An Updated Systematic Review and Meta-Analysis of the Association between the De Ritis Ratio and Disease Severity and Mortality in Patients with COVID-19
Abstract
1. Introduction
2. Methods
2.1. Systematic Literature Search
2.2. Statistical Analysis
3. Results
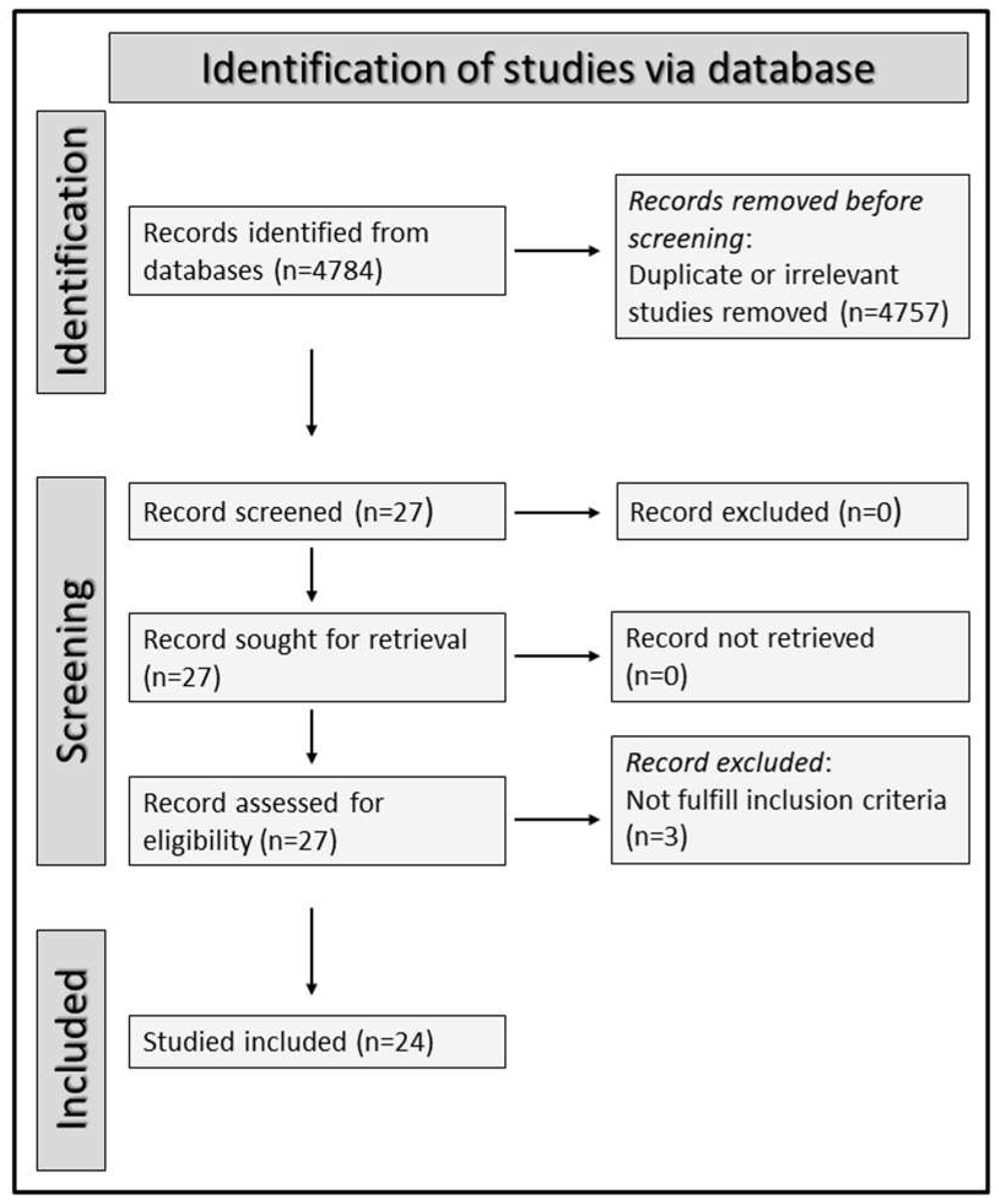
3.1. Selection of Studies
3.2. Pooled Mean Differences
3.2.1. Characteristics of Studies
3.2.2. Risk of Bias
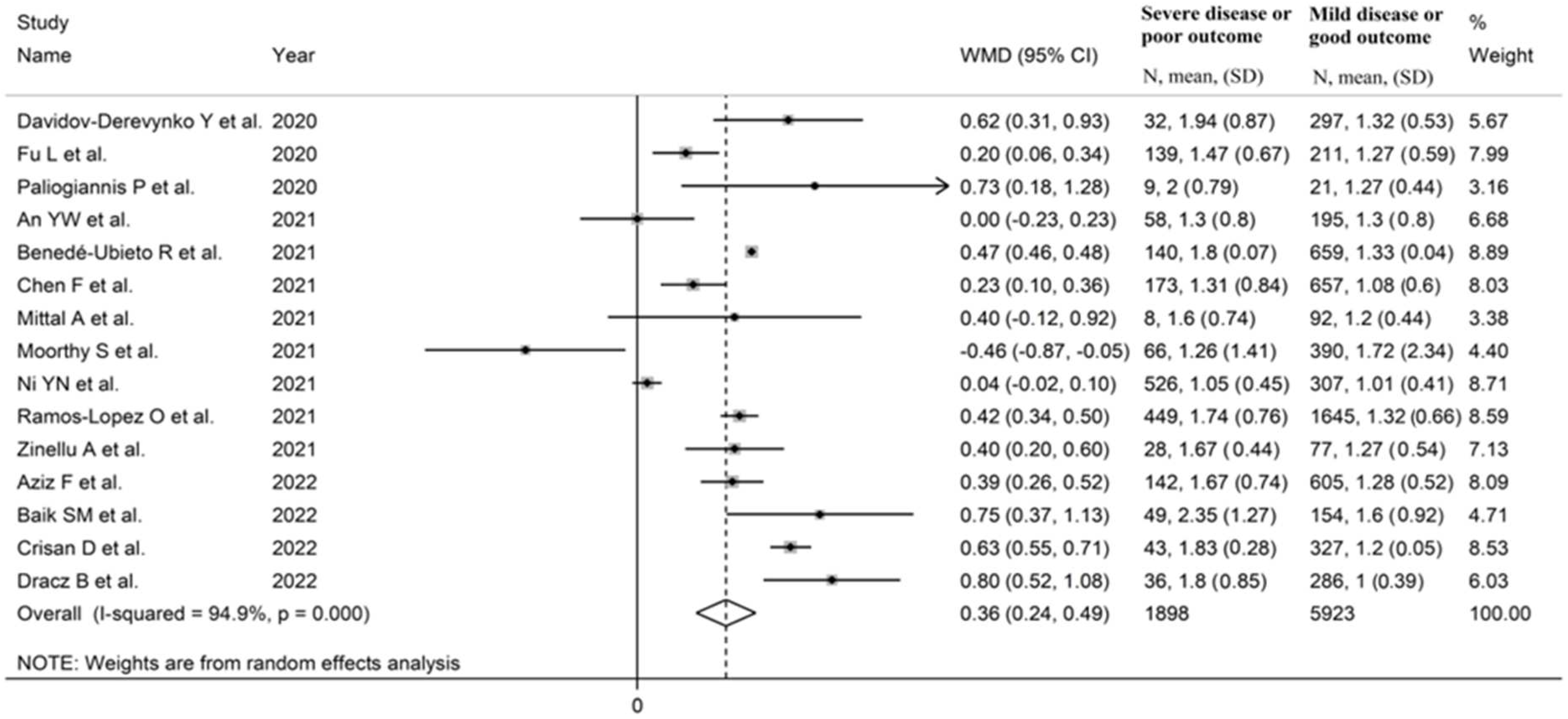
3.2.3. Results of Individual Studies and Syntheses
3.2.4. Publication Bias
3.2.5. Subgroup and Meta-Regression Analysis
3.2.6. Certainty of Evidence
3.3. Pooled Odds Ratios
3.3.1. Characteristics of Studies
3.3.2. Risk of Bias
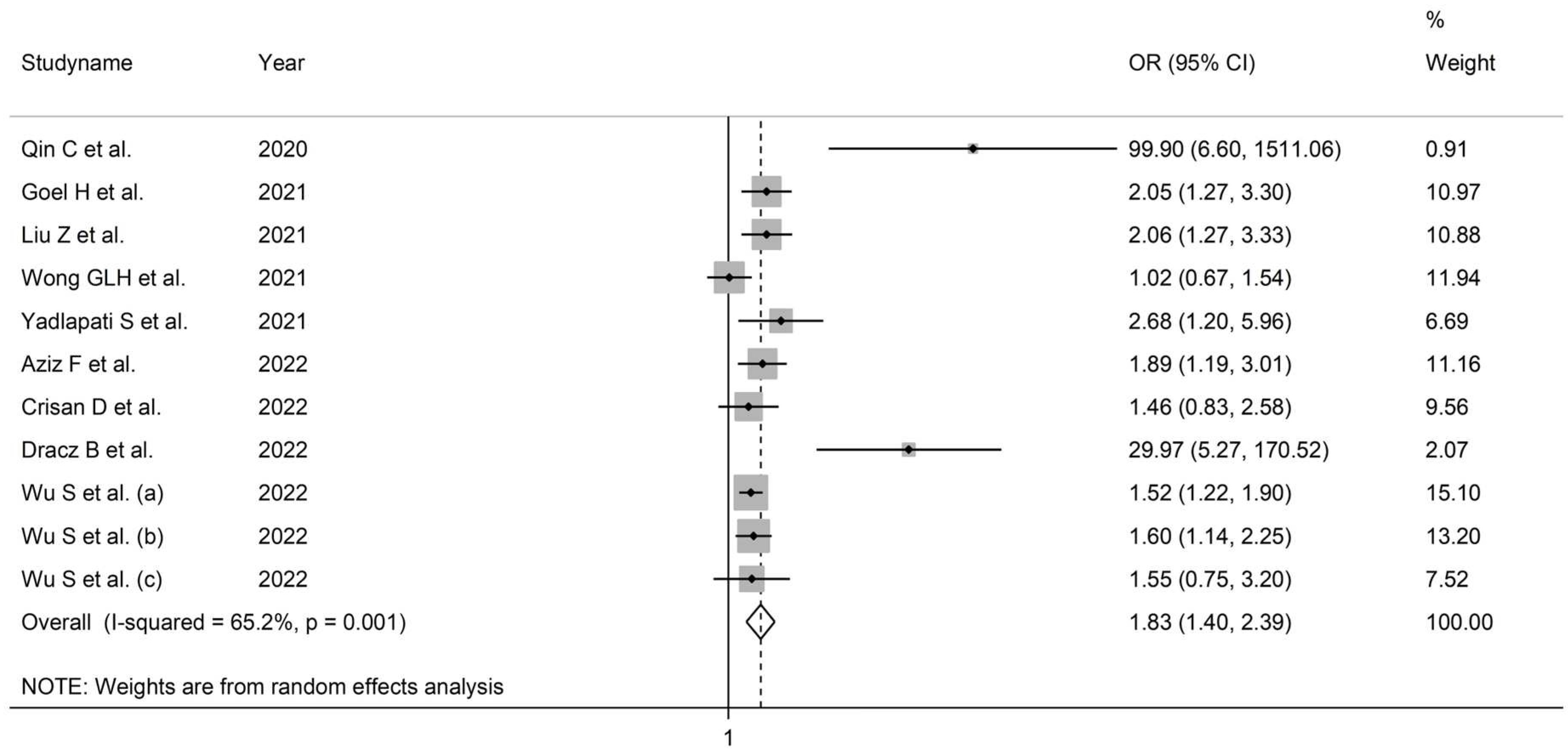
3.3.3. Results of Individual Studies and Syntheses
3.3.4. Publication Bias
3.3.5. Subgroup and Meta-Regression Analysis
3.3.6. Certainty of Evidence
3.4. Pooled Hazard Ratios
3.4.1. Study Characteristics
3.4.2. Risk of Bias
3.4.3. Results of Individual Studies and Syntheses
3.4.4. Publication Bias, Subgroup, and Meta-Regression Analysis
3.4.5. Certainty of Evidence
3.5. Prognostic Accuracy of the De Ritis Ratio
3.5.1. Characteristics of Studies
3.5.2. Risk of Bias
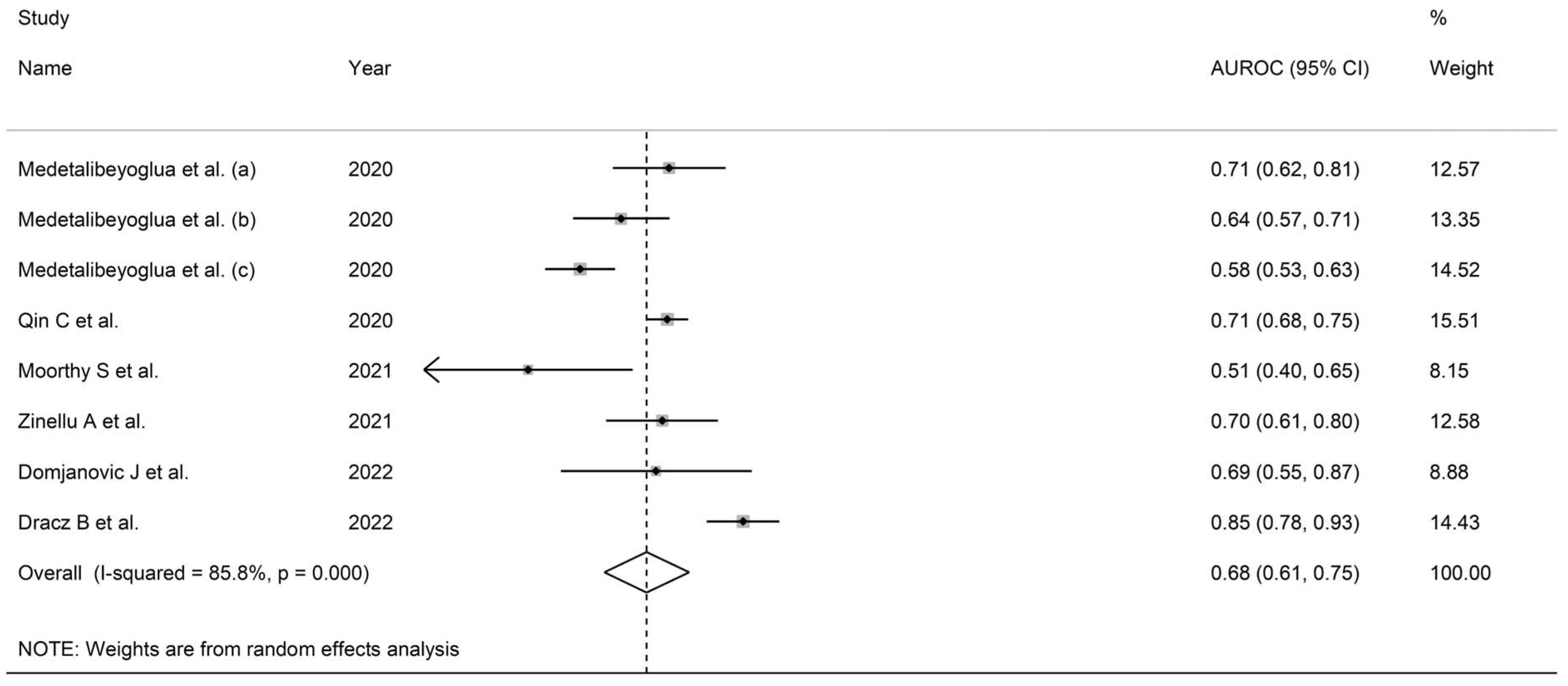
3.5.3. Results of Individual Studies and Syntheses
3.5.4. Publication Bias
3.5.5. Subgroup and Meta-Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jee, Y. WHO International Health Regulations Emergency Committee for the COVID-19 outbreak. Epidemiol. Health 2020, 42, e2020013. [Google Scholar] [CrossRef] [PubMed]
- Msemburi, W.; Karlinsky, A.; Knutson, V.; Aleshin-Guendel, S.; Chatterji, S.; Wakefield, J. The WHO estimates of excess mortality associated with the COVID-19 pandemic. Nature 2023, 613, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Lenz, C.; Slack, M.P.E.; Shea, K.M.; Reinert, R.R.; Taysi, B.N.; Swerdlow, D.L. Long-Term effects of COVID-19: A review of current perspectives and mechanistic insights. Crit. Rev. Microbiol. 2023, 1–14. [Google Scholar] [CrossRef]
- Paterson, C.; Davis, D.; Roche, M.; Bissett, B.; Roberts, C.; Turner, M.; Baldock, E.; Mitchell, I. What are the long-term holistic health consequences of COVID-19 among survivors? An umbrella systematic review. J. Med. Virol. 2022, 94, 5653–5668. [Google Scholar] [CrossRef] [PubMed]
- Wiemken, T.L.; Khan, F.; Puzniak, L.; Yang, W.; Simmering, J.; Polgreen, P.; Nguyen, J.L.; Jodar, L.; McLaughlin, J.M. Seasonal trends in COVID-19 cases, hospitalizations, and mortality in the United States and Europe. Sci. Rep. 2023, 13, 3886. [Google Scholar] [CrossRef] [PubMed]
- Battaglini, D.; Lopes-Pacheco, M.; Castro-Faria-Neto, H.C.; Pelosi, P.; Rocco, P.R.M. Laboratory Biomarkers for Diagnosis and Prognosis in COVID-19. Front. Immunol. 2022, 13, 857573. [Google Scholar] [CrossRef]
- Lopes-Pacheco, M.; Silva, P.L.; Cruz, F.F.; Battaglini, D.; Robba, C.; Pelosi, P.; Morales, M.M.; Caruso Neves, C.; Rocco, P.R.M. Pathogenesis of Multiple Organ Injury in COVID-19 and Potential Therapeutic Strategies. Front. Physiol. 2021, 12, 593223. [Google Scholar] [CrossRef]
- Cron, R.Q.; Goyal, G.; Chatham, W.W. Cytokine Storm Syndrome. Annu. Rev. Med. 2023, 74, 321–337. [Google Scholar] [CrossRef]
- Nazerian, Y.; Ghasemi, M.; Yassaghi, Y.; Nazerian, A.; Hashemi, S.M. Role of SARS-CoV-2-induced cytokine storm in multi-organ failure: Molecular pathways and potential therapeutic options. Int. Immunopharmacol. 2022, 113, 109428. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Mangoni, A.A.; Cangemi, M.; Fois, A.G.; Carru, C.; Zinellu, A. Serum albumin concentrations are associated with disease severity and outcomes in coronavirus 19 disease (COVID-19): A systematic review and meta-analysis. Clin. Exp. Med. 2021, 21, 343–354. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Mangoni, A.A.; Dettori, P.; Nasrallah, G.K.; Pintus, G.; Zinellu, A. D-Dimer Concentrations and COVID-19 Severity: A Systematic Review and Meta-Analysis. Front. Public Health 2020, 8, 432. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. Serum Prealbumin Concentrations, COVID-19 Severity, and Mortality: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 638529. [Google Scholar] [CrossRef]
- Zinellu, A.; Paliogiannis, P.; Carru, C.; Mangoni, A.A. INR and COVID-19 severity and mortality: A systematic review with meta-analysis and meta-regression. Adv. Med. Sci. 2021, 66, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Paliogiannis, P.; Carru, C.; Mangoni, A.A. Serum amyloid A concentrations, COVID-19 severity and mortality: An updated systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 105, 668–674. [Google Scholar] [CrossRef]
- Papagiouvanni, I.; Kotoulas, S.C.; Pataka, A.; Spyratos, D.G.; Porpodis, K.; Boutou, A.K.; Papagiouvannis, G.; Grigoriou, I.; Vettas, C.; Goulis, I. COVID-19 and liver injury: An ongoing challenge. World J. Gastroenterol. 2023, 29, 257–271. [Google Scholar] [CrossRef]
- Li, P.; Liu, Y.; Cheng, Z.; Yu, X.; Li, Y. COVID-19-associated liver injury: Clinical characteristics, pathophysiological mechanisms and treatment management. Biomed. Pharm. 2022, 154, 113568. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Ballester, M.P.; Soffientini, U.; Jalan, R.; Mehta, G. SARS-CoV-2 infection and liver involvement. Hepatol. Int. 2022, 16, 755–774. [Google Scholar] [CrossRef]
- Naeem, M.; Bano, N.; Manzoor, S.; Ahmad, A.; Munawar, N.; Razak, S.I.A.; Lee, T.Y.; Devaraj, S.; Hazafa, A. Pathogenetic Mechanisms of Liver-Associated Injuries, Management, and Current Challenges in COVID-19 Patients. Biomolecules 2023, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Sotgia, S.; Mangoni, A.A.; Mellino, S.; Cangemi, M.; Paliogiannis, P.; Fois, A.G.; Carru, C.; Zinellu, A. A meta-regression study of the clinical significance of serum aminotransferases in COVID-19. Eur. Rev. Med. Pharm. Sci. 2021, 25, 6813–6824. [Google Scholar] [CrossRef]
- Kumar, M.P.; Mishra, S.; Jha, D.K.; Shukla, J.; Choudhury, A.; Mohindra, R.; Mandavdhare, H.S.; Dutta, U.; Sharma, V. Coronavirus disease (COVID-19) and the liver: A comprehensive systematic review and meta-analysis. Hepatol. Int. 2020, 14, 711–722. [Google Scholar] [CrossRef]
- Ghahramani, S.; Tabrizi, R.; Lankarani, K.B.; Kashani, S.M.A.; Rezaei, S.; Zeidi, N.; Akbari, M.; Heydari, S.T.; Akbari, H.; Nowrouzi-Sohrabi, P.; et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: A systematic review and meta-analysis. Eur. J. Med. Res. 2020, 25, 30. [Google Scholar] [CrossRef]
- Mao, R.; Qiu, Y.; He, J.S.; Tan, J.Y.; Li, X.H.; Liang, J.; Shen, J.; Zhu, L.R.; Chen, Y.; Iacucci, M.; et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zuo, Z.; Kang, S.; Jiang, L.; Luo, X.; Xia, Z.; Liu, J.; Xiao, X.; Ye, M.; Deng, M. Multi-organ Dysfunction in Patients with COVID-19: A Systematic Review and Meta-analysis. Aging Dis. 2020, 11, 874–894. [Google Scholar] [CrossRef] [PubMed]
- Ampuero, J.; Sanchez, Y.; Garcia-Lozano, M.R.; Maya-Miles, D.; Romero Gomez, M. Impact of liver injury on the severity of COVID-19: A systematic review with meta-analysis. Rev. Esp. Enferm. Dig. 2021, 113, 125–135. [Google Scholar] [CrossRef]
- Aziz, M.; Haghbin, H.; Lee-Smith, W.; Goyal, H.; Nawras, A.; Adler, D.G. Gastrointestinal predictors of severe COVID-19: Systematic review and meta-analysis. Ann. Gastroenterol. 2020, 33, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jaiswal, P.; Kerakhan, Y.; Saravanan, L.; Murtaza, Z.; Zergham, A.; Honganur, N.S.; Akbar, A.; Deol, A.; Francis, B.; et al. Liver disease and outcomes among COVID-19 hospitalized patients—A systematic review and meta-analysis. Ann. Hepatol. 2021, 21, 100273. [Google Scholar] [CrossRef]
- Wong, Y.J.; Tan, M.; Zheng, Q.; Li, J.W.; Kumar, R.; Fock, K.M.; Teo, E.K.; Ang, T.L. A systematic review and meta-analysis of the COVID-19 associated liver injury. Ann. Hepatol. 2020, 19, 627–634. [Google Scholar] [CrossRef]
- De Ritis, F.; Coltorti, M.; Giusti, G. An enzymic test for the diagnosis of viral hepatitis; the transaminase serum activities. Clin. Chim. Acta 1957, 2, 70–74. [Google Scholar] [CrossRef]
- Botros, M.; Sikaris, K.A. The de ritis ratio: The test of time. Clin. Biochem. Rev. 2013, 34, 117–130. [Google Scholar]
- Davidov-Derevynko, Y.; Ben Yakov, G.; Wieder, A.; Segal, G.; Naveh, L.; Orlova, N.; Gringauz, I.; Amit, S.; Mor, O.; Klempfner, R.; et al. The liver in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Eur. J. Gastroenterol. Hepatol. 2021, 33, e313–e319. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Zinellu, A.; Scano, V.; Mulas, G.; De Riu, G.; Pascale, R.M.; Arru, L.B.; Carru, C.; Pirina, P.; Mangoni, A.A.; et al. Laboratory test alterations in patients with COVID-19 and non COVID-19 interstitial pneumonia: A preliminary report. J. Infect. Dev. Ctries. 2020, 14, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Wei, Y.; Lyu, X.; Zhao, B.; Feng, Y.; Li, T.; Cao, H.; Yang, X.; Zhou, X.; Wang, W.; et al. High aspartate aminotransferase to alanine aminotransferase ratio on admission as risk factor for poor prognosis in COVID-19 patients. Sci. Rep. 2020, 10, 16496. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, D.; Li, J.; Xia, Q.; Gong, Y.; Li, Z.; Wu, Q.; Yi, M.; Huang, Y.; Wu, M.; et al. Prognostic Potential of Liver Enzymes in Patients with COVID-19 at the Leishenshan Hospital in Wuhan. Front. Cell. Infect. Microbiol. 2021, 11, 636999. [Google Scholar] [CrossRef]
- Zinellu, A.; Arru, F.; De Vito, A.; Sassu, A.; Valdes, G.; Scano, V.; Zinellu, E.; Perra, R.; Madeddu, G.; Carru, C.; et al. The De Ritis ratio as prognostic biomarker of in-hospital mortality in COVID-19 patients. Eur. J. Clin. Investig. 2021, 51, e13427. [Google Scholar] [CrossRef]
- Pranata, R.; Huang, I.; Lim, M.A.; Yonas, E.; Vania, R.; Lukito, A.A.; Nasution, S.A.; Siswanto, B.B.; Kuswardhani, R.A.T. Elevated De Ritis Ratio Is Associated With Poor Prognosis in COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 676581. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; Johanna Briggs Institute: Adelaide, Australia, 2017. [Google Scholar]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2021. [Google Scholar]
- Tobias, A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech. Bull. 1999, 47, 15–17. [Google Scholar]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.H.; Obuchowski, N.A.; McClish, D.K. Statistical Methods in Diagnostic Medicine, 2nd ed.; Wiley: Hoboken, NJ, USA, 2011; p. 592. [Google Scholar]
- An, Y.W.; Song, S.; Li, W.X.; Chen, Y.X.; Hu, X.P.; Zhao, J.; Li, Z.W.; Jiang, G.Y.; Wang, C.; Wang, J.C.; et al. Liver function recovery of COVID-19 patients after discharge, a follow-up study. Int. J. Med. Sci. 2021, 18, 176–186. [Google Scholar] [CrossRef]
- Aziz, F.; Stocher, H.; Brauer, A.; Ciardi, C.; Clodi, M.; Fasching, P.; Karolyi, M.; Kautzky-Willer, A.; Klammer, C.; Malle, O.; et al. Biomarkers Predictive for In-Hospital Mortality in Patients with Diabetes Mellitus and Prediabetes Hospitalized for COVID-19 in Austria: An Analysis of COVID-19 in Diabetes Registry. Viruses 2022, 14, 1285. [Google Scholar] [CrossRef]
- Baik, S.M.; Lee, M.; Hong, K.S.; Park, D.J. Development of Machine-Learning Model to Predict COVID-19 Mortality: Application of Ensemble Model and Regarding Feature Impacts. Diagnostics 2022, 12, 1464. [Google Scholar] [CrossRef] [PubMed]
- Benede-Ubieto, R.; Estevez-Vazquez, O.; Flores-Perojo, V.; Macias-Rodriguez, R.U.; Ruiz-Margain, A.; Martinez-Naves, E.; Regueiro, J.R.; Avila, M.A.; Trautwein, C.; Banares, R.; et al. Abnormal Liver Function Test in Patients Infected with Coronavirus (SARS-CoV-2): A Retrospective Single-Center Study from Spain. J. Clin. Med. 2021, 10, 1039. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, W.; Chen, J.; Xu, D.; Xie, W.; Wang, X.; Xie, Y. Clinical features and risk factors of COVID-19-associated liver injury and function: A retrospective analysis of 830 cases. Ann. Hepatol. 2021, 21, 100267. [Google Scholar] [CrossRef]
- Crisan, D.; Avram, L.; Grapa, C.; Dragan, A.; Radulescu, D.; Crisan, S.; Grosu, A.; Militaru, V.; Buzdugan, E.; Stoicescu, L.; et al. Liver Injury and Elevated FIB-4 Define a High-Risk Group in Patients with COVID-19. J. Clin. Med. 2021, 11, 153. [Google Scholar] [CrossRef]
- Domjanovic, J.; Domjanovic Skopinic, T.; Radic, J.; Luketin, M.; Jelicic, I.; Matetic, A. Performance of Derived Laboratory Biomarkers with Regard to 30-Day Mortality in Kidney Transplant Recipients with COVID-19. Life 2022, 12, 2068. [Google Scholar] [CrossRef]
- Dracz, B.; Czompa, D.; Mullner, K.; Hagymasi, K.; Miheller, P.; Szekely, H.; Papp, V.; Horvath, M.; Hritz, I.; Szijarto, A.; et al. The Elevated De Ritis Ratio on Admission Is Independently Associated with Mortality in COVID-19 Patients. Viruses 2022, 14, 2360. [Google Scholar] [CrossRef]
- Fu, L.; Fei, J.; Xu, S.; Xiang, H.X.; Xiang, Y.; Hu, B.; Li, M.D.; Liu, F.F.; Li, Y.; Li, X.Y.; et al. Liver Dysfunction and Its Association with the Risk of Death in COVID-19 Patients: A Prospective Cohort Study. J. Clin. Transl. Hepatol. 2020, 8, 246–254. [Google Scholar] [CrossRef]
- Fu, Y.; Du, S.; Liu, X.; Cao, L.; Yang, G.; Chen, H. A linear relationship between De Ritis ratio and mortality in hospitalized patients with COVID-19: A secondary analysis based on a large retrospective cohort study. iLIVER 2022, 1, 169–175. [Google Scholar] [CrossRef]
- Goel, H.; Harmouch, F.; Garg, K.; Saraiya, P.; Daly, T.; Kumar, A.; Hippen, J.T. The liver in COVID-19: Prevalence, patterns, predictors, and impact on outcomes of liver test abnormalities. Eur. J. Gastroenterol. Hepatol. 2021, 33, e274–e281. [Google Scholar] [CrossRef]
- Medetalibeyoglu, A.; Catma, Y.; Senkal, N.; Ormeci, A.; Cavus, B.; Kose, M.; Bayramlar, O.F.; Yildiz, G.; Akyuz, F.; Kaymakoglu, S.; et al. The effect of liver test abnormalities on the prognosis of COVID-19. Ann. Hepatol. 2020, 19, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Farooqui, M.S. Study of dynamic changes in the parameters of liver function tests in COVID-19 patients: A hospital-based study in Eastern Nepal. J. Biomed. Sci. 2021, 8, 60–64. [Google Scholar] [CrossRef]
- Moorthy, S.; Koshy, T.; Silambanan, S. Role of inflammatory and liver function markers in assessing the prognosis of patients with COVID-19. World Acad. Sci. J. 2021, 3, 52. [Google Scholar] [CrossRef]
- Ni, Y.N.; Wang, T.; Liang, B.M.; Liang, Z.A. The independent factors associated with oxygen therapy in COVID-19 patients under 65 years old. PLoS ONE 2021, 16, e0245690. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; San-Cristobal, R.; Martinez-Urbistondo, D.; Mico, V.; Colmenarejo, G.; Villares-Fernandez, P.; Daimiel, L.; Martinez, J.A. Proinflammatory and Hepatic Features Related to Morbidity and Fatal Outcomes in COVID-19 Patients. J. Clin. Med. 2021, 10, 3112. [Google Scholar] [CrossRef]
- Wong, G.L.; Yip, T.C.; Wong, V.W.; Tse, Y.K.; Hui, D.S.; Lee, S.S.; Yeoh, E.K.; Chan, H.L.; Lui, G.C. SARS-CoV-2 Viral Persistence Based on Cycle Threshold Value and Liver Injury in Patients with COVID-19. Open Forum Infect. Dis. 2021, 8, ofab205. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, K.; Misra-Hebert, A.; Bena, J.; Kashyap, S.R. Impact of Metabolic Syndrome on Severity of COVID-19 Illness. Metab. Syndr. Relat. Disord. 2022, 20, 191–198. [Google Scholar] [CrossRef]
- Yadlapati, S.; Lo, K.B.; DeJoy, R.; Gul, F.; Peterson, E.; Bhargav, R.; Salacup, G.F.; Pelayo, J.; Azmaiparashvilli, Z.; Patarroyo-Aponte, G. Prevailing patterns of liver enzymes in patients with COVID-19 infection and association with clinical outcomes. Ann. Gastroenterol. 2021, 34, 224–228. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Liver enzymes, metabolomics and genome-wide association studies: From systems biology to the personalized medicine. World J. Gastroenterol. 2015, 21, 711–725. [Google Scholar] [CrossRef]
- Esani, M.A. The Physiological Sources of, Clinical Significance of, and Laboratory-Testing Methods for Determining Enzyme Levels. Lab. Med. 2014, 45, e16–e18. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Anderson, F.H.; Zeng, L.; Rock, N.R.; Yoshida, E.M. An assessment of the clinical utility of serum ALT and AST in chronic hepatitis C. Hepatol. Res. 2000, 18, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Nyblom, H.; Bjornsson, E.; Simren, M.; Aldenborg, F.; Almer, S.; Olsson, R. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int. 2006, 26, 840–845. [Google Scholar] [CrossRef]
- Mera, J.R.; Dickson, B.; Feldman, M. Influence of gender on the ratio of serum aspartate aminotransferase (AST) to alanine aminotransferase (ALT) in patients with and without hyperbilirubinemia. Dig. Dis. Sci. 2008, 53, 799–802. [Google Scholar] [CrossRef]
- Montori, M.; Baroni, G.S.; Santori, P.; Di Giampaolo, C.; Ponziani, F.; Abenavoli, L.; Scarpellini, E. Liver Damage and COVID-19: At Least a “Two-Hit” Story in Systematic Review. Curr. Issues Mol. Biol. 2023, 45, 3035–3047. [Google Scholar] [CrossRef]
- Yang, R.; Feng, J.; Wan, H.; Zeng, X.; Ji, P.; Zhang, J. Liver injury associated with the severity of COVID-19: A meta-analysis. Front. Public Health 2023, 11, 1003352. [Google Scholar] [CrossRef]
- Choaib, A.; Issa, E.; El Choueiry, F.; Eldin, J.N.; Shbaklo, K.; Alhajj, M.; Sawaya, R.T.; Assi, G.; Nader, M.; Chatila, R.; et al. SARS-CoV-2-mediated liver injury: Pathophysiology and mechanisms of disease. Inflamm. Res. 2023, 72, 301–312. [Google Scholar] [CrossRef]
- Chornenkyy, Y.; Mejia-Bautista, M.; Brucal, M.; Blanke, T.; Dittmann, D.; Yeldandi, A.; Boike, J.R.; Lomasney, J.W.; Nayar, R.; Jennings, L.J.; et al. Liver Pathology and SARS-CoV-2 Detection in Formalin-Fixed Tissue of Patients with COVID-19. Am. J. Clin. Pathol. 2021, 155, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Liu, H.; Li, W.; Lin, F.; Jiang, L.; Li, X.; Xu, P.; Zhang, L.; Zhao, L.; et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020, 73, 807–816. [Google Scholar] [CrossRef]
- Wanner, N.; Andrieux, G.; Badia, I.M.P.; Edler, C.; Pfefferle, S.; Lindenmeyer, M.T.; Schmidt-Lauber, C.; Czogalla, J.; Wong, M.N.; Okabayashi, Y.; et al. Molecular consequences of SARS-CoV-2 liver tropism. Nat. Metab. 2022, 4, 310–319. [Google Scholar] [CrossRef]
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020, 526, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, M.; Abomhya, A.; Elkhattib, I.; Dawoud, N.; Doshi, R. COVID-19 and liver diseases, what we know so far. World J. Clin. Cases 2022, 10, 3969–3980. [Google Scholar] [CrossRef] [PubMed]
- Pirola, C.J.; Sookoian, S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int. 2020, 40, 2038–2040. [Google Scholar] [CrossRef]
- Seow, J.J.W.; Pai, R.; Mishra, A.; Shepherdson, E.; Lim, T.K.H.; Goh, B.K.P.; Chan, J.K.Y.; Chow, P.K.H.; Ginhoux, F.; DasGupta, R.; et al. Single-Cell RNA-seq Reveals Angiotensin-Converting Enzyme 2 and Transmembrane Serine Protease 2 Expression in TROP2(+) Liver Progenitor Cells: Implications in Coronavirus Disease 2019-Associated Liver Dysfunction. Front. Med. 2021, 8, 603374. [Google Scholar] [CrossRef]
- Simpson, J.D.; Ray, A.; Marcon, C.; Dos Santos Natividade, R.; Dorrazehi, G.M.; Durlet, K.; Koehler, M.; Alsteens, D. Single-Molecule Analysis of SARS-CoV-2 Binding to C-Type Lectin Receptors. Nano Lett. 2023, 23, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Wade, H.; Duan, Q.; Su, Q. Interaction between SARS-CoV-2 structural proteins and host cellular receptors: From basic mechanisms to clinical perspectives. Adv. Protein Chem. Struct. Biol. 2022, 132, 243–277. [Google Scholar] [CrossRef]
- Li, D.; Ding, X.; Xie, M.; Tian, D.; Xia, L. COVID-19-associated liver injury: From bedside to bench. J. Gastroenterol. 2021, 56, 218–230. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Aleya, L.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ. 2022, 808, 152072. [Google Scholar] [CrossRef]
- Idalsoaga, F.; Ayares, G.; Arab, J.P.; Diaz, L.A. COVID-19 and Indirect Liver Injury: A Narrative Synthesis of the Evidence. J. Clin. Transl. Hepatol. 2021, 9, 760–768. [Google Scholar] [CrossRef] [PubMed]
- McGrowder, D.A.; Miller, F.; Anderson Cross, M.; Anderson-Jackson, L.; Bryan, S.; Dilworth, L. Abnormal Liver Biochemistry Tests and Acute Liver Injury in COVID-19 Patients: Current Evidence and Potential Pathogenesis. Diseases 2021, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Franza, L.; Esperide, A.; Gasparrini, I.; Gasbarrini, A.; Franceschi, F.; On Behalf Of Gemelli Against, C. Liver Injury in Patients Hospitalized for COVID-19: Possible Role of Therapy. Vaccines 2022, 10, 192. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangoni, A.A.; Zinellu, A. An Updated Systematic Review and Meta-Analysis of the Association between the De Ritis Ratio and Disease Severity and Mortality in Patients with COVID-19. Life 2023, 13, 1324. https://doi.org/10.3390/life13061324
Mangoni AA, Zinellu A. An Updated Systematic Review and Meta-Analysis of the Association between the De Ritis Ratio and Disease Severity and Mortality in Patients with COVID-19. Life. 2023; 13(6):1324. https://doi.org/10.3390/life13061324
Chicago/Turabian StyleMangoni, Arduino A., and Angelo Zinellu. 2023. "An Updated Systematic Review and Meta-Analysis of the Association between the De Ritis Ratio and Disease Severity and Mortality in Patients with COVID-19" Life 13, no. 6: 1324. https://doi.org/10.3390/life13061324
APA StyleMangoni, A. A., & Zinellu, A. (2023). An Updated Systematic Review and Meta-Analysis of the Association between the De Ritis Ratio and Disease Severity and Mortality in Patients with COVID-19. Life, 13(6), 1324. https://doi.org/10.3390/life13061324








