The Effect of Omega-3 Fatty Acids on Insulin Resistance
Abstract
1. Introduction
2. Materials and Methods
3. Polyunsaturated Fatty Acid (PUFA)
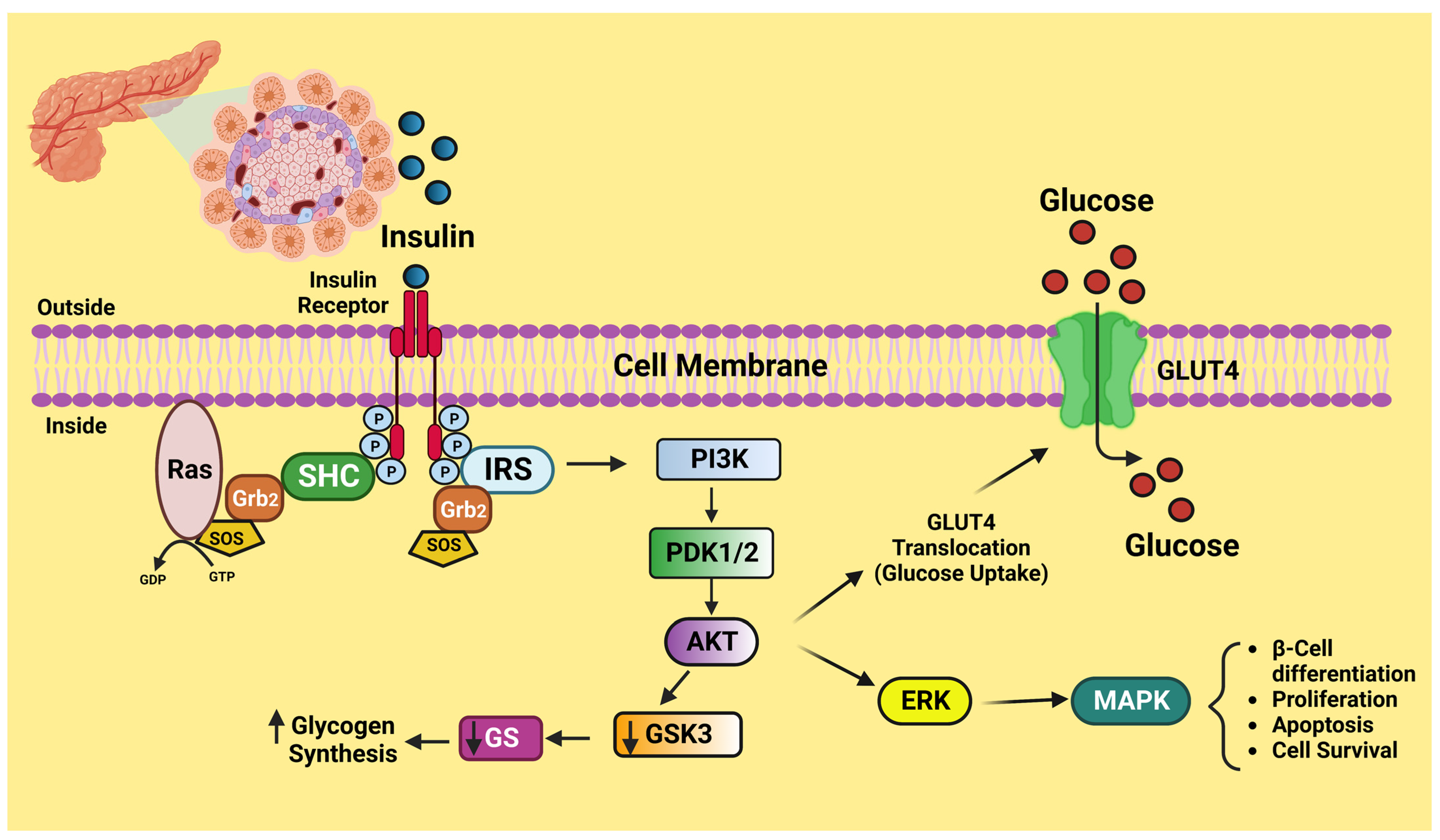
4. Insulin Signaling
5. Insulin Resistance and Its Molecular Mechanism
5.1. Mitochondria and Insulin Resistance
5.2. Mitochondrial Dynamics and Insulin Resistance
6. Endoplasmic Reticulum and Insulin Resistance
7. The Role of Omega-3 PUFA in Insulin Resistance
8. Omega Oil and Diabetes Mellitus
9. Omega-3 FA and Nonalcoholic Fatty Liver Disease
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef] [PubMed]
- Dal Canto, E.; Ceriello, A.; Rydén, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 2019, 26 (Suppl. S2), 25–32. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.; Durstine, J.L. Physical activity, exercise, and chronic diseases: A brief review. Sports Med. Health Sci. 2019, 1, 3–10. [Google Scholar] [CrossRef]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [Google Scholar] [CrossRef]
- Gómez-Hernández, A.; Beneit, N.; Díaz-Castroverde, S.; Escribano, Ó. Differential Role of Adipose Tissues in Obesity and Related Metabolic and Vascular Complications. Int. J. Endocrinol. 2016, 2016, 1216783. [Google Scholar] [CrossRef]
- Islam, H.; Neudorf, H.; Mui, A.L.; Little, J.P. Interpreting ‘anti-inflammatory’ cytokine responses to exercise: Focus on interleukin-10. J. Physiol. 2021, 599, 5163–5177. [Google Scholar] [CrossRef]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef]
- de Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all-cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- Pereira-Dutra, F.S.; Teixeira, L.; de Souza Costa, M.F.; Bozza, P.T. Fat, fight, and Beyond: The multiple roles of lipid droplets in Infections and Inflammation. J. Leukoc. Biol. 2019, 106, 563–580. [Google Scholar] [CrossRef]
- Kalra, S.; Unnikrishnan, A.G.; Baruah, M.P.; Sahay, R.; Bantwal, G. Metabolic and Energy Imbalance in Dysglycemia-Based Chronic Disease. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 165–184. [Google Scholar] [CrossRef]
- Paniagua, J.A. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J. Diabetes 2016, 7, 483–514. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Lorente-Cebrián, S.; Costa, A.G.V.; Navas-Carretero, S.; Zabala, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef]
- Drenjančević, I.; Pitha, J. Omega-3 Polyunsaturated Fatty Acids—Vascular and Cardiac Effects on the Cellular and Molecular Level (Narrative Review). Int. J. Mol. Sci. 2022, 23, 2104. [Google Scholar] [CrossRef]
- Chen, G.-C.; Arthur, R.; Qin, L.-Q.; Chen, L.-H.; Mei, Z.; Zheng, Y.; Li, Y.; Wang, T.; Rohan, T.E.; Qi, Q. Association of Oily and Nonoily Fish Consumption and Fish Oil Supplements With Incident Type 2 Diabetes: A Large Population-Based Prospective Study. Diabetes Care 2021, 44, 672–680. [Google Scholar] [CrossRef]
- Briggs, M.A.; Petersen, K.S.; Kris-Etherton, P.M. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef]
- Yilmaz, M.I.; Romano, M.; Basarali, M.K.; Elzagallaai, A.; Karaman, M.; Demir, Z.; Demir, M.F.; Akcay, F.; Seyrek, M.; Haksever, N.; et al. The Effect of Corrected Inflammation, Oxidative Stress and Endothelial Dysfunction on Fmd Levels in Patients with Selected Chronic Diseases: A Quasi-Experimental Study. Sci. Rep. 2020, 10, 9018. [Google Scholar] [CrossRef]
- Gil, A.; Gil, F. Fish, a Mediterranean source of n-3 PUFA: Benefits do not justify limiting consumption. Br. J. Nutr. 2015, 113 (Suppl. S2), S58–S67. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; de Marañón, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Bi, Y.; Sowers, J.R.; Hetz, C.; Zhang, Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat. Rev. Cardiol. 2021, 18, 499–521. [Google Scholar] [CrossRef] [PubMed]
- Jayarathne, S.; Koboziev, I.; Park, O.-H.; Oldewage-Theron, W.; Shen, C.-L.; Moustaid-Moussa, N. Anti-Inflammatory and Anti-Obesity Properties of Food Bioactive Components: Effects on Adipose Tissue. Prev. Nutr. Food Sci. 2017, 22, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, L.; Wang, D.; Yan, N.; Li, C.; Wu, M.; Wang, F.; Mi, B.; Chen, F.; Jia, W.; et al. Omega-3 polyunsaturated fatty acid biomarkers and risk of type 2 diabetes, cardiovascular disease, cancer, and mortality. Clin. Nutr. 2022, 41, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.-Y.; Filaire, E. Microalgae n-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Takic, M.; Pokimica, B.; Petrovic-Oggiano, G.; Popovic, T. Effects of Dietary α-Linolenic Acid Treatment and the Efficiency of Its Conversion to Eicosapentaenoic and Docosahexaenoic Acids in Obesity and Related Diseases. Molecules 2022, 27, 4471. [Google Scholar] [CrossRef]
- Ganguly, B.B.; Kadam, N.N. Therapeutics for mitochondrial dysfunction-linked diseases in Down syndrome. Mitochondrion 2023, 68, 25–43. [Google Scholar] [CrossRef]
- Sinha, S.; Haque, M. Insulin Resistance Is Cheerfully Hitched with Hypertension. Life 2022, 12, 564. [Google Scholar] [CrossRef]
- Saltiel, A.R. Insulin signaling in health and disease. J. Clin. Investig. 2021, 131, e142241. [Google Scholar] [CrossRef]
- Kma, L.; Baruah, T.J. The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol. Appl. Biochem. 2021, 69, 248–264. [Google Scholar] [CrossRef]
- Sinha, S.; Haque, M. Insulin Resistance and Type 2 Diabetes Mellitus: An Ultimatum to Renal Physiology. Cureus 2022, 14, e28944. [Google Scholar] [CrossRef]
- Whitticar, N.B.; Nunemaker, C.S. Reducing Glucokinase Activity to Enhance Insulin Secretion: A Counterintuitive Theory to Preserve Cellular Function and Glucose Homeostasis. Front. Endocrinol. 2020, 11, 378. [Google Scholar] [CrossRef]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1273–1298. [Google Scholar] [CrossRef]
- Tumova, J.; Andel, M.; Trnka, J. Excess of Free Fatty Acids as a Cause of Metabolic Dysfunction in Skeletal Muscle. Physiol. Res. 2016, 65, 193–207. [Google Scholar] [CrossRef]
- Lepretti, M.; Martucciello, S.; Burgos Aceves, M.A.; Putti, R.; Lionetti, L. Omega-3 Fatty Acids and Insulin Resistance: Focus on the Regulation of Mitochondria and Endoplasmic Reticulum Stress. Nutrients 2018, 10, 350. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef]
- Sánchez-Aguilera, P.; Diaz-Vegas, A.; Campos, C.; Quinteros-Waltemath, O.; Cerda-Kohler, H.; Barrientos, G.; Contreras-Ferrat, A.; Llanos, P. Role of ABCA1 on membrane cholesterol content, insulin-dependent Akt phosphorylation and glucose uptake in adult skeletal muscle fibers from mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1469–1477. [Google Scholar] [CrossRef]
- Di Meo, S.; Iossa, S.; Venditti, P. Skeletal muscle insulin resistance: Role of mitochondria and other ROS sources. J. Endocrinol. 2017, 233, R15–R42. [Google Scholar] [CrossRef]
- Ježek, P.; Holendová, B.; Jabůrek, M.; Tauber, J.; Dlasková, A.; Plecitá-Hlavatá, L. The Pancreatic β-Cell: The Perfect Redox System. Antioxidants 2021, 10, 197. [Google Scholar] [CrossRef]
- Roger, A.J.; Muñoz-Gómez, S.A.; Kamikawa, R. The Origin and Diversification of Mitochondria. Curr. Biol. 2017, 27, R1177–R1192. [Google Scholar] [CrossRef] [PubMed]
- Parry, H.A.; Glancy, B. Energy transfer between the mitochondrial network and lipid droplets in insulin-resistant skeletal muscle. Curr. Opin. Physiol. 2021, 24, 100487. [Google Scholar] [CrossRef] [PubMed]
- Macasoi, I.; Mioc, A.; Mioc, M.; Racoviceanu, R.; Soica, I.; Chevereșan, A.; Dehelean, C.; Dumitrașcu, V. Targeting Mitochondria through the Use of Mitocans as Emerging Anticancer Agents. Curr. Med. Chem. 2020, 27, 5730–5757. [Google Scholar] [CrossRef] [PubMed]
- Tilokani, L.; Russell, F.M.; Hamilton, S.; Virga, D.M.; Segawa, M.; Paupe, V.; Gruszczyk, A.V.; Protasoni, M.; Tabara, L.C.; Johnson, M.; et al. AMPK-dependent phosphorylation of MTFR1L regulates mitochondrial morphology. Sci. Adv. 2022, 8, eabo7956. [Google Scholar] [CrossRef]
- Andreadi, A.; Bellia, A.; Di Daniele, N.; Meloni, M.; Lauro, R.; Della-Morte, D.; Lauro, D. The molecular link between oxidative stress, insulin resistance, and type 2 diabetes: A target for new therapies against cardiovascular diseases. Curr. Opin. Pharmacol. 2022, 62, 85–96. [Google Scholar] [CrossRef]
- Vezza, T.; Díaz-Pozo, P.; Canet, F.; de Marañón, A.M.; Abad-Jiménez, Z.; García-Gargallo, C.; Roldan, I.; Solá, E.; Bañuls, C.; López-Domènech, S.; et al. The Role of Mitochondrial Dynamic Dysfunction in Age-Associated Type 2 Diabetes. World J. Mens Health 2022, 40, 399–411. [Google Scholar] [CrossRef]
- Houzelle, A.; Jörgensen, J.A.; Schaart, G.; Daemen, S.; van Polanen, N.; Fealy, C.E.; Hesselink, M.K.C.; Schrauwen, P.; Hoeks, J. Human skeletal muscle mitochondrial dynamics in relation to oxidative capacity and insulin sensitivity. Diabetologia 2021, 64, 424–436. [Google Scholar] [CrossRef]
- Picard, M.; Turnbull, D.M. Linking the metabolic state and mitochondrial DNA in chronic disease, health, and aging. Diabetes 2013, 62, 672–678. [Google Scholar] [CrossRef]
- de Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Moreno-Fernández, S.; Garcés-Rimón, M.; Vera, G.; Astier, J.; Landrier, J.F.; Miguel, M. High Fat/High Glucose Diet Induces Metabolic Syndrome in an Experimental Rat Model. Nutrients 2018, 10, 1502. [Google Scholar] [CrossRef]
- Fealy, C.E.; Mulya, A.; Axelrod, C.L.; Kirwan, J.P. Mitochondrial dynamics in skeletal muscle insulin resistance and type 2 diabetes. Transl. Res. 2018, 202, 69–82. [Google Scholar] [CrossRef]
- Chen, E.; Tsai, T.H.; Li, L.; Saha, P.; Chan, L.; Chang, B.H. PLIN2 is a Key Regulator of the Unfolded Protein Response and Endoplasmic Reticulum Stress Resolution in Pancreatic β Cells. Sci. Rep. 2017, 7, 40855. [Google Scholar] [CrossRef]
- Kimura, T.; Kagami, K.; Sato, A.; Osaki, A.; Ito, K.; Horii, S.; Toya, T.; Masaki, N.; Yasuda, R.; Nagatomo, Y.; et al. Sarco/Endoplasmic Reticulum Ca2+ ATPase 2 Activator Ameliorates Endothelial Dysfunction; Insulin Resistance in Diabetic Mice. Cells 2022, 11, 1488. [Google Scholar] [CrossRef]
- López-Crisosto, C.; Bravo-Sagua, R.; Rodriguez-Peña, M.; Mera, C.; Castro, P.F.; Quest, A.F.; Rothermel, B.A.; Cifuentes, M.; Lavandero, S. ER-to-mitochondria miscommunication and metabolic diseases. Biochim. Biophys. Acta 2015, 1852 Pt 10A, 2096–2105. [Google Scholar] [CrossRef]
- Benchoula, K.; Mediani, A.; Hwa, W.E. The functions of Ca2+/calmodulin-dependent protein kinase II (CaMKII) in diabetes progression. J. Cell Commun. Signal. 2022, 17, 25–34. [Google Scholar] [CrossRef]
- Ly, L.D.; Xu, S.; Choi, S.-K.; Ha, C.-M.; Thoudam, T.; Cha, S.-K.; Wiederkehr, A.; Wollheim, C.B.; Lee, I.-K.; Park, K.-S. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp. Mol. Med. 2017, 49, e291. [Google Scholar] [CrossRef]
- González-Rodríguez, L.G.; Aparicio, A.; López-Sobaler, A.M.; Ortega, R.M. Omega 3 and omega 6 fatty acids intake and dietary sources in a representative sample of Spanish adults. Int. J. Vitam. Nutr. Res. 2013, 83, 36–47. [Google Scholar] [CrossRef]
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marin, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef]
- Liu, R.; Chen, L.; Wang, Z.; Zheng, X.; Hou, Z.; Zhao, D.; Long, J.; Liu, J. Omega-3 polyunsaturated fatty acids prevent obesity by improving tricarboxylic acid cycle homeostasis. J. Nutr. Biochem. 2020, 88, 108503. [Google Scholar] [CrossRef]
- Ren, G.; Rimando, A.M.; Mathews, S.T. AMPK activation by pterostilbene contributes to suppression of hepatic gluconeogenic gene expression and glucose production in H4IIE cells. Biochem. Biophys. Res. Commun. 2018, 498, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sadoshima, J. Cardiomyopathy in obesity, insulin resistance and diabetes. J. Physiol. 2019, 598, 2977–2993. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, B.F.; Gosmann, G.; Zimmer, A.R. Modeling Diet-Induced Metabolic Syndrome in Rodents. Mol. Nutr. Food Res. 2020, 64, e2000249. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Chen, M.; Li, Y.; Li, Q.; Jiang, X.; Yang, Y.; Ling, W. Fish oil supplementation inhibits endoplasmic reticulum stress and improves insulin resistance: Involvement of AMP-activated protein kinase. Food Funct. 2017, 8, 1481–1493. [Google Scholar] [CrossRef]
- Martínez-Fernández, L.; Laiglesia, L.M.; Huerta, A.E.; Martínez, J.A.; Moreno-Aliaga, M.J. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat. 2015, 121 Pt A, 24–41. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.-L. Redox Regulation of NLRP3 Inflammasomes: ROS as Trigger or Effector? Antioxidants Redox Signal. 2015, 22, 1111–1129. [Google Scholar] [CrossRef]
- Rocha, M.; Apostolova, N.; Diaz-Rua, R.; Muntane, J.; Victor, V.M. Mitochondria and T2D: Role of Autophagy, ER Stress, and Inflammasome. Trends Endocrinol. Metab. 2020, 31, 725–741. [Google Scholar] [CrossRef]
- Dos Santos, N.C.C.; Andere, N.M.R.B.; Araujo, C.F.; De Marco, A.C.; Kantarci, A.; Van Dyke, T.E.; Santamaria, M.P. Omega-3 PUFA and aspirin as adjuncts to periodontal debridement in patients with periodontitis and type 2 diabetes mellitus: Randomized clinical trial. J. Periodontol. 2020, 91, 1318–1327. [Google Scholar] [CrossRef]
- Djoussé, L.; Cook, N.R.; Kim, E.; Walter, J.; Al-Ramady, O.T.; Luttmann-Gibson, H.; Albert, C.M.; Mora, S.; Buring, J.E.; Gaziano, J.M.; et al. Diabetes Mellitus, Race, and Effects of Omega-3 Fatty Acids on Incidence of Heart Failure Hospitalization. JACC Hear. Fail. 2022, 10, 227–234. [Google Scholar] [CrossRef]
- Ascend Study Collaborative Group; Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef]
- Raygan, F.; Taghizadeh, M.; Mirhosseini, N.; Akbari, E.; Bahmani, F.; Memarzadeh, M.R.; Sharifi, N.; Jafarnejad, S.; Banikazemi, Z.; Asemi, Z. A comparison between the effects of flaxseed oil and fish oil supplementation on cardiovascular health in type 2 diabetic patients with coronary heart disease: A randomized, double-blinded, placebo-controlled trial. Phytother. Res. 2019, 33, 1943–1951. [Google Scholar] [CrossRef]
- Dakin, H.A.; Farmer, A.; Gray, A.M.; Holman, R.R. Economic Evaluation of Factorial Trials: Cost-Utility Analysis of the Atorvastatin in Factorial with Omega EE90 Risk Reduction in Diabetes 2 × 2 × 2 Factorial Trial of Atorvastatin, Omega-3 Fish Oil, and Action Planning. Value Heal. 2020, 23, 1340–1348. [Google Scholar] [CrossRef]
- Liu, K.; Bin Wang, B.; Zhou, R.; Lang, H.-D.; Ran, L.; Wang, J.; Li, L.; Kang, C.; Zhu, X.-H.; Zhang, Q.-Y.; et al. Effect of combined use of a low-carbohydrate, high-protein diet with omega-3 polyunsaturated fatty acid supplementation on glycemic control in newly diagnosed type 2 diabetes: A randomized, double-blind, parallel-controlled trial. Am. J. Clin. Nutr. 2018, 108, 256–265. [Google Scholar] [CrossRef]
- Jamilian, M.; Tabassi, Z.; Mansournia, M.A.; Memarzadeh, M.R.; Asemi, Z.; Reiner, Ž.; Panahandeh, I.; Naderi, F.; Aghadavood, E.; Amirani, E.; et al. The effects of n-3 fatty acids from flaxseed oil on genetic and metabolic profiles in patients with gestational diabetes mellitus: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2020, 123, 792–799. [Google Scholar] [CrossRef]
- Britten-Jones, A.C.; Kamel, J.T.; Roberts, L.J.; Braat, S.; Craig, J.P.; MacIsaac, R.J.; Downie, L.E. Investigating the Neuroprotective Effect of Oral Omega-3 Fatty Acid Supplementation in Type 1 Diabetes (nPROOFS1): A Randomized Placebo-Controlled Trial. Diabetes 2021, 70, 1794–1806. [Google Scholar] [CrossRef]
- O’Mahoney, L.L.; Dunseath, G.; Churm, R.; Holmes, M.; Boesch, C.; Stavropoulos-Kalinoglou, A.; Ajjan, R.A.; Birch, K.M.; Orsi, N.M.; Mappa, G.; et al. Omega-3 polyunsaturated fatty acid supplementation versus placebo on vascular health, glycaemic control, and metabolic parameters in people with type 1 diabetes: A randomized controlled preliminary trial. Cardiovasc. Diabetol. 2020, 19, 127. [Google Scholar] [CrossRef]
- Naeini, Z.; Toupchian, O.; Vatannejad, A.; Sotoudeh, G.; Teimouri, M.; Ghorbani, M.; Nasli-Esfahani, E.; Koohdani, F. Effects of DHA-enriched fish oil on gene expression levels of p53 and NF-κB and PPAR-γ activity in PBMCs of patients with T2DM: A randomized, double-blind, clinical trial. Nutr. Metab. Cardiovasc. Dis. 2019, 30, 441–447. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Liu, X.; Xie, Y.; Xia, H.; Wang, S.; Sun, G. Effects of marine-derived and plant-derived omega-3 polyunsaturated fatty acids on erythrocyte fatty acid composition in type 2 diabetic patients. Lipids Heal. Dis. 2022, 21, 20. [Google Scholar] [CrossRef]
- Burhop, M.; Schuchardt, J.P.; Nebl, J.; Müller, M.; Lichtinghagen, R.; Hahn, A. Marine Oil from C. finmarchicus Enhances Glucose Homeostasis and Liver Insulin Resistance in Obese Prediabetic Individuals. Nutrients 2022, 14, 396. [Google Scholar] [CrossRef]
- Abbott, K.; Burrows, T.; Acharya, S.; Thota, R.; Garg, M. DHA-enriched fish oil reduces insulin resistance in overweight and obese adults. Prostaglandins Leukot. Essent. Fat. Acids 2020, 159, 102154. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-S.; Chen, J.; Wang, L.; Yang, H.; Fang, L.; Yu, Y.; Yuan, L.; Feng, J.; Li, K.; Tang, J.; et al. Replication of a Gene-Diet Interaction at CD36, NOS3 and PPARG in Response to Omega-3 Fatty Acid Supplements on Blood Lipids: A Double-Blind Randomized Controlled Trial. Ebiomedicine 2018, 31, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.N.; Dias, C.B.; Abbott, K.A.; Acharya, S.H.; Garg, M.L. Curcumin alleviates postprandial glycaemic response in healthy subjects: A cross-over, randomized controlled study. Sci. Rep. 2018, 8, 13679. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Best, K.; Yelland, L.; McPhee, A.; Zhou, S.; Quinlivan, J.; Dodd, J.; Atkinson, E.; Safa, H.; van Dam, J.; et al. A Randomized Trial of Prenatal n−3 Fatty Acid Supplementation and Preterm Delivery. N. Engl. J. Med. 2019, 381, 1035–1045. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.C.; Liu, X.S.; Dong, S.N.; Pan, D.; Yang, L.G.; Sun, G.J. [Effects of ω-3 polyunsaturated fatty acids from different sources on glucolipid metabolism in type 2 diabetic patients with dyslipidemia]. Zhonghua yu fang yi xue za zhi Chin. J. Prev. Med. 2019, 53, 570–575. (In Chinese) [Google Scholar] [CrossRef]
- Talari, H.R.; Najafi, V.; Raygan, F.; Mirhosseini, N.; Ostadmohammadi, V.; Amirani, E.; Taghizadeh, M.; Hajijafari, M.; Shafabakhash, R.; Asemi, Z. Long-term vitamin D and high-dose n-3 fatty acids’ supplementation improve markers of cardiometabolic risk in type 2 diabetic patients with CHD. Br. J. Nutr. 2019, 122, 423–430. [Google Scholar] [CrossRef]
- Wasserfurth, P.; Nebl, J.; Schuchardt, J.P.; Müller, M.; Boßlau, T.K.; Krüger, K.; Hahn, A. Effects of Exercise Combined with a Healthy Diet or Calanus finmarchicus Oil Supplementation on Body Composition and Metabolic Markers—A Pilot Study. Nutrients 2020, 12, 2139. [Google Scholar] [CrossRef]
- Ruan, Y.; Zheng, J.; Ren, Y.; Tang, J.; Li, J.; Li, D. Changes of urine metabolites in response to n-3 fatty acid supplements and their correlation with metabolic risk factors in patients with type 2 diabetes. Food Funct. 2019, 10, 2471–2479. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and metabolic partitioning of fatty acids within the liver in the context of nonalcoholic fatty liver disease. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 248–255. [Google Scholar] [CrossRef]
- Magkos, F. Basal very low-density lipoprotein metabolism in response to exercise: Mechanisms of hypotriacylglycerolemia. Prog. Lipid Res. 2009, 48, 171–190. [Google Scholar] [CrossRef]
- Hodson, L.; Bhatia, L.; Scorletti, E.; Smith, D.E.; Jackson, N.C.; Shojaee-Moradie, F.; Umpleby, M.; Calder, P.C.; Byrne, C.D. Docosahexaenoic acid enrichment in NAFLD is associated with improvements in hepatic metabolism and hepatic insulin sensitivity: A pilot study. Eur. J. Clin. Nutr. 2017, 71, 973–979. [Google Scholar] [CrossRef]
- Tobin, D.; Brevik-Andersen, M.; Qin, Y.; Innes, J.K.; Calder, P.C. Evaluation of a High Concentrate Omega-3 for Correcting the Omega-3 Fatty Acid Nutritional Deficiency in Non-Alcoholic Fatty Liver Disease (CONDIN). Nutrients 2018, 10, 1126. [Google Scholar] [CrossRef]
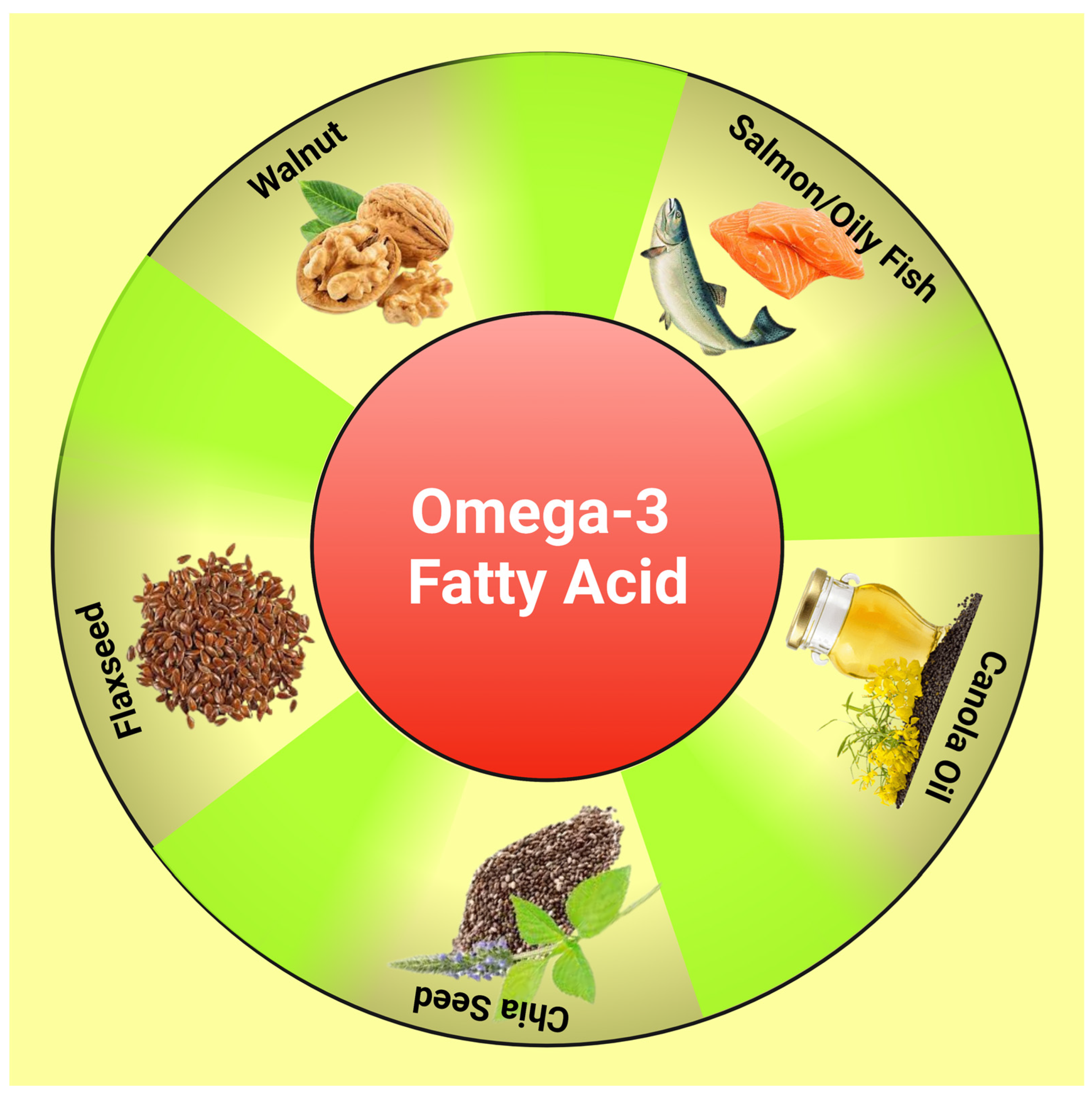


| Authors Name | Journal Details | Study Details (Study Design/Study Subjects/Dose and Duration/Effect) | Background | Result | Conclusion |
|---|---|---|---|---|---|
| Nidia et al. [69] | J Periodontol. 2020;91(10):1318–1327. | Placebo-controlled, double-blind, randomized clinical study/human subjects/test group (TG)1: 3 PUFA + aspirin (3 g of fish oil per day + 100 mg of aspirin per day for two months), or test group (TG)2: 3 PUFA + aspirin (3 g of fish oil per day + 100 mg of aspirin per day for two months) prior to periodontal exfoliation/both test groups’ IFN- and IL-8 levels dropped with time. For test group 1, IL-6 levels were lower, and HbA1c values dropped. | Low-dose aspirin (ASA) and omega-3 polyunsaturated fatty acid (-3 PUFA) supplements have been suggested as a host modulation strategy to manage chronic inflammatory disorders. | Clinical attachment gain for TG1 occurred in moderate and deep pockets. IFN- and interleukin (IL)-8 levels dropped with time for both test groups, and IFN- and interleukin (IL)-8 levels dropped with time. | Patients with type 2 diabetes who receive adjunctive -3 and ASA following periodontal debridement benefit clinically and immunologically from the medical management of periodontitis. |
| Djoussé et al. [70] | JACC Heart Fail. 2022;10(4):227–234. | Randomized, double-blind, placebo-controlled trial/human subjects/1 g of fish omega-3 fatty acids per day and 2000 IU of vitamin D3 (cholecalciferol) every day from 2011 to 2017/omega-3 supplementation reduced recurrent HF hospitalization exclusively in Black subjects. | It is uncertain if race and T2DM affect how often people have heart failure (HF) after taking omega-3 supplements. | The HR for the first HF hospitalization when omega-3 supplements were compared to placebo was 0.69 (95% CI: 0.50–0.95) in patients with prevalent T2D and 1.09 (95% CI: 0.88–1.34) in participants without T2D (p for interaction = 0.019). | Omega-3 fatty acid supplements had a beneficial impact on the frequency of HF hospitalization in individuals with T2DM but not in those without T2DM. These advantages seemed to be more pronounced in Black participants with T2DM. |
| ASCEND Study Collaborative Group; Bowman et al. [71] | N Engl J Med. 2018;379(16):1540–1550. | Randomized, double-blind, placebo-controlled trial/human subjects/1 g capsules containing either omega-3 fatty acids or a matching placebo (olive oil) daily for 4.4 years/there were no discernible variations in the frequency of major nonfatal complications comparing groups. | Increased intake of n-3 fatty acids has been associated with a reduced risk of cardiovascular disease in observational studies, but this finding has not been confirmed in randomized trials. It remains unclear whether omega-3 fatty acid supplementation has cardiovascular benefits in patients with diabetes mellitus | A major vascular incident occurred in 689 patients (8.9%) in the fatty acid group during a mean follow-up of 7.4 years (adherence rate, 76%) and in 712 patients (9.2%) in the placebo group (rate ratio, 0.97; 95% confidence interval (CI), 0.87 to 1.08; p = 0.55). | There was no discernible difference in the incidence of major vascular events among diabetic individuals without signs of cardiovascular disease between those who were given n-3 fatty acid supplements and those who received a placebo. |
| Raygan et al. [72] | Phytother Res. 2019;33(7):1943–1951. | Randomized control trial/human subjects/for a period of 12 weeks, 3 intervention groups were given either 1000 mg of omega-3 fatty acids from flaxseed oil, 1000 mg of omega-3 fatty acids from fish oil, or a placebo/comparable to fish oil; flaxseed oil reduced insulin while enhancing total nitrite and total antioxidant properties. | In diabetic patients with coronary heart disease, this study examined the effects of flaxseed and fish oil supplementation on cardiovascular risk factors. | Compared to the placebo, flaxseed and fish oil supplementation significantly decreased insulin levels (0.04). | This study indicated that supplementing with fish and flaxseed oils had advantageous effects on specific metabolic profiles. According to this research, flaxseed oil has a similar impact to fish oil in terms of lowering insulin and raising total nitrite and antioxidant capacity. |
| Dakin et al. [73] | 2020;23(10):1340–1348 | Randomized control trial/human subjects/individually randomized to receive 20 mg/day atorvastatin or omega-3 EE90 or a matching placebo observed over the first 16 weeks of the 1-year trial/atorvastatin would be more cost-effective than omega-3 fatty acid. | More elaborate factorial designs and the necessity for extrapolating results after the trial’s end complicates the analytical approaches. From the viewpoint of the UK National Health Service, this study evaluated the cost-effectiveness of atorvastatin, omega-3 fish oil, and an action-planning leaflet, both individually and collectively. | The results were unaffected by various methods of factorial design analysis. At GBP 20,000/QALY, there was a 99% likelihood that atorvastatin would be more cost-effective than omega-3 fatty acid. | Atorvastatin monotherapy was the most economical option when comparing the three study therapies on a GBP 20,000/QALY basis. Omega-3 fish oil was not cost-effective, and there was insufficient information to make definite statements on action planning. |
| Liu et al. [74] | Am J Clin Nutr. 2018;108(2):256–265. | Randomized controlled trial/human subjects/low-protein diet with low ω-3 PUFAs (control), a low-carbohydrat–high-protein (LCHP) diet, ω-3, or LCHP+ω-3 diet for 12 wk/greater reductions in fasting glucose levels and glycated hemoglobin (HbA1c) were seen. | It is unknown how people with type 2 diabetes (T2D) would respond to a low-carb, high-protein (LCHP) diet paired with omega-3 (n-3) polyunsaturated fatty acid (PUFA) supplementation. | Compared with the control diet group, more decreases in glycated hemoglobin (HbA1c) and fasting glucose were detected in all the other 3 diet groups at 12 weeks. | There may be a need to combine an LCHP diet with omega-3-3 PUFAs in managing T2D because the LCHP+omega-3 diet had more substantial effects on HbA1c and fasting glucose and faster effects on fasting glucose than both the LCHP and omega-3 diets. |
| Jamilian et al. [75] | Br J Nutr. 2020;123(7):792–799. | Randomized controlled trial/60 women with GDM/consumption of either 2 × 1000 mg/d omega-3 fatty acids from flaxseed oil containing 400 mg α-linolenic acid in each capsule (n = 30) or placebo (n = 30) for 6 weeks/omega-3 fatty acid supplementation for 6 weeks demonstrated positive benefits on gene expression linked to insulin, lipids, glycemic management, inflammatory markers, and oxidative stress in women with GDM. | Researchers looked at how n-3 fatty acids from flaxseed oil affected patients with genetic and metabolic gestational diabetes mellitus (GDM) characteristics. | In peripheral blood mononuclear cells from people with GDM, n-3 fatty acid intake increased PPAR- gamma (p < 0.001) and LDL receptor (p = 0.004) and decreased the gene expression of IL-1 (p = 0.002) and TNF-alpha (p = 0.001). Additionally, as compared to the placebo, n-3 fatty acid supplementation decreased fasting plasma glucose (p = 0.001), insulin levels (p = 0.001), insulin resistance (p = 0.001), and enhanced insulin sensitivity (p = 0.005). | Omega-3 fatty acid supplementation demonstrated positive benefits on gene expression linked to insulin, lipid and inflammation, glycemic control, lipids, inflammatory markers, and oxidative stress in women with GDM for six weeks. |
| Britten-Jones et al. [76] | Diabetes. 2021;70(8):1794–1806. | Randomized, double-musked, placebo-controlled trial/human subjects with type 1 DM/omega-3 (1800 mg/day fish oil) or placebo (600 mg/day olive oil) supplements for 180 days/in type 1 diabetes, long-chain omega-3 supplements promoted corneal neuro-regeneration. | Omega-3 (n-3) fatty acid oral supplementation’s effects on peripheral nerves in type 1 diabetes were examined in this placebo-controlled experiment. | Compared to the placebo, the Omega-3 Index increased by 3.3% (95% CI: 2.4, 4.2). Most functional properties of tiny and large nerve fibers were similar. | This randomized controlled research discovered that long-chain n-3 supplements cause neurodegenerative changes in the corneas of people with type 1 diabetes, suggesting a potential function in regulating the health of peripheral nerves. |
| O’Mahoney et al. [77] | Cardiovasc Diabetol. 2020;19(1):127. | Randomized controlled trial/human subjects with type 1 DM/3.3 g/day of encapsulated omega-3 PUFA or encapsulated 3.0 g/day corn oil placebo for 6 months, with follow-up at 9 months after 3 months washout/in subjects with T1D, daily high-dose bolus omega-3 PUFA supplementation did not improve glucose homeostasis, vascular health, or metabolic markers. | The significance of omega-3 polyunsaturated fatty acids and the possible effects of omega-3 PUFA supplementation in managing and treating type 1 diabetes (T1D) is still unclear and debatable. | Overall acquaintance with omega-3PUFA over the 6 months was 14.27 ± 3.05% per month under omega-3 PUFA and 9.11 ± 2.74% per month below PLA (p < 0.001). VCAM-1, ICAM-1, E-selectin, P-selectin, pentraxin-3, VEGF, TNFα, CIMT, FMD, blood pressure, HbA1c, FPG, and postprandial metabolism did not vary between or within groups after treatment (p > 0.05). | According to this study, supplementing with omega-3 PUFAs at a high dose each day for six months did not improve vasculature condition, glucose homeostasis, or metabolic parameters in Type 1 diabetes patients. |
| Naeini et al. [78] | Nutr Metab Cardiovasc Dis. 2020;30(3):441–447. | Randomized controlled trial/human subjects with T2DM/2400 mg/d DHA-rich fish oil or placebo for 8 weeks/short-term administration of docosahexaenoic acid (DHA) rich fish oil may modify PPAR activity in peripheral blood mononuclear cells. | Omega-3 polyunsaturated fatty acids (PUFAs) are peroxisome proliferator-activated receptor gamma (PPAR-γ) ligands. | Raised peroxisome proliferator-activated receptor gamma (PPAR-gamma) activity in peripheral blood mononuclear cells (PBMCs) (p = 0.01) compared to placebo (p = 0.4), while mRNA expression levels of the p53 and nuclear factor kappa-B (NFk-B) genes did not demonstrate significant variations between the study groups. | Short-term DHA-rich fish oil supplementation may modulate PPAR-γ activity in PBMCs. |
| Liu et al. [79] | Lipids Health Dis. 2022;21(1):20. | Randomized double-blinded trial/human subjects with T2DM/administration of fish oil at a dose of 3 g/day containing eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), perilla oil at a dose of 3 g/day containing α-linolenic (ALA), linseed, and fish oil with mixed linseed and fish oil at a dose of 3 g/day containing EPA, DHA, and ALA for six months/perilla oil administration reduced fasting blood glucose levels while fish oil supplement reduced TG levels. | The composition of erythrocyte fatty acids is influenced by dietary fatty acid consumption, and abnormalities of glycolipid metabolism are highly associated with this. | There were significant effects of treatment–time interaction on fasting blood glucose (FBG), insulin, HOMA-IR and C-peptide, total cholesterol, and triglycerides (TG), and there were significant effects of time–treatment interaction (p = 0.001). Following the intervention, serum TG in the fish oil group dramatically dropped (p = 0.001), as did glucose and C-peptide in the perilla oil and linseed and fish oil groups. | Perilla oil supplementation reduced fasting blood glucose, while fish oil supplementation reduced TG. Omega-3 PUFAs from plants and animals have diverse impacts on the erythrocytes’-controlled glycolipid metabolism and fatty acid compositions. |
| Burhop et al. [80] | Nutrients. 2022;14(2):396. | Randomized controlled trial/43 obese human subjects/the calanus oil group received LC n-3 FAs-rich oil extracted from C. finmarchicus, while the control group was supplemented with placebo capsules (filled with paraffin oil)/in obese people, calanus oil improves insulin resistance and glucose metabolism. | Calanus finmarchicus oil is a source of long-chain omega-3 PUFAs that have shown encouraging results on glucose homeostasis in preclinical investigations because of its anti-obesity and/or anti-inflammatory qualities. | Fasting insulin, HOMA-IR, and the hepatic insulin resistance index significantly improved after a 12-week CO intervention. At the same time, there were no differences in HbA1c, AUC0–2 h glucose, AUC0–2 h insulin, 2 h plasma glucose, or muscle insulin sensitivity index. | In obese people, calanus oil improves insulin resistance and glucose metabolism. |
| Abbott et al. [81] | Prostaglandins Leukot Essent Fatty Acids. 2020; 159:102154. | Randomized controlled trial/men and women with abdominal obesity without diabetes/2 g fish oil/day (860 mg DHA + 120 mg EPA) (intervention, n = 38) or 2 g corn oil (CO)/day (control, n = 35) for 12 weeks/higher insulin and HOMA-IR at baseline were linked to significant decreases in the fish oil group. | The inflammation of adipose tissue primarily causes insulin resistance (IR). Docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), and other long-chain omega-3 polyunsaturated fatty acids (LCn-3PUFA) are anti-inflammatory bioactive lipids that may delay the onset of type 2 diabetes (T2D). | Fish oil substantially decreased HOMA-IR by −0.40 units and fasting insulin by −1.62 IU/L when compared to corn oil. At baseline, higher levels of insulin and HOMA-IR were linked to more considerable decreases in the fish oil group. There was no connection between sex and the medication that caused insulin levels to change. | In individuals with abdominal obesity, DHA-enriched fish oil decreases IR; sex-dependent variations were not observed in this investigation. |
| Zheng et al. [82] | EBioMedicine. 2018; 31:150–156. | Randomized controlled trial/150 humans with T2DM/for 180 days, participants in each group received 4 capsules each day, which equaled 2 g of C20:5n-3 and C22:6n-3 in the case of fish oil and 2.5 g of C18:3n-3 in the case of flaxseed oil. Corn oil served as the control oil/omega-3 supplementation has an impact on blood lipid profiles differently in T2D patients with various genotypes at CD36, NOS3, and PPARG. | How genetic variations affect omega-3 fatty acid supplements affect blood lipids is unknown. | The lipid profiles of T2D patients with the CD36-G, PPARG-G, and NOS3-A alleles tended to improve more quickly after treatment with omega-3 fatty acids. The omega-3 fatty acid group’s interaction outcomes were primarily related to fish oil supplementation. | According to this study, omega-3 supplements had a distinct effect on blood lipid profiles in T2D patients with diverse genotypes at CD36, NOS3, and PPARG. |
| Thota et al. [83] | Sci Rep. 2018;8(1):13679. | Randomized, placebo-controlled, and crossover study/human subject/on four test days separated by a week, participants took a placebo, 180 mg of curcumin in the form of tablets, 1.2 g of long-chain omega-3 PUFA in the form of capsules, and 180 mg of fish oil plus curcumin/curcumin reduces postprandial glycemic response and insulin demand for glucose control. | In preclinical investigations, curcumin, a dietary bio-active produced from turmeric and fish oil, reduced fasting blood sugar and insulin resistance. | The curcumin and curcumin + fish oil groups had significantly lower postprandial glucose concentrations. | The postprandial glycemic response and insulin demand for glucose control are decreased by curcumin but not by fish oil. |
| Makrides et al. [84] | N Engl J Med. 2019;381(11):1035–1045. | Randomized controlled trial/human pregnant women/fish oil capsules contained 900 mg of omega-3 PUFA or vegetable oil capsules contained trace omega-3 PUFA (control group) daily, beginning before 20 weeks of gestation and continuing to 34 weeks of gestation or delivery/the omega-3 group reported more minor gastrointestinal issues than the control group did. | N-3 long-chain polyunsaturated fatty acid supplementation during pregnancy may decrease the likelihood of preterm birth and cause the pregnancy to last beyond term. | In the n-3 group, early premature delivery occurred in 61 of 2734 pregnancies and 55 of 2752 pregnancies. | N-3 long-chain polyunsaturated fatty acid supplementation from the beginning of pregnancy to 34 weeks of gestation did not lead to a greater incidence of interventions in post-term deliveries or a lower incidence of early preterm deliveries than control. |
| Wang et al. [85] | Zhonghua Yu Fang Yi Xue Za Zhi. 2019;53(6):570–575. | Randomized controlled trial/human subjects/3 g/day fish oil (FO), perilla oil (PO), or fish oil mixed with linseed oil (FLO) for 6 months/omega-3 PUFA from various sources had similar impacts on glucose metabolism in type 2 diabetics with dyslipidemia. | Impact of different sources of omega-3 polyunsaturated fatty acids on the glucolipid metabolism in patients with dyslipidemia who have type 2 diabetes. | After 6 months, there were no appreciable differences in serum glucose levels, glycated hemoglobin, C-peptide, insulin, or homeostasis model assessment-insulin resistance between the fish oil, perilla oil, or fish oil combined with linseed oil groups. | Omega-3 PUFA from various sources had comparable effects on glucose metabolism in type 2 diabetics with dyslipidemia. Each of these has a promising future for use in enhancing lipid metabolism. |
| Talari et al. [86] | Br J Nutr. 2019;122(4):423–430. | Randomized controlled trial/vitamin D deficient diabetic human subjects/50,000 IU vitamin D supplements every 2 weeks plus 2× 1000 mg/d n-3 fatty acids from flaxseed oil (n = 30) or placebo (n = 31) for 6 months/co-supplementation of vitamin D and omega-3 fatty acids improved markers of cardiometabolic risk. | This study looked at the impact of co-supplementing with vitamin D and n-3 fatty acids on cardiometabolic risk markers in diabetic patients with CHD. | Compared to the placebo, the co-supplementation of vitamin D and n-3 fatty acids significantly decreased the mean and maximum levels of left carotid intima-media thickness (CIMT) and the mean and maximum levels of right CIMT. | The co-supplementation of n-3 fatty acids and vitamin D reduced cardiometabolic risk markers. |
| Wasserfurth et al. [87] | Nutrients. 2020;12(7):2139. | Randomized controlled trial/healthy human subjects/there were four study groups: (1) a control group receiving no intervention; (2) aerobic and resistance training only for two weeks; (3) exercise combined with dietary advice following the recommendations of the German Nutrition Society; and (4) exercise combined with the consumption of 2 g of Calanus finmarchicus oil daily/in senior untrained overweight people, a combination of moderate activity, consumption of Calanus finmarchicus oil, and a good diet may improve fat loss. | Harmful changes brought on by aging, such as the emergence of diseases such as type-2 diabetes mellitus or persistent low-grade inflammation, include a steady loss of muscle mass and an increase in fat mass. | The exercise regimen combined with an intake of 2 g/day of oil from the Calanus finmarchicus group and the exercise regimen combined with dietary counseling following the recommendations of the German Nutrition Society group showed the most significant reductions in body fat. Blood lipids and glucose metabolism indicators remained constant across all groups. | In untrained, overweight seniors, moderate activity; consumption of Calanus finmarchicus oil; or a good diet may improve fat loss. |
| Ruan et al. [88] | Food Funct. 2019;10(5):2471–2479. | Randomized controlled trial/human subjects with T2DM/fish oil (FO, 4 capsules per day, 50% of EPA + DHA), flaxseed oil (FSO, 4 capsules per day, 63% of ALA), and corn oil (CO, 4 capsules per day, serving as a control) for 180 days/as a potent biomarker of fish oil, 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid suggested that marine omega-3 PUFA intake may have a positive impact on lipid metabolism and renal function in T2D patients. | The impact of n-3 fatty acid supplements on urine metabolite profile and how they relate to metabolic risk factors in individuals with type 2 diabetes in China. | Compared to the corn oil group, levels of 2-hexenoylcarnitine and 3-carboxy-4-methyl-5-propyl-2-furan propanoic acid (CMPF) were significantly higher in the fish oil group, whereas those of hydroxyisovaleroyl carnitine were much lower. | Marine omega-3 PUFA intake may positively impact lipid metabolism and kidney health in T2D individuals. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinha, S.; Haque, M.; Lugova, H.; Kumar, S. The Effect of Omega-3 Fatty Acids on Insulin Resistance. Life 2023, 13, 1322. https://doi.org/10.3390/life13061322
Sinha S, Haque M, Lugova H, Kumar S. The Effect of Omega-3 Fatty Acids on Insulin Resistance. Life. 2023; 13(6):1322. https://doi.org/10.3390/life13061322
Chicago/Turabian StyleSinha, Susmita, Mainul Haque, Halyna Lugova, and Santosh Kumar. 2023. "The Effect of Omega-3 Fatty Acids on Insulin Resistance" Life 13, no. 6: 1322. https://doi.org/10.3390/life13061322
APA StyleSinha, S., Haque, M., Lugova, H., & Kumar, S. (2023). The Effect of Omega-3 Fatty Acids on Insulin Resistance. Life, 13(6), 1322. https://doi.org/10.3390/life13061322






