Association between Mortality and Lung Low Attenuation Areas in NSCLC Treated by Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CT and LAAs Analysis
2.3. Lung Surgery
2.4. Statistical Analysis
3. Results
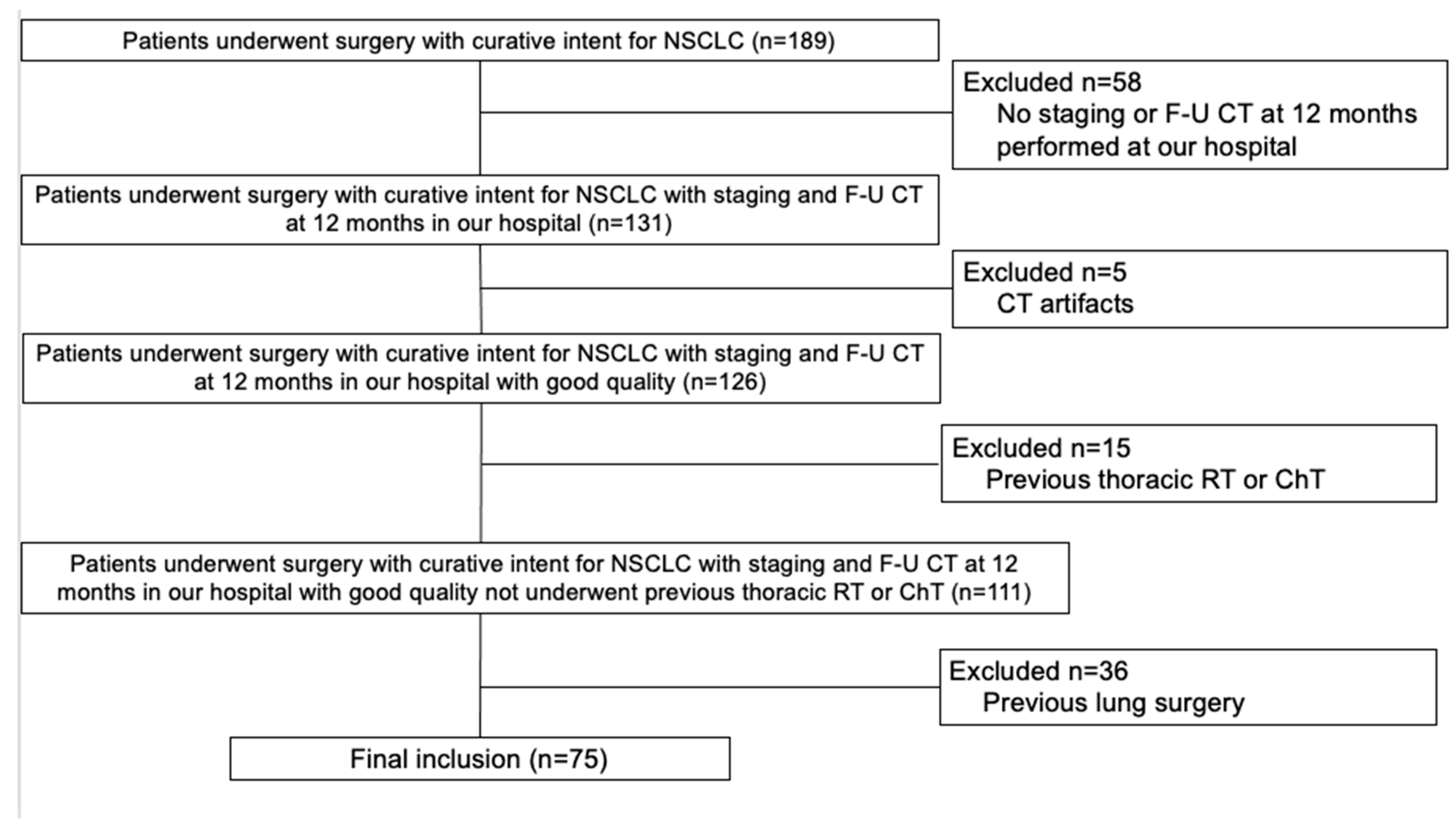
3.1. Study Population
3.2. CT Analysis
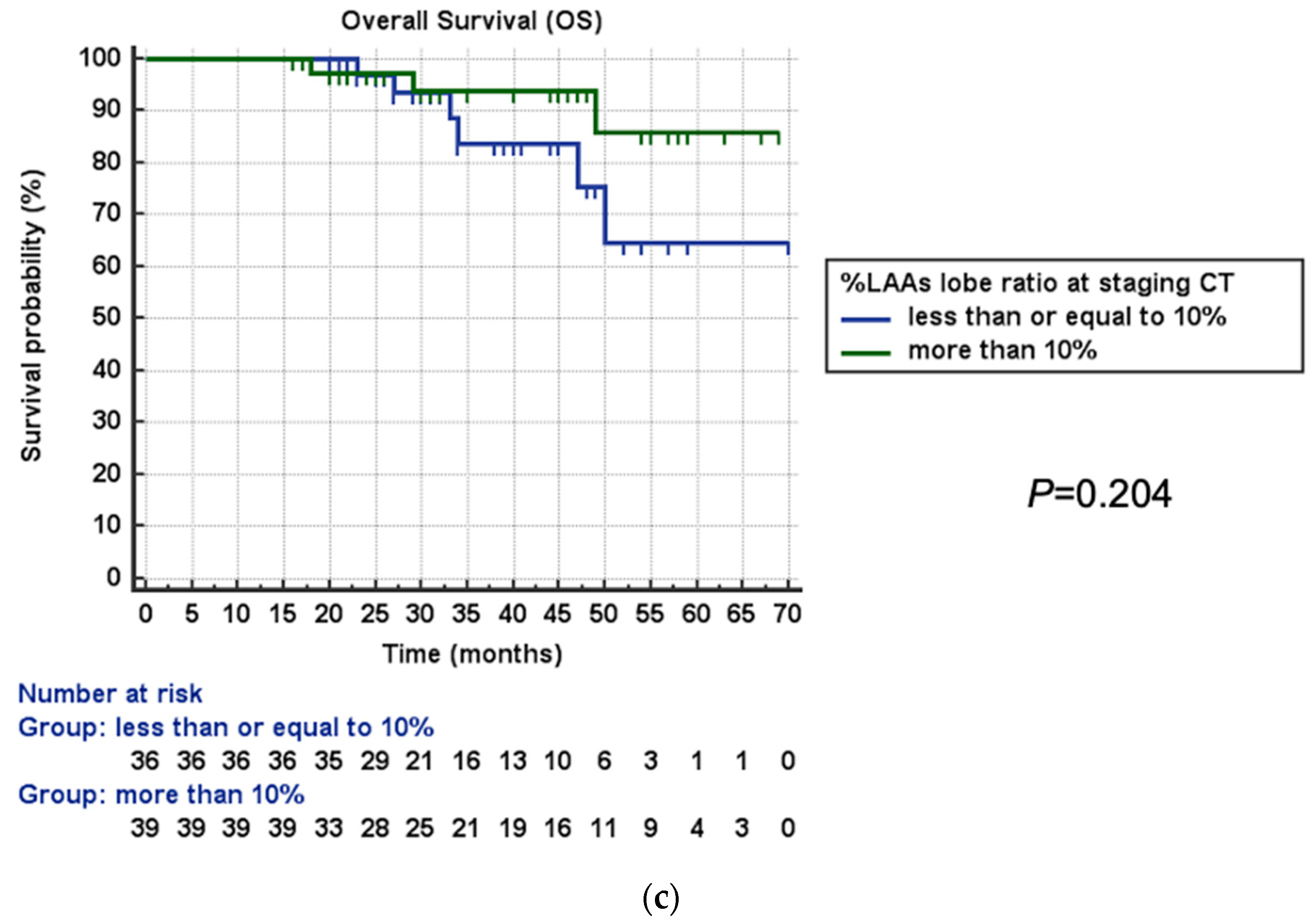
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Colombi, D.; Di Lauro, E.; Silva, M.; Manna, C.; Rossi, C.; De Filippo, M.; Zompatori, M.; Ruffini, L.; Sverzellati, N. Non-Small Cell Lung Cancer after Surgery and Chemoradiotherapy: Follow-up and Response Assessment. Diagn. Interv. Radiol. 2013, 19, 447–456. [Google Scholar] [CrossRef]
- Jia, B.; Zheng, Q.; Wang, J.; Sun, H.; Zhao, J.; Wu, M.; An, T.; Wang, Y.; Zhuo, M.; Li, J.; et al. A Nomogram Model to Predict Death Rate among Non-Small Cell Lung Cancer (NSCLC) Patients with Surgery in Surveillance, Epidemiology, and End Results (SEER) Database. BMC Cancer 2020, 20, 666. [Google Scholar] [CrossRef]
- Silva, M.; Bankier, A.A.; Centra, F.; Colombi, D.; Ampollini, L.; Carbognani, P.; Sverzellati, N. Longitudinal Evolution of Incidentally Detected Solitary Pure Ground-Glass Nodules on Ct: Relation to Clinical Metrics. Diagn. Interv. Radiol. 2015, 21, 385. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, E.Y.; Ahn, H.K.; Cho, E.K.; Jeong, Y.M.; Kim, J.H. Prognostic Significance of CT-Emphysema Score in Patients with Advanced Squamous Cell Lung Cancer. J. Thorac. Dis. 2016, 8, 1966–1973. [Google Scholar] [CrossRef]
- McKenna, R.J.J.; Fischel, R.J.; Brenner, M.; Gelb, A.F. Combined Operations for Lung Volume Reduction Surgery and Lung Cancer. Chest 1996, 110, 885–888. [Google Scholar] [CrossRef]
- Edwards, J.G.; Duthie, D.J.R.; Waller, D.A. Lobar Volume Reduction Surgery: A Method of Increasing the Lung Cancer Resection Rate in Patients with Emphysema. Thorax 2001, 56, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Baldi, S.; Ruffini, E.; Harari, S.; Roviaro, G.C.; Nosotti, M.; Bellaviti, N.; Venuta, F.; Diso, D.; Rea, F.; Schiraldi, C.; et al. Does Lobectomy for Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease Affect Lung Function? A Multicenter National Study. J. Thorac. Cardiovasc. Surg. 2005, 130, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, J.J.; Wisnivesky, J.P.; Henschke, C.I.; Yip, R.; Farooqi, A.O.; McCauley, D.I.; Chen, M.; Libby, D.M.; Smith, J.P.; Pasmantier, M.W.; et al. Emphysema Scores Predict Death from COPD and Lung Cancer. Chest 2012, 141, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Nakamura, M.; Shimizu, Y.; Goto, T.; Koike, T.; Ishikawa, H.; Tsuchida, M. The Impact of Emphysema on Surgical Outcomes of Early-Stage Lung Cancer: A Retrospective Study. BMC Pulm. Med. 2019, 19, 73. [Google Scholar] [CrossRef]
- Sverzellati, N.; Colombi, D.; Randi, G.; Pavarani, A.; Silva, M.; Walsh, S.L.; Pistolesi, M.; Alfieri, V.; Chetta, A.; Vaccarezza, M.; et al. Computed Tomography Measurement of Rib Cage Morphometry in Emphysema. PLoS ONE 2013, 8, e68546. [Google Scholar] [CrossRef]
- Johannessen, A.; Skorge, T.D.; Bottai, M.; Grydeland, T.B.; Nilsen, R.M.; Coxson, H.; Dirksen, A.; Omenaas, E.; Gulsvik, A.; Bakke, P. Mortality by Level of Emphysema and Airway Wall Thickness. Am. J. Respir. Crit. Care Med. 2013, 187, 602–608. [Google Scholar] [CrossRef]
- Lynch, D.A.; Moore, C.M.; Wilson, C.; Nevrekar, D.; Jennermann, T.; Humphries, S.M.; Austin, J.H.M.; Grenier, P.A.; Kauczor, H.U.; Han, M.L.K.; et al. CT-Based Visual Classification of Emphysema: Association with Mortality in the COPDGene Study. Radiology 2018, 288, 859–866. [Google Scholar] [CrossRef]
- Klooster, K.; Slebos, D.J. Endobronchial Valves for the Treatment of Advanced Emphysema. Chest 2021, 159, 1833–1842. [Google Scholar] [CrossRef]
- Yokoba, M.; Ichikawa, T.; Harada, S.; Shiomi, K.; Mikubo, M.; Ono, M.; Sonoda, D.; Satoh, Y.; Hanawa, H.; Naoki, K.; et al. Comparison between Quantitative Computed Tomography, Scintigraphy, and Anatomical Methods for Prediction of Postoperative FEV1 and DLCO: Effects of Chronic Obstructive Pulmonary Disease Status and Resected Lobes. J. Thorac. Dis. 2020, 12, 5269–5280. [Google Scholar] [CrossRef]
- Colombi, D.; Risoli, C.; Delfanti, R.; Chiesa, S.; Morelli, N.; Petrini, M.; Capelli, P.; Franco, C.; Michieletti, E. Software-Based Assessment of Well-Aerated Lung at CT for Quantification of Predicted Pulmonary Function in Resected NSCLC. Life 2023, 13, 198. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Bolejack, V.; Arenberg, D.A.; Crowley, J.; Donington, J.S.; Franklin, W.A.; Girard, N.; Marom, E.M.; Mazzone, P.J.; Nicholson, A.G.; et al. The IASLC Lung Cancer Staging Project: Background Data and Proposals for the Classification of Lung Cancer with Separate Tumor Nodules in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Franklin, W.A.; Nicholson, A.G.; Girard, N.; Arenberg, D.A.; Travis, W.D.; Mazzone, P.J.; Marom, E.M.; Donington, J.S.; Tanoue, L.T.; et al. The IASLC Lung Cancer Staging Project: Background Data and Proposed Criteria to Distinguish Separate Primary Lung Cancers from Metastatic Foci in Patients with Two Lung Tumors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 651–665. [Google Scholar] [CrossRef]
- Silvestri, G.A.; Gonzalez, A.V.; Jantz, M.A.; Margolis, M.L.; Gould, M.K.; Tanoue, L.T.; Harris, L.J.; Detterbeck, F.C. Methods for Staging Non-Small Cell Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013, 143, e211S–e250S. [Google Scholar] [CrossRef]
- Brunelli, A.; Charloux, A.; Bolliger, C.T.; Rocco, G.; Sculier, J.P.; Varela, G.; Licker, M.; Ferguson, M.K.; Faivre-Finn, C.; Huber, R.M.; et al. ERS/ESTS Clinical Guidelines on Fitness for Radical Therapy in Lung Cancer Patients (Surgery and Chemo-Radiotherapy). Eur. Respir. J. 2009, 34, 17–41. [Google Scholar] [CrossRef]
- Lynch, D.A.; Austin, J.H.M.; Hogg, J.C.; Grenier, P.A.; Kauczor, H.U.; Bankier, A.A.; Barr, R.G.; Colby, T.V.; Galvin, J.R.; Gevenois, P.A.; et al. CT-Definable Subtypes of Chronic Obstructive Pulmonary Disease: A Statement of the Fleischner Society1. Radiology 2015, 277, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of Stage I and II Non-Small Cell Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013, 143, e278S–e313S. [Google Scholar] [CrossRef]
- Schober, P.; Mascha, E.J.; Vetter, T.R. Statistics from a (agreement) to Z (z score): A guide to interpreting common measures of association, agreement, diagnostic accuracy, effect size, heterogeneity, and reliability in medical research. Anesth. Analg. 2021, 133, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, W.N.; Meyers, B.F. Surgical Resection in Combination with Lung Volume Reduction Surgery for Stage I Non-Small Cell Lung Cancer. Semin. Thorac. Cardiovasc. Surg. 2010, 22, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Krass, S.; Lassen-Schmidt, B.; Schenk, A. Computer-Assisted Image-Based Risk Analysis and Planning in Lung Surgery—A Review. Front. Surg. 2022, 9, 920457. [Google Scholar] [CrossRef]
- Kitazawa, S.; Wijesinghe, A.I.; Maki, N.; Yanagihara, T.; Saeki, Y.; Kobayashi, N.; Kikuchi, S.; Goto, Y.; Ichimura, H.; Sato, Y. Predicting Respiratory Complications Following Lobectomy Using Quantitative CT Measures of Emphysema. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 2523–2531. [Google Scholar] [CrossRef]
- Pompe, E.; Strand, M.; van Rikxoort, E.M.; Hoffman, E.A.; Barr, R.G.; Charbonnier, J.P.; Humphries, S.; Han, M.L.K.; Hokanson, J.E.; Make, B.J.; et al. Five-Year Progression of Emphysema and Air Trapping at Ct in Smokers with and Those without Chronic Obstructive Pulmonary Disease: Results from the COPDGene Study. Radiology 2020, 295, 218–226. [Google Scholar] [CrossRef]
- De Boer, E.; Nijholt, I.M.; Jansen, S.; Edens, M.A.; Walen, S.; van den Berg, J.W.K.; Boomsma, M.F. Optimization of Pulmonary Emphysema Quantification on CT Scans of COPD Patients Using Hybrid Iterative and Post Processing Techniques: Correlation with Pulmonary Function Tests. Insights Imaging 2019, 10, 4–11. [Google Scholar] [CrossRef]


| Parameter | All Patients (n = 75) | Survivors (n = 66) | Non-Survivors (n = 9) | p-Value |
|---|---|---|---|---|
| Age (years) | 70 (63–75) | 69 (62–75) | 71 (67–75) | 0.230 |
| Gender | ||||
| Male (n) | 46/75 (61%) | 39/66 (59%) | 7/9 (78%) | 0.468 |
| Female (n) | 29/75 (39%) | 27/66 (41%) | 2/9 (22%) | |
| History of other cancer than NSCLC (n) | 26/75 (35%) | 22/66 (33%) | 4/9 (44%) | 0.710 |
| Smoking history | ||||
| Never (n) | 14/75 (19%) | 13/66 (20%) | 1/9 (11%) | 1.000 |
| Current or former (n) | 61/75 (81%) | 53/66 (80%) | 8/9 (89%) | |
| Pack-years (n) | 30 (11–40) | 30 (10–40) | 30 (30–42) | 0.311 |
| COPD (n) | 37/75 (50%) | 33/66 (50%) | 4/9 (44%) | 1.000 |
| LAAs category | ||||
| trace emphysema (LAAs ≤ 0.5%; n) | 52/75 (69%) | 48/66 (72%) | 4/9 (44%) | 0.122 |
| mild emphysema (LAAs 0.5–5%; n) | 14/75 (19%) | 12/66 (18%) | 2/9 (22%) | 0.671 |
| moderate emphysema (LAAs ≥ 5%; n) | 9/75 (12%) | 6/66 (10%) | 3/9 (34%) | 0.070 |
| LAAs surgery lobe/LAAs whole lung (%) | 10 (0.25–24) | 11 (0–25) | 9 (0.75–12) | 0.445 |
| Cancer pathological stage | ||||
| stage IA (n) | 53/75 (71%) | 47/66 (71%) | 6/9 (67%) | 0.716 |
| stage IB (n) | 10/75 (13%) | 9/66 (14%) | 1/9 (11%) | 1.000 |
| stage II (n) | 5/75 (7%) | 5/66 (8%) | 0/9 (0%) | 1.000 |
| stage III (n) | 7/75 (9%) | 5/66 (7%) | 2/9 (22%) | 0.195 |
| Cancer histology | ||||
| adenocarcinoma (n) | 60/75 (80%) | 53/66 (80%) | 7/9 (78%) | 1.000 |
| squamous cell carcinoma (n) | 9/75 (12%) | 7/66 (10%) | 2/9 (22%) | 0.293 |
| other (n) | 6/75 (8%) | 6/66 (10%) | 0/9 (0%) | 1.000 |
| Upgrade in LAAs category between staging and follow-up CT scans (n) | 19/75 (25%) | 17/66 (26%) | 2/9 (22%) | 1.000 |
| Difference in LAAs between staging and follow-up CT scans (%) | −0.01 (−1.74 ± 0.18) | −0.04 (−1.78 ± 0.10) | 0.11 (−1.23 ± 15.12) | 0.142 |
| Variable | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age > 70 years-old | 1.75 (0.47–6.52) | 0.400 | - | NR |
| Males | 1.97 (0.41–9.43) | 0.397 | - | NR |
| History of other cancer | 1.93 (0.52–7.20) | 0.326 | - | NR |
| Current or former smoker | 1.53 (0.19–12.17) | 0.687 | - | NR |
| Pack years > 30 | 1.24 (0.31–5.01) | 0.755 | - | NR |
| COPD | 0.85 (0.23–3.17) | 0.816 | - | NR |
| LAA category (reference trace emphysema LAAs ≤ 0.5%) | ||||
| mild emphysema (LAAs 0.5–5%) | 1.58 (0.29–8.57) | 0.595 | - | NR |
| moderate emphysema (LAAs ≥ 5%) | 5.27 (1.18–23.49) | 0.029 | 7.27 (1.60–32.96) | 0.010 |
| LAA surgery lobe/LAA whole lung ≥10% | 0.41 (0.10–0.96) | 0.048 | 0.24 (0.05–0.94) | 0.046 |
| Cancer pathological stage III | 4.35 (1.02–21.06) | 0.041 | 6.50 (1.11–37.92) | 0.038 |
| Adenocarcinoma histology | 1.51 (0.30–7.41) | 0.610 | - | NR |
| Upgrade in LAAs category between staging and follow-up CT scans (n) | 1.34 (0.27–6.56) | 0.712 | - | NR |
| Difference in LAAs between staging and follow-up CT scans >−0.01% | 1.54 (0.38–6.13) | 0.541 | - | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombi, D.; Adebanjo, G.A.R.; Delfanti, R.; Chiesa, S.; Morelli, N.; Capelli, P.; Franco, C.; Michieletti, E. Association between Mortality and Lung Low Attenuation Areas in NSCLC Treated by Surgery. Life 2023, 13, 1377. https://doi.org/10.3390/life13061377
Colombi D, Adebanjo GAR, Delfanti R, Chiesa S, Morelli N, Capelli P, Franco C, Michieletti E. Association between Mortality and Lung Low Attenuation Areas in NSCLC Treated by Surgery. Life. 2023; 13(6):1377. https://doi.org/10.3390/life13061377
Chicago/Turabian StyleColombi, Davide, Ganiyat Adenike Ralitsa Adebanjo, Rocco Delfanti, Sara Chiesa, Nicola Morelli, Patrizio Capelli, Cosimo Franco, and Emanuele Michieletti. 2023. "Association between Mortality and Lung Low Attenuation Areas in NSCLC Treated by Surgery" Life 13, no. 6: 1377. https://doi.org/10.3390/life13061377
APA StyleColombi, D., Adebanjo, G. A. R., Delfanti, R., Chiesa, S., Morelli, N., Capelli, P., Franco, C., & Michieletti, E. (2023). Association between Mortality and Lung Low Attenuation Areas in NSCLC Treated by Surgery. Life, 13(6), 1377. https://doi.org/10.3390/life13061377






