Abstract
Policosanol consumption has been associated with treating blood pressure and dyslipidemia by increasing the level of high-density lipoproteins-cholesterol (HDL-C) and HDL functionality. Although policosanol supplementation also ameliorated liver function in animal models, it has not been reported in a human clinical study, particularly with a 20 mg doage of policosanol. In the current study, twelve-week consumption of Cuban policosanol (Raydel®) significantly enhanced the hepatic functions, showing remarkable decreases in hepatic enzymes, blood urea nitrogen, and glycated hemoglobin. From the human trial with Japanese participants, the policosanol group (n = 26, male 13/female 13) showed a remarkable decrease in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) from baseline up to 21% (p = 0.041) and 8.7% (p = 0.017), respectively. In contrast, the placebo group (n = 26, male 13/female 13) showed almost no change or slight elevation. The policosanol group showed a 16% decrease in γ-glutamyl transferase (γ-GTP) at week 12 from the baseline (p = 0.015), while the placebo group showed a 1.2% increase. The policosanol group exhibited significantly lower serum alkaline phosphatase (ALP) levels at week 8 (p = 0.012), week 12 (p = 0.012), and after 4-weeks (p = 0.006) compared to those of the placebo group. After 12 weeks of policosanol consumption, the ferric ion reduction ability and paraoxonase of serum were elevated by 37% (p < 0.001) and 29% (p = 0.004) higher than week 0, while placebo consumption showed no notable changes. Interestingly, glycated hemoglobin (HbA1c) in serum was lowered significantly in the policosanol group 4 weeks after consumption, which was approximately 2.1% (p = 0.004) lower than the placebo group. In addition, blood urea nitrogen (BUN) and uric acid levels were significantly lower in the policosanol group after 4 weeks: 14% lower (p = 0.002) and 4% lower (p = 0.048) than those of the placebo group, respectively. Repeated measures of ANOVA showed that the policosanol group had remarkable decreases in AST (p = 0.041), ALT (p = 0.008), γ-GTP (p = 0.016), ALP (p = 0.003), HbA1c (p = 0.010), BUN (p = 0.030), and SBP (p = 0.011) from the changes in the placebo group in point of time and group interaction. In conclusion, 12 weeks of 20 mg consumption of policosanol significantly enhanced hepatic protection by lowering the serum AST, ALT, ALP, and γ-GTP via a decrease in glycated hemoglobin, uric acid, and BUN with an elevation of serum antioxidant abilities. These results suggest that improvements in blood pressure by consumption of 20 mg of policosanol (Raydel®) were accompanied by protection of liver function and enhanced kidney function.
1. Introduction
Liver damage is associated with many diseases with metabolic disorders or acute and chronic infections, which can be linked with life-threatening events [1,2]. Although the warning signs of liver diseases are jaundice, vomiting blood, and abdomen swelling, but most liver diseases often show no symptoms until they have progressed to significant damage [3]. Many blood biomarkers, such as liver aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (γ-GTP), and alkaline phosphatase (ALP) have been used clinically [4,5] to monitor liver function for regular check-ups. Many liver diseases are caused by viral infections, inherited genetic factors, obesity, exposure to xenobiotics, and misuse of alcohol [6]. On the other hand, except for infections and genetic factors, liver damage, including fatty liver changes, is intimately associated with lifestyle, such as lack of exercise [7], alcohol consumption [8], and frequent use of drugs [9]. In particular, underlying diseases, such as diabetes, hypertension, and coronary heart disease, are risk factors for liver damage in middle-aged populations [10,11].
Many functional foods, such as milk thistle, ginseng, licorice, and turmeric, have been developed and marketed to protect against liver damage and enhance hepatic functions, such as lowering the serum AST and ALT levels [12]. In the Republic of Korea, 13 kinds of herbal and fermented extracts have been registered in the Ministry of Food and Drug Safety (MFDS) for hepatic health, https://www.foodsafetykorea.go.kr/portal/healthyfoodlife/functionalityView.do?viewNo=13 (accessed on 22 February 2023). On the other hand, their recommended dosage is too high, approximately 300 mg (Pinitol)–3150 mg (Rubus coreanus extract powder) per day, suggesting low efficacy. The common concerns of herbal medicine or folk medicine were associated with causing hepatotoxicity due to the hidden or unidentified ingredients and adulterants in the extracts, as summarized previously [13,14]. Therefore, to avoid herb-induced liver injury (HILI) and drug-induced liver injury (DILI), it is necessary to develop new agents to improve the hepatic functions without liver and kidney toxicity.
Because liver damage is closely associated with the progression of metabolic syndrome [15], including hypertension, diabetes, and dyslipidemia, improvement of the diabetic parameters and kidney functions should be accompanied by improvements in hepatic functions. Many studies to develop nutraceuticals to protect against liver damage could not focus only on lowering the AST, ALT, and γ-GTP levels without comparing the glycated hemoglobin and kidney function parameters. In a Chinese and Japanese study, dyslipidemia and nephrolithiasis were correlated to have a co-incidental risk factor of being overweight, hypertension [16], and glycated hemoglobin [17].
Cuban policosanol (Raydel®) consumption (10–20 mg/day) for 8–12 weeks was associated with the treatment of prehypertension [18,19] and dyslipidemia [20] via raising HDL-C and enhancing HDL functionality in randomized human studies in healthy Korean participants. Long-term clinical studies for 24 weeks also showed that policosanol consumption exerted an anti-glycation activity and antioxidant activity to protect HDL and LDL [21], which made a good agreement with the protection of apoA-I and apo-B from proteolytic degradation from in vitro studies [22,23]. Furthermore, in vitro and in vivo studies showed that reconstituted HDL containing Cuban policosanol exerted potent antioxidant, anti-glycation, and anti-inflammatory activities [18,19,20,21,22,23] with cholesterol efflux ability [19]. Recently, the reconstituted HDL-containing Cuban policosanol displayed a larger particle size with potent anti-glycation activity to protect apoA-I and antioxidant activity to protect LDL, while three reconstituted HDLs, each containing Chinese policosanol, did not [24]. These in vitro potentials of policosanol to enhance the HDL functionality are linked with the in vivo efficacy in a human clinical study to improve blood pressure and dyslipidemia in healthy Korean and Japanese participants [18,19,20,21,25].
A recent clinical study with healthy middle-aged Japanese participants showed that 12 weeks of policosanol (Raydel®) consumption significantly improved blood pressure, hepatic parameters, BUN, and HbA1c with enhanced HDL functionalities [25]. These results suggest that the efficacy of policosanol consumption in hypertension and dyslipidemia might be associated with the protection of liver function by improving HDL functionalities and kidney function parameters.
A desirable therapeutic agent should simultaneously protect against liver and kidney damage by improving fatty liver disease and hypertension without adverse effects. Based on the previous study, this study analyzed the effects of policosanol consumption on hepatic parameters, such as AST, ALT, γ-GTP, and ALP, as well as kidney parameters, HbA1c, uric acid, and blood urea nitrogen during 16 weeks, including 12 weeks of consumption and four weeks post-consumption.
2. Materials and Methods
2.1. Policosanol
Raydel® policosanol tablet (10 mg/tablet, two tablets for total 20 mg per day), which was manufactured with Cuban policosanol at Raydel Australia (Thornleigh, Sydney, Australia), was obtained from Raydel Japan (Tokyo, Japan). Cuban policosanol was defined as genuine policosanol with a specific ratio of each ingredient [26]: 1-tetracosanol (C24H49OH, 0.1–20 mg/g); 1-hexacosanol (C26H53OH, 30.0–100.0 mg/g); 1-heptacosanol (C27H55OH, 1.0–30.0 mg/g); 1-octacosanol (C28H57OH, 600.0–700.0 mg/g); 1-nonacosanol (C29H59OH, 1.0–20.0 mg/g); 1-triacontanol (C30H61OH, 100.0–150.0); 1-dotriacontanol (C32H65OH, 50.0–100.0 mg/g); and 1-tetratriacontanol (C34H69OH, 1.0–50.0 mg/g).
2.2. Participants, Study Design, and Analysis
Healthy male and female volunteers with normal lipid levels and normal blood pressure were recruited nationwide in Japan via newspaper and internet advertisements between September 2021 and May 2022, as described in the preceding paper [25]. The inclusion criteria were LDL-C levels in the normal range (120–159 mg/dL) and age between 20 and 65 years old. The exclusion criteria were as follows: (1) maintenance treatment for metabolic disorder, including dyslipidemia, hypertension, and diabetes; (2) severe hepatic, renal, cardiac, respiratory, endocrinological, and metabolic disorder disease; (3) allergies; (4) heavy drinkers (more than 30 g of alcohol per day); (5) taking medicine or functional food products that may affect the lipid metabolism, including raising HDL-C or lowering LDL-C concentration, and lowering triglyceride concentration; (6) current or past smoker; (7) women in pregnancy, lactation, or planning to become pregnant during the study period; (8) person who had more than 200 mL of blood donation within one month or 400 mL of blood within three months before starting this clinical trial; (9) a person who participated in another clinical trial within the last three months or currently is participating in another clinical trial; (10) those who consumed more 2000 kcal per day; and (11) others considered unsuitable for the study at the discretion of the principal investigator. The study was approved by the Koseikai Fukuda Internal Medicine Clinic (Osaka, Japan, IRB approval number 15000074, approval date on 18 September 2021).
As shown in Figure 1A, this study was a double-blinded, randomized, and placebo-controlled trial with a 12-week treatment period. The selected participants were healthy male and female volunteers (n = 52, average age 52.1 ± 1.3 years old) with a sedentary lifestyle and without hypertension or any complaint of endocrinological disorder. All participants had unremarkable medical records without illicit drug use or a past history of chronic diseases. All participants received advice to avoid excess food (1800 kcal and 1500 kcal for men and women, respectively, per day), cholesterol (600 mg per day), alcohol drinking (<30 g and <15 g of ethanol for men and women, respectively, per day), and smoking, both direct and indirect, which can interfere with liver and kidney metabolism.
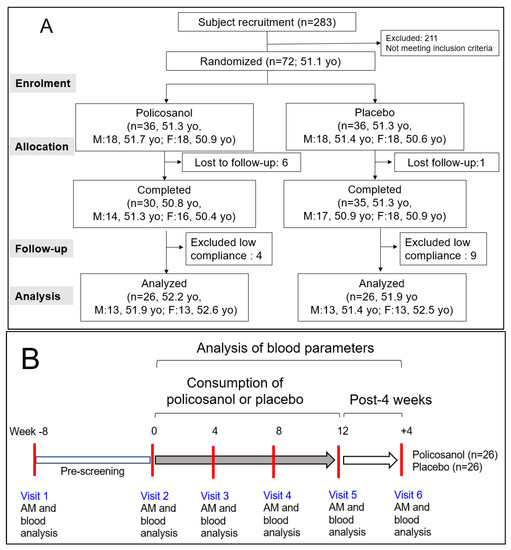
Figure 1.
Study design and participant allocation for analysis (A) and visiting schedule of participants (B). AM, anthropometric measurements; yo, years old.
After allocating the participants into two groups, they were directed to take two tablets per day containing policosanol 10 mg/tablet (Raydel®) or a placebo. The tablet for the policosanol group included policosanol (10 mg/tablet), hydroxypropyl cellulose, carboxymethyl cellulose, maltodextrin, lactose, and crystalline cellulose. The tablet for the placebo group contained maltodextrin (10 mg/tablet) instead of policosanol. The blood parameters of all participants who completed the program were analyzed after 12 weeks of consumption and four weeks post-consumption (Figure 1B).
2.3. Anthropometric Analysis and Blood Analysis
The blood pressure was measured using an Omron HEM-907 (Kyoto, Japan) with three measurements with the average recorded. The height, bodyweight, and body mass index (BMI) were measured individually using a DST-210N (Muratec KDS Co., Ltd., Kyoto, Japan).
After fasting overnight, blood samples were collected in ethylenediaminetetraacetic acid (EDTA)-coated tubes and centrifuged at 3000× g for 15 min at 4 °C for the plasma assays. The samples were subjected to 19 blood biochemical assays by BML Inc. (Tokyo, Japan): total protein, albumin, aspartate transferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (γ-GTP), creatinine, glucose, uric acid, blood urea nitrogen (BUN), lactate dehydrogenase (LDH), total bilirubin, glycated hemoglobin (hemoglobin A1c, HbA1c), and high sensitivity C-reactive protein (hsCRP). The protocol of human blood donation was conducted according to the guidelines of the Declaration of Helsinki and approved by the Koseikai Fukuda Internal Medicine Clinic (Osaka, Japan), with the IRB approval number 15000074.
2.4. Ferric Ion Reducing Ability Assay
The ferric ion-reducing ability (FRA) was determined using the method reported by Benzie and Strain [27]. Briefly, the FRA reagents were prepared freshly by mixing 20 mL of 0.2 M acetate buffer (pH 3.6), 2.5 mL of 10 mM 2,4,6-tripyridyl-S-triazine (Fluka Chemicals, Buchs, Switzerland), and 2.5 mL of 20 mM FeCl3∙6H2O. The antioxidant activities of serum were estimated by measuring the increase in absorbance induced by the ferrous ions generated. Freshly prepared FRA reagent (300 μL) was mixed with serum as an antioxidant source. The FRA was determined by measuring the absorbance at 593 nm every two min over a 60 min period at 25 °C using a UV-2600i spectrophotometer.
2.5. Paraoxonase Assay
The paraoxonase-1 (PON-1) activity in serum toward paraoxon was determined by evaluating the hydrolysis of paraoxon into p-nitrophenol and diethylphosphate, which was catalyzed by the enzyme [28]. The PON-1 activity was determined by measuring the initial velocity of p-nitrophenol production at 37 °C, as determined by the absorbance at 415 nm (microplate reader, Bio-Rad model 680; Bio-Rad, Hercules, CA, USA).
2.6. Data Analysis
All analyses in the Tables were normalized using a homogeneity test of the variances through Levene’s statistics. Nonparametric statistics were performed using a Kruskal–Wallis test if not normalized. For Table 1, repeated measure ANOVA was used to compare the score changes in the hepatic parameters, renal parameters, and SBP between the two groups during the same period. The differences in the placebo or policosanol groups over the follow-up time were analyzed to compare in point of time and group interaction. Significant changes between the baseline and follow-up values within the groups were assessed using a paired t-test.
For Supplemental Tables S1–S5, comparisons between the policosanol and placebo with respect to BP, anthropological assessments, hematological data in blood, protein data in serum, and inflammatory assessments were analyzed using an analysis of covariance (ANCOVA) with the independent variable as the baseline and treatment. As a post hoc analysis, the Bonferroni test was used to determine the significance of the differences in the continuous variables to identify the differences between the two groups. Spearman rank correlation analysis was carried out to find a positive or negative association. The statistical power was estimated using the program G*Power 3.1.9.7 (G*Power from the University of Düsseldorf, Düsseldorf, Germany). All tests were two-tailed, and the statistical significance was p < 0.05. The data were analyzed using the SPSS software version 29.0 (IBM, Chicago, IL, USA).
3. Results
3.1. Improvements in Hepatic Functions and Kideny Functions in the Policosanol Group
At baseline (week 0), all subjects showed a normal range of each parameter in blood and serum data without any difference between the policosanol group and the placebo group, as summarized in the Supplemental Table S1. However, after 12 weeks of consumption, the policosanol group showed significantly lower AST, ALT, γ-GTP, and ALP than those of the placebo group in a time-dependent manner, particularly at week 12 and after 4 weeks, as shown in Table 1.

Table 1.
Repeated measures ANOVA of blood parameters with hepatic functions, biliary systems, and kidney functions between placebo group and policosanol (PCO) group during 16 weeks ¶.
Table 1.
Repeated measures ANOVA of blood parameters with hepatic functions, biliary systems, and kidney functions between placebo group and policosanol (PCO) group during 16 weeks ¶.
| Variables | Groups | Week 0 | Week 4 | Week 8 | Week 12 | Post 4 Weeks | Sources | F | p ‡ |
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |||||
| AST | placebo | 20.1 ± 1.0 | 20.4 ± 1.1 | 20.3 ± 1.2 | 20.9 ± 1.2 | 22.3 ± 1.5 | Time × Group | 2.715 | 0.041 |
| PCO 20 mg | 20.8 ± 1.6 | 19.8 ± 0.8 | 19.1 ± 0.7 | 18.6 ± 0.8 | 18.6 ± 0.8 * | ||||
| p † | 0.724 | 0.312 | 0.196 | 0.017 | 0.008 | ||||
| ALT | placebo | 18.2 ± 1.7 | 19.1 ± 1.9 | 19.7 ± 2.5 | 18.8 ± 1.8 | 20.4 ± 2.1 | Time × Group | 3.954 | 0.008 |
| PCO 20 mg | 21.5 ± 3.3 | 18.7 ± 1.2 | 17.4 ± 0.9 | 17.1 ± 1.6 | 15.8 ± 1.0 | ||||
| p † | 0.367 | 0.176 | 0.124 | 0.041 | 0.001 | ||||
| ALT/AST (ratio) | placebo | 0.88 ± 0.06 | 0.92 ± 0.07 | 0.91 ± 0.06 | 0.88 ± 0.06 | 0.90 ± 0.06 | Time × Group | 3.404 | 0.010 |
| PCO 20 mg | 0.98 ± 0.06 | 0.94 ± 0.04 | 0.91 ± 0.04 | 0.89 ± 0.05 | 0.84 ± 0.04 | ||||
| p † | 0.258 | 0.256 | 0.112 | 0.145 | 0.001 | ||||
| γ-GTP | placebo | 26.2 ± 3.6 | 28 ± 3.9 | 26.9 ± 4.1 | 27.8 ± 4.3 | 30.0 ± 5.2 | Time × Group | 3.312 | 0.016 |
| PCO 20 mg | 28.7 ± 4.3 | 27.4 ± 3.3 | 24.2 ± 2.7 | 24.1 ± 2.7 | 23.7 ± 2.5 | ||||
| p † | 0.666 | 0.128 | 0.039 | 0.015 | 0.011 | ||||
| ALP | placebo | 64.8 ± 3.9 | 66.0 ± 3.5 | 66.7 ± 4.2 | 66.1 ± 3.8 | 67.0 ± 3.8 | Time × Group | 4.240 | 0.003 |
| PCO 20 mg | 69.0 ± 2.6 | 69.3 ± 2.3 | 66.6 ± 2.3 | 65.9 ± 2.5 | 65.7 ± 2.1 | ||||
| p † | 0.414 | 0.958 | 0.012 | 0.012 | 0.006 | ||||
| HbA1c | placebo | 5.48 ± 0.05 | 5.48 ± 0.06 | 5.35 ± 0.05 | 5.45 ± 0.07 | 5.46 ± 0.06 | Time × Group | 7.129 | 0.010 |
| PCO 20 mg | 5.46 ± 0.05 | 5.42 ± 0.05 | 5.30 ± 0.04 | 5.42 ± 0.05 | 5.34 ± 0.04 | ||||
| p † | 0.791 | 0.230 | 0.250 | 0.599 | 0.004 | ||||
| BUN | placebo | 13.6 ± 0.6 | 14.5 ± 0.7 | 14.0 ± 0.7 | 14.2 ± 0.7 | 14.8 ± 0.7 | Time × Group | 2.944 | 0.030 |
| PCO 20 mg | 13.4 ± 0.4 | 13.4 ± 0.6 | 13.3 ± 0.5 | 12.8 ± 0.6 | 12.6 ± 0.6 * | ||||
| p † | 0.767 | 0.193 | 0.411 | 0.053 | 0.002 | ||||
| Uric acid (mg/dL) | placebo | 5.1 ± 0.3 | 5.0 ± 0.3 | 4.9 ± 0.3 | 5.1 ± 0.3 | 5.2 ± 0.3 | Time × Group | 1.437 | 0.241 |
| PCO 20 mg | 5.2 ± 0.2 | 5.0 ± 0.3 | 5.0 ± 0.3 | 5.1 ± 0.2 | 5.0 ± 0.2 * | ||||
| p | 0.859 | 0.528 | 0.708 | 0.418 | 0.048 | ||||
| SBP (mmHg) | placebo | 112.0 ± 2.1 | 114.7 ± 2.5 | 105.9 ± 3.1 | 110.4 ± 2.2 | 115.7 ± 2.2 | Time × Group | 3.359 | 0.011 |
| PCO 20 mg | 114.0 ± 3.1 | 111.0 ± 3.3 | 104.0 ± 2.8 | 104.7 ± 2.9 | 107.4 ± 2.6 * | ||||
| p † | 0.586 | 0.045 | 0.231 | 0.004 | 0.001 |
¶ Data are expressed as the mean ± SEM. Estimated statistical power was 99.8% from the selected participants in both group (n = 52) based on calculations using the program G*Power 3.1.9.7 (G*Power from the University of Düsseldorf, Düsseldorf, Germany). The participants meet the inclusion criteria and were instructed to avoid alcohol drinking (<30 g and <15 g of ethanol for men and women, respectively, per day), and smoking, both direct and indirect. p † indicates whether the ANCOVA is statistically significant. p ‡ indicates whether the repeated measures ANOVA is statistically significant. *, Statistically significantly different mean value by independent t-test between the placebo group and policosanol 20 mg group. AST, alanine transaminase; ALT, alanine aminotransferase; γ-GTP, gamma-glutamyl transferase; ALP, alkaline phosphatase; HbA1c, glycated hemoglobin; BUN, blood urea nitrogen; SBP, systolic blood pressure; PCO, policosanol.
Repeated measures ANOVA revealed that there were significant differences in serum AST, ALT, ALT/AST (ratio), γ-GTP, and ALP between the placebo and policosanol groups in a time-dependent manner. The policosanol group significantly lowered the hepatic function parameters, especially in week 12 and after 4 weeks of consumption in terms of time and group interaction. The diabetic marker (HbA1c) and kidney function parameters (BUN and uric acid) were also significantly decreased in the policosanol group in a time-dependent manner (Table 1). Repeated measures ANOVA revealed that HbA1c and BUN showed significant differences between the placebo and policosanol groups in terms of time and group interaction.
As shown in Table 1, serum AST levels were 11% (p = 0.017) and 17% (p = 0.008) lower in the policosanol group than the placebo group at week 12 and after 4 weeks, respectively. The policosanol group also exhibited significantly lower AST in a time-dependent manner: up to 17% decrease at week 16 (p † = 0.008) compared to week 0, as shown in Table 1 and Supplemental Figure S1. Serum ALT levels were 9% (p † = 0.041) and 23% (p † < 0.001) lower in the policosanol group than the placebo group at week 12 and after 4 weeks, respectively. Repeated measures ANOVA revealed that policosanol group showed significantly lower AST (p ‡ = 0.041) and ALT (p ‡ = 0.008) than the placebo group, as shown in Table 1.
Interestingly, although the two groups had similar alcohol consumption of 9–10 g of ethanol/day during the 16 weeks, γ-GTP was 10% (p † = 0.039), 14% (p † = 0.015), and 23% (p † = 0.011) lower in the policosanol group than those of placebo group at week 8, week 12, and after 4 weeks, respectively, in a time-dependent manner, as shown in Table 1 and Supplemental Figure S2. The serum alkaline phosphatase (ALP) level was decreased in the policosanol group in a time-dependent manner during the 16 weeks: approximately 4.8% lower than week 0 (p † = 0.006), while the placebo group showed a 3.3% increase from week 0 to week 16 (Table 1). The policosanol group showed significantly lower ALP at week 8 (p † = 0.012), week 12 (p † = 0.012), and after 4 weeks (p † = 0.006) compared with those of the placebo group, although both groups showed a normal range of ALP during the 16 weeks. At week 16, the policosanol group showed a 2.1% lower ALP level (p † = 0.006) than that of the placebo group, but policosanol group showed a 6.5% higher level than the placebo group at week 0. Repeated measures ANOVA revealed that the policosanol group showed significantly lower γ-GTP (p ‡ = 0.016) and ALP (p ‡ = 0.003) than placebo group as shown in Table 1.
These results suggest that liver function is protected by policosanol consumption for 12 weeks: lowered serum levels of hepatic enzymes, AST, ALT, γ-GTP, and ALP, in the policosanol group. Interestingly, the hepatic protection effects of policosanol were maintained at post-4 week consumption.
3.2. Improvements in Kidney Functions in the Policosanol Group
As a kidney function parameter, the blood urea nitrogen (BUN) decreased in the policosanol group in a time-dependent manner with up to a 6% decrease (p † = 0.002) from weeks 0 to 16, while the placebo group showed a 9% increase from week 0 to 16 (Table 1 and Supplemental Figure S3A). The policosanol group also exhibited a 15% lower BUN (p † = 0.002) than the placebo group at week 16. Repeated measures ANOVA of BUN showed that the policosanol group showed a significant difference (p ‡ = 0.030) from the placebo group in the point of time and group interaction (Table 1). On the other hand, uric acid was also decreased in the policosanol group in a time-dependent manner: 4% lower than week 0 and the placebo group at week 16 (p † = 0.048), as shown in Table 1 and Supplemental Figure S3B. Although repeated measures ANOVA showed no significant difference (p ‡ = 0.241) in the group and time interactions during the 16 weeks between the two groups, the policosanol group showed a significant difference between week 0 and 16 (after 4 weeks of consumption) compared to the placebo group. The other parameters for kidney functions, such as electrolytes, inorganic phosphorus (P), calcium (Ca), sodium (Na), potassium (K), and chloride (Cl), were similar in both groups, which fell in the normal range, as listed in Supplemental Table S2. These results suggested that the policosanol consumption induced enhancement of kidney function via a decrease of BUN and uric acid without impairment of electrolyte metabolism in kidney.
3.3. Decrease of ALT/AST Ratio and SBP in the Policosanol Group
The ALT/AST ratio decreased gradually and significantly in the policosanol group from 0.98 ± 0.06 at week 0 to 0.86 ± 0.05 at week 16 and after four weeks of consumption, while the placebo group did not show a change around 0.88~0.90 during the 16 weeks, as shown in Table 1 and Supplemental Figure S4A. Repeated measures ANOVA showed that there was a significant difference (p = 0.010) in the ALT/AST ratio between the two groups in the group and the time interactions during the 16 weeks (Table 1). The SBP decreased gradually and significantly to an 8.2% reduction in the policosanol group from 114.0 ± 3.1 mmHg at week 0 and to 104.7 ± 2.9 mmHg at week 12, as shown in Table 1 and Supplemental Figure S4B. On the other hand, the placebo group did not show a notable change in the SBP during 16 weeks––approximately 112–115 mmHg. Repeated measures ANOVA showed a significant difference (p = 0.011) in the SBP between the two groups in the group and time interactions during the 16 weeks (Table 1). These results suggest positive correlations between the decrease in the ALT/AST ratio and SBP.
There was no age difference (approximately 52.1 ± 1.3 years old) between the policosanol group (n = 26, M13/F13) and the placebo group (n = 26, M13/F13) during the consumption period. Although there was no difference in SBP between the groups at week 0, the policosanol group showed a 6.2% (p = 0.005) and 7.2% (p = 0.004) lower SBP than that of the placebo group at week 12 and week 16 (after 4 weeks), respectively, as shown in Supplemental Table S3. The policosanol group also exhibited a significantly lower SBP time-dependent manner: up to a 5.2% (p = 0.004) and 7.2% (p < 0.001) decrease in the SBP at week 12 and week 16, respectively, compared with week 0 (Supplemental Table S1). Although the policosanol group showed a significant decrease in the SBP at week 12 and 16, after 12 weeks of consumption, the SBP remained in the normal range without an abrupt decrease below hypotension (<90 mmHg).
The DBP and pulse rate were similar in both groups at approximately 63–69 mmHg and 68–73 bpm, respectively, in the normal ranges during the 16 weeks. Interestingly, the policosanol group showed 2% lower body weight (p = 0.031) and BMI (p = 0.022) than the placebo group only at week 4. The two groups showed no difference with normal ranges of total serum protein, albumin, creatinine, glucose, and lactate dehydrogenase (LDH) levels, which were similar to the group during the 16 weeks (Supplemental Tables S3–S5). These results suggest that policosanol consumption caused an improvement in the BP without significant impairment of the protein and carbohydrate metabolism in the liver and kidney, as shown in Table 1 and Supplemental Tables S2–S5. Except for the decrease in SBP in the policosanol group, there was no difference in DBP, pulse rate, body weight, or BMI between the placebo and policosanol groups during the 16 weeks.
Overall, these results suggest that the consumption of policosanol caused several beneficial effects to protect liver functions (lowering AST, ALT, and γ-GTP), hepatobiliary systems (lowering ALP), and kidney functions (lowering BUN, uric acid, and HbA1c)0. These beneficial activities contributed to the enhancement of the liver function and kidney function, which are connected to the decrease in SBP, without impairment of electrolyte metabolism. These results suggest that policosanol consumption may prevent or attenuate the incidence of liver disease, kidney disease, and diabetes, which can explain why blood pressure improves.
3.4. Enhancement of the Antioxidant Abilities of Serum
As shown in Figure 2A, at week 12, the policosanol group showed a 37% increase in the ferric ion reduction ability (FRA) around 118 μM of ferrous equivalents than that of week 0 (p < 0.001). In contrast, the placebo group did not change (63–70 μM of ferrous equivalents). The paraoxonase (PON) activity was elevated 29% in the policosanol group to approximately 116 μU/L/min at week 12 compared with week 0 (p = 0.004). In contrast, the placebo group did not change: it was approximately 88–90 μU/L/min (Figure 2B). These results suggest that the consumption of policosanol for 12 weeks was linked with the enhancement of the serum antioxidant abilities, such as FRA and PON.
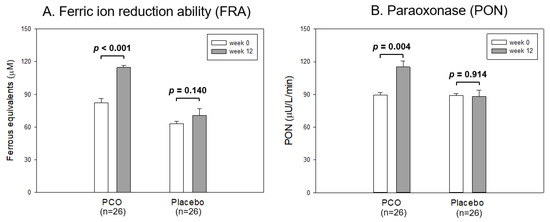
Figure 2.
Antioxidant abilities of serum from each group between week 0 and 12. The data are expressed as the mean ± SD from three independent experiments with duplicate samples. FRA and PON activity in each group between week 0 and week 12 were compared using a paired t-test. (A) Comparison of the ferric ion reduction ability (FRA). FRA was expressed as the concentration of vitamin C (mM), equivalent to reducing the amount of ferric ion (μM) per hour. (B) Comparison of the paraoxonase (PON) activity. PON activity was expressed as the initial velocity of p-nitrophenol production per min (μU/L/min) at 37 °C during 60 min incubation.
3.5. Changes of Hematological Data and Serum Protein Data
As shown in Supplemental Table S4, the policosanol and placebo groups showed a normal range of numbers in white blood cells (WBC), hematocrit (Hct), and platelets (Plt) between during the 16 weeks. These results suggest that there was no difference in complete blood count between the two groups without indication of leukemia, anemia, or thrombocytosis. Although the mechanism is unclear, a mild increase in RBC number and Hb number was associated with an enhancement of oxygen carrying ability. Other hematologic parameters, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were similar in both groups within normal range during 16 weeks (Supplemental Table S4). These results suggest that policosanol consumption did not impair the red blood cell size and volume parameters; all values were in the normal range without risk of anemia.
Interestingly, the glycated hemoglobin (HbA1c) level was significantly lower in the policosanol group, even though the policosanol group showed a higher hemoglobin (Hb) level than the placebo group during 16 weeks (Supplemental Figure S5A). At week 16, the policosanol group showed a 2.2% lower HbA1c level (p = 0.004) than the placebo group, as shown in Table 1 and Supplemental Figure S5B. During the 16 weeks, repeated measures ANOVA of HbA1c showed that the policosanol group showed a significant difference (p = 0.010) from the placebo group in point of time and group interaction, as shown in Table 1. These results suggest that policosanol consumption inhibited glycation in blood proteins, particularly hemoglobin, in a time-dependent manner at week 16 after four weeks of consumption. These decreases in glycated hemoglobin were closely associated with the decrease in SBP (r = 0.197, p = 0.161) by policosanol consumption, but the mechanism is unclear.
As shown in Supplemental Table S5, the placebo group and policosanol group showed normal levels of total protein and albumin in the blood, around 7.0–7.1 g/dL and 4.3–4.4 g/dL, respectively, without any difference between the groups during the 16 weeks. Lactate dehydrogenase (LDH) and creatinine showed normal ranges around 155–168 mg/dL and 0.71–0.77 mg/dL, respectively, in both groups without any difference between the groups. The acute inflammatory parameter, hsCRP, was no different between the groups within the normal range around 0.03–0.22 mg/dL during the 16 weeks, suggesting that there were no remarkable infections or autoimmune inflammatory responses such as rheumatoid arthritis. Overall, these results suggest that policosanol consumption did not impair protein synthesis and nutritional metabolism in liver and kidney functions.
4. Discussion
Patients with dyslipidemia are frequently associated with nonalcoholic fatty liver disease (NAFLD) accompanied by impairment of hepatic functions: elevation of hepatic parameters including AST, ALT, ALP, and γ-GTP [29,30]. Therefore, lowering the hepatic parameters has been recognized as a potent efficacy of nutraceuticals to protect against liver damage in NAFLD [31]. The blood biomarkers level of liver health should be checked regularly to diagnose the progression of liver damage or diseases, such as fatty liver disease, hepatitis, and cirrhosis, particularly in middle-aged populations. Globally, NAFLD and metabolic syndrome have increased rapidly [32], particularly due to the westernized transition, and are more prevalent in middle-aged populations (45–65 years old) to exhibit hypertension, dyslipidemia (low HDL-C and high triglyceride), diabetes, and chronic kidney disease to impair the quality of life [33]. More importantly, improving the serum lipid profile by increasing HDL-C and decreasing the TG and glucose levels in middle-aged adults was associated with an enhancement of cognition and lower Alzheimer’s disease risk [34].
In the middle-aged Japanese participants, the consumption of Cuban policosanol lowered blood pressure and glycated hemoglobin by raising HDL-C with an improvement in the HDL quality and functionality [25]. The current results also revealed the policosanol group to have a 2.2% lower HbA1c level than the placebo group during the 16 weeks in point of group and time interaction (p ‡ = 0.010), and a 2.2% decrease in HbA1c from baseline (Supplemental Figure S5 and Supplemental Tables S1 and S4). Glycated hemoglobin is a risk factor for cardiovascular diseases, all-cause mortality [35], and hypertension [36]. Therefore, lowering HbA1c by policosanol consumption might help alleviate cardiovascular mortality because HbA1c is a reliable risk factor for all-cause mortality and cardiovascular mortality in nondiabetic and diabetic populations [37]. Indeed, in vitro tests and human clinical studies showed that Cuban policosanol exhibited potent anti-glycation activity against fructation [22,23,24] and less glycation extent of apoA-I in HDL [21] and apo-B in LDL/VLDL [25]. A comparison study with various origins of policosanol showed that Cuban policosanol inhibited glycation by protecting apoA-I from proteolytic degradation. In contrast, three Chinese policosanols did not inhibit fructose-mediated glycation [24]. The in vitro anti-glycation activities of Cuban policosanol agreed with in vivo protection of apoA-I with less multimerization of apoA-I from human studies with Korean [20,21] and Japanese participants [25].
Although a few of results look similar and overlap with the preceding report [25], however, there are many different points between the preceding paper and current paper. In the previous paper, change in blood pressure, glycated hemoglobin, AST, ALT, and γ-GTP were reported via analysis of covariance between week 0 and week 12 without data from repeated measures ANOVA of a 4 week interval. The preceding paper [25] focused mainly on improvement of lipid profile, lipoprotein properties, HDL and LDL quality, and HDL functionality.
The current study is very different from the preceding paper [25] in terms of its longer analysis period and different parameters of repeated measures ANOVA. In the current study, 16 weeks of total data were analyzed by repeated measures ANOVA with blood pressure, glycated hemoglobin, liver function, and kidney function parameters. However, the preceding paper [25] showed total 12-week data, which was analyzed by ANCOVA with the independent variables as baseline (week 0) and treatment (week 12) in each group.
Many efficacy studies with functional foods have focused only on the lowering effects of AST and ALT and γ-GTP, without comparing ALP, glycated hemoglobin, and kidney function parameters, such as blood urea nitrogen (BUN) and uric acid. ALP is a diagnostic marker of cholestatic hepatitis and hepatic fibrosis in patients with nonalcoholic steatohepatitis [37] and an independent predictor for hepatic disease-related death [38]. BUN is elevated in patients with nonalcoholic fatty liver disease [39] and is positively associated with the elevation of HbA1c in the older population [40]. In addition to protecting against liver function, enhancing kidney function and blood pressure is more desirable to prevent NAFLD and metabolic syndrome in middle-aged populations.
High serum ALT and AST were associated with a high risk of hypertension and increased BP in young Chinese populations [41]. A clinical study with Korean subjects suggested that AST, ALT, and γ-GTP were positively associated with SBP and DBP from correlation analysis of the liver enzyme and cardiovascular factors [42]. As shown in Supplemental Figures S1 and S4 a decrease in AST, ALT, and AST/ALT ratio all correlate well with a decrease in BP in the policosanol group. Interestingly, among four liver enzymes (AST, ALT, γ-GTP, and ALP), only ALT was negatively associated with the serum apoA-I level (r = −0.028, p = 0.010) and HDL-C (r = −0.224, p < 0.001) and positively associated with the apo-B level (r = 0.114, p = 0.022) and SBP (r = 0.148, p = 0.002) from the clinical observations [42]. A recent randomized and double-blinded clinical study with Japanese participants also showed that 12 weeks of policosanol consumption resulted in an increase in apoA-I, a decrease in ALT, and a decrease in SBP [25]. Overall, these reports strongly suggest that enhancing the HDL quantity and quality were related to protecting against liver function and preventing hypertension.
The current results agree well with previous reports, which showed the hepatoprotective activity of policosanol with antioxidant activity. Policosanol (25 and 100 mg/kg) protected against acute liver injury, carbon tetrachloride (CCl4)-induced hepatic injury in rats, and a model of hepatotoxicity in which the process of lipid peroxidation [43]. Oral supplementation (100 mg/kg) of policosanol alleviates CCl4-induced liver fibrosis by lowering AST, ALT, ALP, and γ-GTP [41]. The hepatoprotection activity was also linked with the reduction of serum levels of interleukin (IL-6), tumor necrosis factor (TNF-α), and malondialdehyde (MDA) [44]. Without fatty liver change, interestingly, serum AST and ALT were decreased significantly by policosanol supplementation for eight weeks in hyperlipidemic zebrafish [45]. In spontaneously hypertensive rats (SHR), eight weeks of feeding the policosanol resulted in a remarkable lowering of the BP with a lowering of the serum CRP [46]. The zebrafish and SHR also significantly decreased fatty streak lesions, inflammatory cell infiltration, and reactive oxygen species [45,46]. These ameliorations of the fatty liver changes agreed with another report that showed policosanol alleviated hepatic lipid accumulation in mice models by regulating bile acids metabolism [47]. In the same context, policosanol attenuated cholesterol synthesis via AMP-activated protein kinase (AMPK) in hepatoma cells [48] and hypercholesterolemic rats [49]. Overall, the policosanol consumption ameliorated the fatty liver change and lowered the AST and ALT levels in human and various animal models.
A new therapeutic agent with identified active ingredients was developed to protect against liver and kidney damage with a relatively lower dosage (<20–100 mg/day) without adverse effects. On the other hand, the almost registered ingredients of functional foods in MFDS of Korea have a higher dosage (e.g., 3150 mg/day of Rubus coreanus Extract) and many unknown and unidentified ingredients, which can help induce HILI and DILI [50,51]. Moreover, heavy metal contamination of registered herbal supplements and synthetic drugs as common adulterants in herbal products are frequently associated with high morbidity and mortality from HILI [52,53].
As far as we know, this paper is the first report to show that short-term (12 week) consumption of 20 mg of policosanol resulted in improved liver functions and kidney functions simultaneously by lowering the serum AST, ALT, γ-GTP, ALP, HbA1c, BUN, and SBP in a time- and group-dependent manner. These enhancements can explain why policosanol supplementation ameliorated fatty liver change, inhibited the inflammatory response and ROS production in hepatic tissue, and lowered BP in animal and human studies, as reported previously [21,22,23,24,25].
In conclusion, 12 weeks of 20 mg consumption of policosanol (Raydel®) protected liver function and enhanced kidney functions, and improved blood pressure (SBP) from randomized, placebo-controlled, and double-blinded trials with healthy Japanese participants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life13061319/s1, Figure S1. Graphical expression of change in the parameters in hepatic function, serum AST (A), and ALT (B), during the 16 weeks between the policosanol and placebo group. AST, Aspartate transaminase; ALT, Alanine aminotransferase. *, p < 0.05 versus placebo; **, p < 0.01 versus placebo; ***, p < 0.001 versus placebo from the analysis of covariance (ANCOVA) model with the independent variable as the baseline and treatment. p ‡ value in blue font indicates the significance of time and group interaction during 16 weeks from repeated measurement ANOVA. Figure S2. Graphical expression of change in the parameters in hepatic function, serum γ-GTP (A) and ALP (B), during the 16 weeks between the policosanol and placebo group. γ-GTP, Gamma-glutamyl transferase; ALP, Alkaline phosphatase. *, p < 0.05 versus placebo from the analysis of covariance (ANCOVA) model with the independent variable as the baseline and treatment. p ‡ value in blue font indicates the significance of time and group interaction during 16 weeks from repeated measurement ANOVA. Figure S3. Graphical expression of change in the parameters of the kidney functions, blood urea nitrogen (A), and uric acid (B), during the 16 weeks between the policosanol and placebo group. BUN, blood urea nitrogen. *, p < 0.05 versus placebo; **, p < 0.01 versus placebo from the analysis of covariance (ANCOVA) model with the independent variable as the baseline and treatment. p ‡ value in blue font indicates the significance of time and group interaction during 16 weeks from repeated measurement ANOVA. Figure S4. Graphical expression of change in the hepatic parameters, ALT/AST ratio, (A) and SBP (B) during the 16 weeks between the policosanol and placebo group. *, p < 0.05 versus placebo; **, p < 0.01 versus placebo; ***, p < 0.001 versus placebo from the analysis of covariance (ANCOVA) model with the independent variable as the baseline and treatment. p ‡ value in blue font indicates the significance of time and group interaction during 16 weeks from repeated measurement ANOVA. Figure S5. Graphical expression of change in the blood hemoglobin (A) and glycated hemoglobin (B) contents during the 16 weeks between the policosanol and placebo groups. Hb, hemoglobin; HbA1c, glycated hemoglobin. *, p < 0.05 versus the placebo from the analysis of covariance (ANCOVA) model with the independent variable as the baseline and treatment. p ‡ value in blue font indicates the significance of the time and group interaction during 16 weeks from repeated measurement ANOVA. Table S1. Comparison of baseline (week 0) data between placebo and policosanol 20 mg group ¶. Table S2. Parameters for kidney function test. Table S3. Change in the blood pressure and anthropological data between placebo group and policosanol (PCO) group during 16 weeks ¶. Table S4. Changes in the hematologic data in blood between placebo group and policosanol (PCO) group during 16 weeks ¶. Table S5. Serum proteins, glucose, and inflammatory parameters between placebo group and policosanol (PCO) group during 16 weeks ¶.
Author Contributions
Conceptualization, K.-H.C. and Y.U.; methodology, J.-E.K. and T.K.; writing—original draft preparation, K.-H.C.; supervision, K.-H.C.; data curation and investigation, Y.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The protocol of human blood donation was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Koseikai Fukuda Internal Medicine Clinic (Osaka, Japan), with the IRB approval number 15000074, approval date on 18 September 2021.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rosselli, M.; Lotersztajn, S.; Vizzutti, F.; Arena, U.; Pinzani, M.; Marra, F. The metabolic syndrome and chronic liver disease. Curr. Pharm. Des. 2014, 20, 5010–5024. [Google Scholar] [CrossRef] [PubMed]
- Talwani, R.; Gilliam, B.L.; Howell, C. Infectious diseases and the liver. Clin. Liver Dis. 2011, 15, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Castera, L.; Loomba, R. Impact of non-invasive biomarkers on hepatology practice: Past, present and future. J. Hepatol. 2022, 76, 1362–1378. [Google Scholar] [CrossRef]
- Limdi, J.; Hyde, G. Evaluation of abnormal liver function tests. Postgrad. Med. J. 2003, 79, 307–312. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016, 15, 817. [Google Scholar] [CrossRef]
- Beier, J.I.; Arteel, G.E. Environmental exposure as a risk-modifying factor in liver diseases: Knowns and unknowns. Acta Pharm. Sin. B 2021, 11, 3768–3778. [Google Scholar] [CrossRef]
- Harrison, S.A.; Day, C.P. Benefits of lifestyle modification in NAFLD. Gut 2007, 56, 1760–1769. [Google Scholar] [CrossRef]
- Osna, N.A.; Donohue, T.M., Jr.; Kharbanda, K.K. Alcoholic liver disease: Pathogenesis and current management. Alcohol Res. Curr. Rev. 2017, 38, 147. [Google Scholar]
- Imani, F.; Motavaf, M.; Safari, S.; Alavian, S.M. The therapeutic use of analgesics in patients with liver cirrhosis: A literature review and evidence-based recommendations. Hepat. Mon. 2014, 14, e23539. [Google Scholar] [CrossRef]
- Chang, W.H.; Mueller, S.H.; Chung, S.-C.; Foster, G.R.; Lai, A.G. Increased burden of cardiovascular disease in people with liver disease: Unequal geographical variations, risk factors and excess years of life lost. J. Transl. Med. 2022, 20, 2. [Google Scholar] [CrossRef]
- Bonora, E.; Targher, G. Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Mega, A.; Marzi, L.; Kob, M.; Piccin, A.; Floreani, A. Food and nutrition in the pathogenesis of liver damage. Nutrients 2021, 13, 1326. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Eickhoff, A. Herbal hepatotoxicity in traditional and modern medicine: Actual key issues and new encouraging steps. Front. Pharmacol. 2015, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-H.; Oh, D.-S.; Hong, S.-H.; Ko, H.; Lee, N.-H.; Park, S.-E.; Han, C.-W.; Kim, S.-M.; Kim, Y.-C.; Kim, K.-S. A nationwide study of the incidence rate of herb-induced liver injury in Korea. Arch. Toxicol. 2017, 91, 4009–4015. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Forlani, G.; Bugianesi, E. Is liver disease a threat to patients with metabolic disorders? Ann. Med. 2005, 37, 333–346. [Google Scholar] [CrossRef]
- Ding, Q.; Ouyang, J.; Fan, B.; Cao, C.; Fan, Z.; Ding, L.; Li, F.; Tu, W.; Jin, X.; Wang, J. Association between dyslipidemia and nephrolithiasis risk in a Chinese population. Urol. Int. 2019, 103, 156–165. [Google Scholar] [CrossRef]
- Kabeya, Y.; Kato, K.; Tomita, M.; Katsuki, T.; Oikawa, Y.; Shimada, A.; Atsumi, Y. Associations of insulin resistance and glycemic control with the risk of kidney stones. Intern. Med. 2012, 51, 699–705. [Google Scholar] [CrossRef]
- Park, H.-J.; Yadav, D.; Jeong, D.-J.; Kim, S.-J.; Bae, M.-A.; Kim, J.-R.; Cho, K.-H. Short-term consumption of Cuban policosanol lowers aortic and peripheral blood pressure and ameliorates serum lipid parameters in healthy Korean participants: Randomized, double-blinded, and placebo-controlled study. Int. J. Environ. Res. Public Health 2019, 16, 809. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, S.-J.; Yadav, D.; Kim, J.-Y.; Kim, J.-R. Consumption of cuban policosanol improves blood pressure and lipid profile via enhancement of HDL functionality in healthy women subjects: Randomized, double-blinded, and placebo-controlled study. Oxid. Med. Cell. Longev. 2018, 2018, 4809525. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, S.-M.; Kim, S.-J.; Lee, E.-Y.; Kim, J.-R.; Cho, K.-H. Consumption of policosanol enhances HDL functionality via CETP inhibition and reduces blood pressure and visceral fat in young and middle-aged subjects. Int. J. Mol. Med. 2017, 39, 889. [Google Scholar] [CrossRef]
- Kim, S.-J.; Yadav, D.; Park, H.-J.; Kim, J.-R.; Cho, K.-H. Long-term consumption of cuban policosanol lowers central and brachial blood pressure and improves lipid profile with enhancement of lipoprotein properties in healthy korean participants. Front. Physiol. 2018, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-M.; Yoo, J.-A.; Lee, E.-Y.; Cho, K.-H. Enhancement of high-density lipoprotein cholesterol functions by encapsulation of policosanol exerts anti-senescence and tissue regeneration effects via improvement of anti-glycation, anti-apoptosis, and cholesteryl ester transfer inhibition. Rejuvenation Res. 2016, 19, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Bae, M.; Kim, J.-R. Cuban sugar cane wax acid and policosanol showed similar atheroprotective effects with inhibition of LDL oxidation and cholesteryl ester transfer via enhancement of high-density lipoproteins functionality. Cardiovasc. Ther. 2019, 2019, 8496409. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Baek, S.H.; Nam, H.-S.; Kim, J.-E.; Kang, D.-J.; Na, H.; Zee, S. Cuban Sugar Cane Wax Alcohol Exhibited Enhanced Antioxidant, Anti-Glycation and Anti-Inflammatory Activity in Reconstituted High-Density Lipoprotein (rHDL) with Improved Structural and Functional Correlations: Comparison of Various Policosanols. Int. J. Mol. Sci. 2023, 24, 3186. [Google Scholar] [CrossRef]
- Cho, K.-H.; Nam, H.-S.; Baek, S.-H.; Kang, D.-J.; Na, H.; Komatsu, T.; Uehara, Y. Beneficial Effect of Cuban Policosanol on Blood Pressure and Serum Lipoproteins Accompanied with Lowered Glycated Hemoglobin and Enhanced High-Density Lipoprotein Functionalities in a Randomized, Placebo-Controlled, and Double-Blinded Trial with Healthy Japanese. Int. J. Mol. Sci. 2023, 24, 5185. [Google Scholar] [CrossRef]
- Canavaciolo, V.L.G.; Gómez, C.V. “Copycat-policosanols” versus genuine policosanol. Rev. CENIC Cienc. Quím. 2007, 38, 207–213. [Google Scholar]
- Benzie, I.F.; Strain, J. [2] Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 15–27. [Google Scholar] [CrossRef]
- Blatter Garin, M.-C.; Moren, X.; James, R.W. Paraoxonase-1 and serum concentrations of HDL-cholesterol and apoA-I. J. Lipid Res. 2006, 47, 515–520. [Google Scholar] [CrossRef]
- Speliotes, E.K.; Balakrishnan, M.; Friedman, L.S.; Corey, K.E. treatment of Dyslipidemia in Common liver Diseases. Clin. Liver Dis. 2019, 14, 161–162. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Faghihimani, E.; Adibi, P. Nonalcoholic fatty liver disease: Diagnostic biomarkers. World J. Gastrointest. Pathophysiol. 2017, 8, 11. [Google Scholar] [CrossRef]
- Cicero, A.F.; Colletti, A.; Bellentani, S. Nutraceutical approach to non-alcoholic fatty liver disease (NAFLD): The available clinical evidence. Nutrients 2018, 10, 1153. [Google Scholar] [CrossRef]
- Zhou, F.; Zhou, J.; Wang, W.; Zhang, X.J.; Ji, Y.X.; Zhang, P.; She, Z.G.; Zhu, L.; Cai, J.; Li, H. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: A systematic review and meta-analysis. Hepatology 2019, 70, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Kuma, A.; Kato, A. Lifestyle-Related Risk Factors for the Incidence and Progression of Chronic Kidney Disease in the Healthy Young and Middle-Aged Population. Nutrients 2022, 14, 3787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tong, T.; Chang, A.; Ang, T.F.A.; Tao, Q.; Auerbach, S.; Devine, S.; Qiu, W.Q.; Mez, J.; Massaro, J. Midlife lipid and glucose levels are associated with Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Cavero-Redondo, I.; Peleteiro, B.; Álvarez-Bueno, C.; Rodriguez-Artalejo, F.; Martínez-Vizcaíno, V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: A systematic review and meta-analysis. BMJ Open 2017, 7, e015949. [Google Scholar] [CrossRef]
- Bower, J.K.; Appel, L.J.; Matsushita, K.; Young, J.H.; Alonso, A.; Brancati, F.L.; Selvin, E. Glycated hemoglobin and risk of hypertension in the atherosclerosis risk in communities study. Diabetes Care 2012, 35, 1031–1037. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, X.; Gu, J.; Yang, M.; Zhang, X.; Zhao, H.; Li, L. Serum alkaline phosphatase levels as a simple and useful test in screening for significant fibrosis in treatment-naive patients with hepatitis B e-antigen negative chronic hepatitis B. Eur. J. Gastroenterol. Hepatol. 2019, 31, 817–823. [Google Scholar] [CrossRef]
- Rafiq, N.; Bai, C.; Fang, Y.; Srishord, M.; McCullough, A.; Gramlich, T.; Younossi, Z.M. Long-term follow-up of patients with nonalcoholic fatty liver. Clin. Gastroenterol. Hepatol. 2009, 7, 234–238. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Liang, J. Blood urea nitrogen is elevated in patients with non-alcoholic fatty liver disease. Hepato-Gastroenterol. 2013, 60, 343–345. [Google Scholar]
- Lan, Q.; Zheng, L.; Zhou, X.; Wu, H.; Buys, N.; Liu, Z.; Sun, J.; Fan, H. The value of blood urea nitrogen in the prediction of risks of cardiovascular disease in an older population. Front. Cardiovasc. Med. 2021, 8, 614117. [Google Scholar] [CrossRef]
- Zhu, L.; Fang, Z.; Jin, Y.; Chang, W.; Huang, M.; He, L.; Chen, Y.; Yao, Y. Association between serum alanine and aspartate aminotransferase and blood pressure: A cross-sectional study of Chinese freshmen. BMC Cardiovasc. Disord. 2021, 21, 472. [Google Scholar] [CrossRef]
- Park, E.-O.; Bae, E.J.; Park, B.-H.; Chae, S.-W. The associations between liver enzymes and cardiovascular risk factors in adults with mild dyslipidemia. J. Clin. Med. 2020, 9, 1147. [Google Scholar] [CrossRef] [PubMed]
- Noa, M.; Mendoza, S.; Mas, R.; Mendoza, N. Effect of policosanol on carbon tetrachloride-induced acute liver damage in Sprague-Dawley rats. Drugs R&D 2003, 4, 29–35. [Google Scholar] [CrossRef]
- Zein, N.; Yassin, F.; Makled, S.; Alotaibi, S.S.; Albogami, S.M.; Mostafa-Hedeab, G.; Batiha, G.E.-S.; Elewa, Y.H.A. Oral supplementation of policosanol alleviates carbon tetrachloride-induced liver fibrosis in rats. Biomed. Pharmacother. 2022, 150, 113020. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-Y.; Yoo, J.-A.; Lim, S.-M.; Cho, K.-H. Anti-aging and tissue regeneration ability of policosanol along with lipid-lowering effect in hyperlipidemic zebrafish via enhancement of high-density lipoprotein functionality. Rejuvenation Res. 2016, 19, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Yadav, D.; Kim, S.-J.; Kim, J.-R. Blood pressure lowering effect of cuban policosanol is accompanied by improvement of hepatic inflammation, lipoprotein profile, and HDL quality in spontaneously hypertensive rats. Molecules 2018, 23, 1080. [Google Scholar] [CrossRef]
- Zhai, Z.; Niu, K.M.; Liu, H.; Lin, C.; Tu, Y.; Liu, Y.; Cai, L.; Ouyang, K.; Liu, J. Policosanol alleviates hepatic lipid accumulation by regulating bile acids metabolism in C57BL6/mice through AMPK–FXR–TGR5 cross-talk. J. Food Sci. 2021, 86, 5466–5478. [Google Scholar] [CrossRef]
- Singh, D.K.; Li, L.; Porter, T.D. Policosanol inhibits cholesterol synthesis in hepatoma cells by activation of AMP-kinase. J. Pharmacol. Exp. Ther. 2006, 318, 1020–1026. [Google Scholar] [CrossRef]
- Nam, D.-E.; Yun, J.-M.; Kim, D.; Kim, O.-K. Policosanol attenuates cholesterol synthesis via AMPK activation in hypercholesterolemic rats. J. Med. Food 2019, 22, 1110–1117. [Google Scholar] [CrossRef]
- Amadi, C.N.; Orisakwe, O.E. Herb-induced liver injuries in developing nations: An update. Toxics 2018, 6, 24. [Google Scholar] [CrossRef]
- Suk, K.T.; Kim, D.J. Drug-induced liver injury: Present and future. Clin. Mol. Hepatol. 2012, 18, 249. [Google Scholar] [CrossRef]
- Obi, E.; Akunyili, D.N.; Ekpo, B.; Orisakwe, O.E. Heavy metal hazards of Nigerian herbal remedies. Sci. Total Environ. 2006, 369, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Adulteration of Chinese herbal medicines with synthetic drugs: A systematic review. J. Intern. Med. 2002, 252, 107–113. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).