaratne, A.; Bhattacharya, R.; Terrill, N.; et al. Nanoscale mechanisms in age-related hip-fractures. Sci. Rep. 2020, 10, 14208. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.A.; Schaible, E.; Gludovatz, B.; Schmidt, F.N.; Riedel, C.; Krause, M.; Vettorazzi, E.; Acevedo, C.; Hahn, M.; Püschel, K.; et al. Intrinsic mechanical behavior of femoral cortical bone in young, osteoporotic and bisphosphonate-treated individuals in low- and high energy fracture conditions. Sci. Rep. 2016, 6, 21072. [Google Scholar] [CrossRef] [PubMed]
- Bonicelli, A.; Tay, T.; Cobb, J.P.; Boughton, O.R.; Hansen, U.; Abel, R.L.; Zioupos, P. Association between nanoscale strains and tissue level nanoindentation properties in age-related hip-fractures. J. Mech. Behav. Biomed. Mater. 2023, 138, 105573. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Alcock, S.G.; Davidson, L.S.; Emmins, J.H.; Hiller Bardsley, J.C.; Holloway, P.; Malfois, M.; Marshall, A.R.; Pizzey, C.L.; Rogers, S.E.; et al. I22: SAXS/WAXS beamline at Diamond Light Source—An overview of 10 years operation. J. Synchrotron. Radiat. 2021, 28, 939–947. [Google Scholar] [CrossRef]
- Basham, M.; Filik, J.; Wharmby, M.T.; Chang, P.C.Y.; El Kassaby, B.; Gerring, M.; Aishima, J.; Levik, K.; Pulford, B.C.A.; Sikharulidze, I.; et al. Data Analysis WorkbeNch (DAWN). J. Synchrotron. Radiat. 2015, 22, 853–858. [Google Scholar] [CrossRef]
- Filik, J.; Ashton, A.W.; Chang, P.C.Y.; Chater, P.A.; Day, S.J.; Drakopoulos, M.; Gerring, M.W.; Hart, M.L.; Magdysyuk, O.V.; Michalik, S.; et al. Processing two-dimensional X-ray diffraction and small-angle scattering data in DAWN 2. J. Appl. Cryst. 2017, 50, 959–966. [Google Scholar] [CrossRef]
- Pauw, B.R.; Smith, A.J.; Snow, T.; Terrill, N.J.; Thünemann, A.F. The modular small-angle X-ray scattering data correction sequence. J. Appl. Cryst. 2017, 50, 1800–1811. [Google Scholar] [CrossRef]
- Barth, H.D.; Launey, M.E.; Macdowell, A.A.; Ager, J.W., 3rd; Ritchie, R.O. On the effect of X-ray irradiation on the deformation and fracture behavior of human cortical bone. Bone 2010, 46, 1475–1485. [Google Scholar] [CrossRef]
- Hulley, S.; Cummings, S.; Browner, W.; Grady, D.; Newman, T. Designing Clinical Research: An Epidemiologic Approach, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Stock, S.R. The Mineral–Collagen Interface in Bone. Calcif. Tissue Int. 2015, 97, 262–280. [Google Scholar] [CrossRef]
- Launey, M.E.; Buehler, M.J.; Ritchie, R.O. On the Mechanistic Origins of Toughness in Bone. Annu. Rev. Mater. Res. 2020, 40, 25–53. [Google Scholar] [CrossRef]
- Ritchie, R.O. Toughening materials: Enhancing resistance to fracture. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2021, 379, 20200437. [Google Scholar] [CrossRef] [PubMed]
- Tertuliano, O.A.; Edwards, B.W.; Meza, L.R.; Deshpande, V.S.; Greer, J.R. Nanofibril-mediated fracture resistance of bone. Bioinspir. Biomim. 2021, 16, 35001. [Google Scholar] [CrossRef]
- Thurner, P.J.; Katsamenis, O.L. The role of nanoscale toughening mechanisms in osteoporosis. Curr. Osteoporos. Rep. 2014, 12, 351–356. [Google Scholar] [CrossRef]
- McCalden, R.W.; McGeough, J.A.; Barker, M.B.; Court-Brown, C.M. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J. Bone Jt. Surg. Am. 1993, 75, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Zioupos, P.; Currey, J.D. Changes in the stiffness, strength, and toughness of human cortical bone with age. Bone 1998, 22, 57–66. [Google Scholar] [CrossRef]
- Zioupos, P.; Kirchner, H.O.K.; Peterlik, H. Ageing bone fractures: The case of a ductile to brittle transition that shifts with age. Bone 2020, 131, 115176. [Google Scholar] [CrossRef] [PubMed]
- Handschin, R.G.; Stern, W.B. X-ray diffraction studies on the lattice perfection of human bone apatite. Bone 1995, 16, S355–S363. [Google Scholar] [CrossRef]
- Jin, A.; Cobb, J.; Hansen, U.; Bhattacharya, R.; Reinhard, C.; Vo, N.; Atwood, R.; Li, J.; Karunaratne, A.; Wiles, C.; et al. The effect of long-term bisphosphonate therapy on trabecular bone strength and microcrack density. Bone Jt. Res. 2017, 6, 602–609. [Google Scholar] [CrossRef]


| Control | Hip-Fx | ||
|---|---|---|---|
| Sex | Age | Sex | Age |
| F | 82 | F | 90 |
| F | 82 | F | 88 |
| F | 73 | F | 82 |
| F | 73 | F | 82 |
| F | 72 | F | 82 |
| F | 72 | F | 81 |
| F | 72 | F | 78 |
| F | 72 | F | 78 |
| M | 84 | F | 76 |
| M | 73 | F | 75 |
| M | 73 | F | 74 |
| M | 71 | F | 74 |
| M | 57 | F | 73 |
| M | 57 | F | 71 |
| M | 57 | M | 94 |
| M | 57 | M | 86 |
| M | 44 | M | 79 |
| M | 78 | ||
| M | 73 | ||
| M | 70 | ||
| Mean | 69 | 79 | |
| SD | 11 | 6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavri, R.; Tay, T.; Wiles, C.C.; Di Federico, E.; Boughton, O.; Ma, S.; Karunaratne, A.; Churchwell, J.H.; Bhattacharya, R.; Terrill, N.J.; et al. A Cross-Sectional Study of Bone Nanomechanics in Hip Fracture and Aging. Life 2023, 13, 1378. https://doi.org/10.3390/life13061378
Stavri R, Tay T, Wiles CC, Di Federico E, Boughton O, Ma S, Karunaratne A, Churchwell JH, Bhattacharya R, Terrill NJ, et al. A Cross-Sectional Study of Bone Nanomechanics in Hip Fracture and Aging. Life. 2023; 13(6):1378. https://doi.org/10.3390/life13061378
Chicago/Turabian StyleStavri, Richard, Tabitha Tay, Crispin C. Wiles, Erica Di Federico, Oliver Boughton, Shaocheng Ma, Angelo Karunaratne, John H. Churchwell, Rajarshi Bhattacharya, Nicholas J. Terrill, and et al. 2023. "A Cross-Sectional Study of Bone Nanomechanics in Hip Fracture and Aging" Life 13, no. 6: 1378. https://doi.org/10.3390/life13061378
APA StyleStavri, R., Tay, T., Wiles, C. C., Di Federico, E., Boughton, O., Ma, S., Karunaratne, A., Churchwell, J. H., Bhattacharya, R., Terrill, N. J., Cobb, J. P., Hansen, U., & Abel, R. L. (2023). A Cross-Sectional Study of Bone Nanomechanics in Hip Fracture and Aging. Life, 13(6), 1378. https://doi.org/10.3390/life13061378






