Diagnostic Capability of Isolated-Check Visual Evoked Potential for Early to Moderate Primary Open-Angle Glaucoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Criteria
2.2. Visual Field Examination
2.3. PVEP Test
2.4. icVEP Examination
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Schimiti, R.B.; Avelino, R.R.; Kara-Jose, N.; Costa, V.P. Full-threshold versus Swedish Interactive Threshold Algorithm (SITA) in normal individuals undergoing automated perimetry for the first time. Ophthalmology 2002, 109, 2084–2092; discussion 2092. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Miglior, S.; Manni, G.; Centofanti, M.; Bucci, M.G. Clinical ability of pattern electroretinograms and visual evoked potentials in detecting visual dysfunction in ocular hypertension and glaucoma. Ophthalmology 2006, 113, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Lek, J.J.; Nguyen, B.N.; McKendrick, A.M.; Vingrys, A.J. An Electrophysiological Comparison of Contrast Response Functions in Younger and Older Adults, and Those with Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, X.; Tang, X. Associations of subjective and objective clinical outcomes of visual functions with quality of life in Chinese glaucoma patients: A cross-sectional study. BMC Ophthalmol. 2019, 19, 166. [Google Scholar] [CrossRef]
- Odom, J.V.; Bach, M.; Brigell, M.; Holder, G.E.; McCulloch, D.L.; Mizota, A.; Tormene, A.P. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc. Ophthalmol. Adv. Ophthalmol. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Zemon, V.; Tsai, J.C.; Forbes, M.; Al-Aswad, L.A.; Chen, C.M.; Gordon, J.; Greenstein, V.C.; Hu, G.; Strugstad, E.C.; Dhrami-Gavazi, E.; et al. Novel electrophysiological instrument for rapid and objective assessment of magnocellular deficits associated with glaucoma. Doc. Ophthalmol. Adv. Ophthalmol. 2008, 117, 233–243. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y. Diagnostic performance of isolated-check visual evoked potential versus retinal ganglion cell-inner plexiform layer analysis in early primary open-angle glaucoma. BMC Ophthalmol. 2017, 17, 77. [Google Scholar] [CrossRef]
- Chen, X.W.; Zhao, Y.X. Comparison of isolated-check visual evoked potential and standard automated perimetry in early glaucoma and high-risk ocular hypertension. Int. J. Ophthalmol. 2017, 10, 599–604. [Google Scholar]
- Xu, L.J.; Zhang, L.; Li, S.L.; Zemon, V.; Virgili, G.; Liang, Y.B. Accuracy of isolated-check visual evoked potential technique for diagnosing primary open-angle glaucoma. Doc. Ophthalmol. Adv. Ophthalmol. 2017, 135, 107–119. [Google Scholar] [CrossRef]
- Fan, X.; Wu, L.L.; Di, X.; Ding, T.; Ding, A.H. Applications of Isolated-Check Visual Evoked Potential in Early Stage of Open-Angle Glaucoma Patients. Chin. Med. J. 2018, 131, 2439–2446. [Google Scholar] [CrossRef] [PubMed]
- Kolomeyer, N.N.; Drinkwater, O.J.; Drivas, E.; Zakik, A.; Zemon, V.; Sidoti, P.A.; Tsai, J.C.; Panarelli, J.F. Utility of the Modified Isolated-check Visual Evoked Potential Technique in Functional Glaucoma Assessment. J. Glaucoma 2020, 29, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wu, L.; Ding, A. Assessing Early Stage Open-Angle Glaucoma in Patients by Isolated-Check Visual Evoked Potential. J. Vis. Exp. 2020, 159. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Y.; Liu, Z.; Li, X. Isolated-check visual evoked potential: A more sensitive tool to detect traumatic optic neuropathy after orbital fracture. Graefes. Arch. Clin. Exp. Ophthalmol. 2021, 259, 547–555. [Google Scholar] [CrossRef]
- Qi, X.; Tong, B.; Hu, W.; Luo, B. Comparing isolated-check visual evoked potential, pattern visual evoked potential, and standard automated perimetry in dysthyroid optic neuropathy eyes. Eye 2020, 35, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Liu, R.; Wang, S.; Hu, W.; Li, Y.; Tong, B.; Zhang, H.; Qi, X. Utility of isolated-check visual evoked potential technique in dysthyroid optic neuropathy. Graefes. Arch. Clin. Exp. Ophthalmol. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E.M. Structure and function of parallel pathways in the primate early visual system. J. Physiol. 2005, 566 Pt 1, 13–19. [Google Scholar] [CrossRef]
- Glovinsky, Y.; Quigley, H.A.; Pease, M.E. Foveal ganglion cell loss is size dependent in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 1993, 34, 395–400. [Google Scholar]
- Weber, A.J.; Chen, H.; Hubbard, W.C.; Kaufman, P.L. Experimental glaucoma and cell size, density, and number in the primate lateral geniculate nucleus. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1370–1379. [Google Scholar]
- Morgan, J.E. Selective cell death in glaucoma: Does it really occur? Br. J. Ophthalmol. 1994, 78, 875–879; discussion 879–880. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Sample, P.A.; Weinreb, R.N. Frequency doubling technology perimetry abnormalities as predictors of glaucomatous visual field loss. Am. J. Ophthalmol. 2004, 137, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Hodapp, E.; Parrish, R.; Anderson, D. Clinical Decision in Glaucoma. In Follow-Up of Primary Open-Angle Glaucoma; Hodapp, E., Parrish, R.K., Anderson, D.R., Eds.; Mosby: St. Louis, MI, USA, 1993; pp. 84–126. [Google Scholar]
- Kerrigan-Baumrind, L.A.; Quigley, H.A.; Pease, M.E.; Kerrigan, D.F.; Mitchell, R.S. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Investig. Ophthalmol. Vis. Sci. 2000, 41, 741–748. [Google Scholar]
- Pierre-Filho Pde, T.; Schimiti, R.B.; de Vasconcellos, J.P.; Costa, V.P. Sensitivity and specificity of frequency-doubling technology, tendency-oriented perimetry, SITA Standard and SITA Fast perimetry in perimetrically inexperienced individuals. Acta. Ophthalmol. Scand. 2006, 84, 345–350. [Google Scholar] [CrossRef]
- King, A.J.; Taguri, A.; Wadood, A.C.; Azuara-Blanco, A. Comparison of two fast strategies, SITA Fast and TOP, for the assessment of visual fields in glaucoma patients. Graefes. Arch. Clin. Exp. Ophthalmol. 2002, 240, 481–487. [Google Scholar] [CrossRef]
- Wadood, A.C.; Azuara-Blanco, A.; Aspinall, P.; Taguri, A.; King, A.J. Sensitivity and specificity of frequency-doubling technology, tendency-oriented perimetry, and Humphrey Swedish interactive threshold algorithm-fast perimetry in a glaucoma practice. Am. J. Ophthalmol. 2002, 133, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.N.; Sanderson, G.F.; James, A.C.; Vaegan; Maddess, T. Visual evoked potential and psychophysical contrast thresholds in glaucoma. Doc. Ophthalmol. Adv. Ophthalmol. 2014, 128, 111–120. [Google Scholar] [CrossRef]
- Pillai, C.; Ritch, R.; Derr, P.; Gonzalez, A.; Kopko Cox, L.; Siegfried, J.; Liebmann, J.M.; Tello, C. Sensitivity and specificity of short-duration transient visual evoked potentials (SD-tVEP) in discriminating normal from glaucomatous eyes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2847–2852. [Google Scholar] [CrossRef]
- Amarasekera, D.C.; Resende, A.F.; Waisbourd, M.; Puri, S.; Moster, M.R.; Hark, L.A.; Katz, L.J.; Fudemberg, S.J.; Mantravadi, A.V. Steady-state pattern electroretinogram and short-duration transient visual evoked potentials in glaucomatous and healthy eyes. Clin. Exp. Ophthalmol. 2018, 46, 54–61. [Google Scholar] [CrossRef]
- Arvind, H.; Klistorner, A.; Grigg, J.; Graham, S.L. Low-luminance contrast stimulation is optimal for early detection of glaucoma using multifocal visual evoked potentials. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3744–3750. [Google Scholar] [CrossRef]
- Zhong, Y.; Min, Y.; Jiang, Y.; Cheng, Y.; Qin, J.; Shen, X. Color Doppler imaging and pattern visual evoked potential in normal tension glaucoma and hypertension glaucoma. Doc. Ophthalmol. Adv. Ophthalmol. 2009, 119, 171–180. [Google Scholar] [CrossRef]
- Yang, L.; Tang, X. Bilateral asymmetry improved accuracy when assessing glaucomatous vision-related quality of life impairment. Medicine 2019, 98, e17924. [Google Scholar] [CrossRef] [PubMed]
- Senger, C.; Moreto, R.; Watanabe, S.E.S.; Matos, A.G.; Paula, J.S. Electrophysiology in Glaucoma. J. Glaucoma 2020, 29, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Pencina, M.J.; D’Agostino, R.B., Sr.; D’Agostino, R.B., Jr.; Vasan, R.S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172; discussion 207–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, R.S.; Wei, Y.H.; Fang, Y.; Tian, T.; Li, M.; Pan, Y.Z. Applications of the isolated-check visual evoked potential in primary open angle glaucoma with or without high myopia. Int. J. Ophthalmol. 2021, 14, 704–713. [Google Scholar] [CrossRef] [PubMed]

| Variables | POAG N = 33 | Control N = 35 | p |
|---|---|---|---|
| Age, year | 56.39 ± 11.3 | 56.11 ± 8.56 | 0.792 c |
| Sex (male/female) | 18/15 | 13/22 | 0.15 b |
| Axial length, mm | 23.92 ± 1.3 | 23.32 ± 1.3 | 0.059 a |
| CCT, μm | 547.91 ± 28.31 | 539.31 ± 35.53 | 0.39 a |
| IOP, mmHg | 14.67 ± 2.15 | 15.11 ± 2.38 | 0.343 c |
| Spherical equivalent, D | −1.46 ± 2.7 | −0.5 ± 2.4 | 0.121 c |
| Average RNFL, μm | 85.22 ± 11.55 | 110.4 ± 10.96 | <0.001 a |
| Variables | POAG N = 33 | Control N = 35 | p |
|---|---|---|---|
| icVEP | |||
| SNR | 0.87 ± 0.32 | 1.57 ± 0.53 | <0.001 b |
| PVEP | |||
| P100 latency (1°), ms | 106.6 ± 9.28 | 103.54 ± 2.9 | 0.246 a |
| P100 latency (0.25°), ms | 116.56 ± 10.07 | 106.96 ± 5.69 | <0.001 b |
| P100 amplitude (1°), μv | 8.62 ± 5.44 | 13.39 ± 4.5 | <0.001 b |
| P100 amplitude (0.25°), μv | 10.18 ± 6.72 | 14.5 ± 4.88 | 0.001 b |
| VF | |||
| MD, dB | −5.55 ± 3.21 | −1.81 ± 1.5 | <0.001 b |
| PSD, dB | 5.32 ± 3.04 | 1.86 ± 0.93 | <0.001 b |
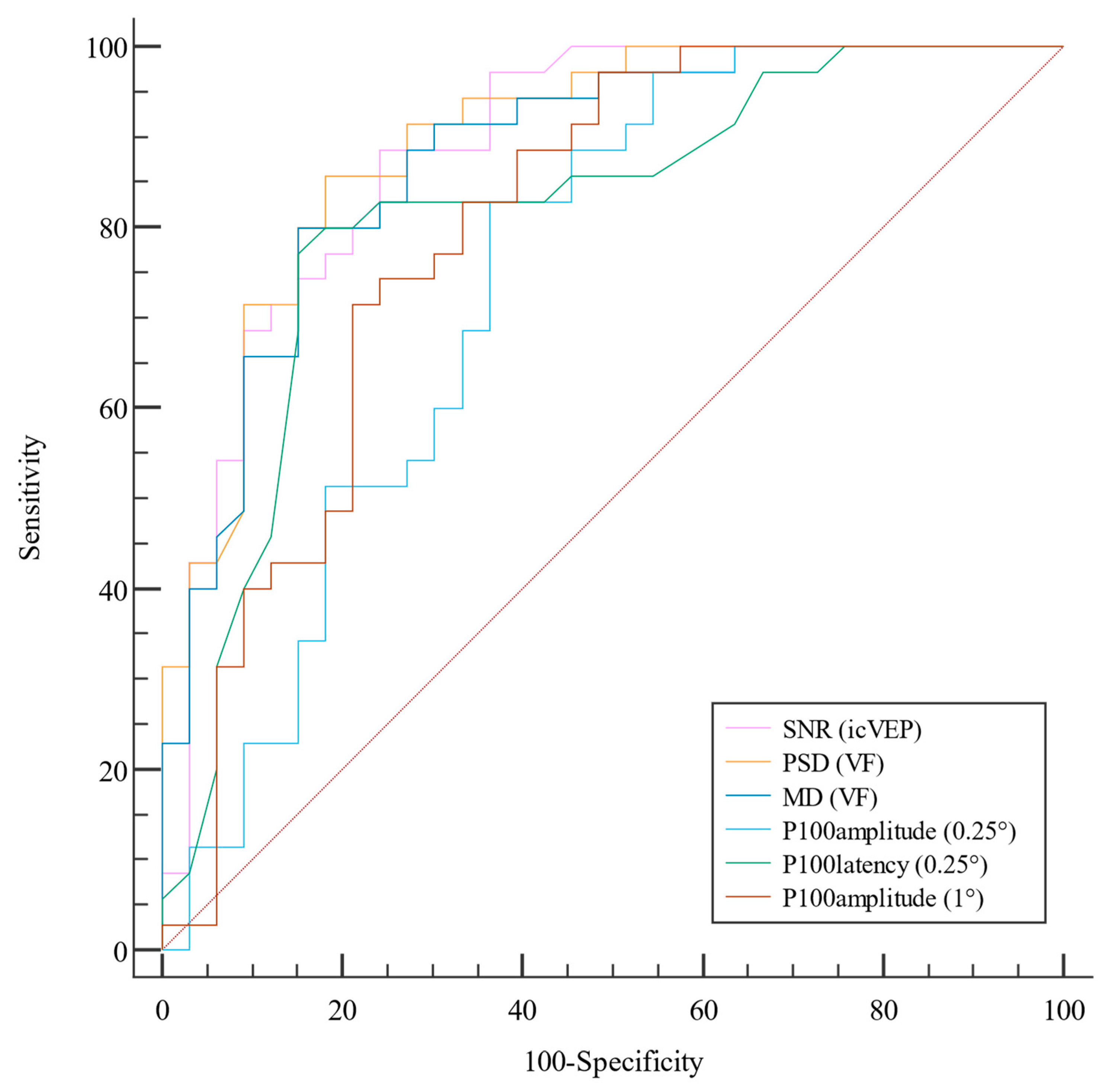
| Parameters | AUC | 95% CI | Best Threshold | Sensitivity, % | Specificity, % | Accuracy | PPV, % | NPV, % |
|---|---|---|---|---|---|---|---|---|
| PSD, db (VF) | 0.895 | (0.821, 0.97) | 2.725 | 81.82 | 85.71 | 0.838 | 84.4 | 83.3 |
| SNR (icVEP) | 0.887 | (0.807, 0.967) | 0.99 | 75.76 | 88.57 | 0.823 | 86.2 | 79.5 |
| MD, db (VF) | 0.877 | (0.796, 0.959) | −2.81 | 84.85 | 80 | 0.824 | 80 | 84.8 |
| P100 latency (0.25°), ms (PVEP) | 0.815 | (0.709, 0.921) | 108.5 | 84.85 | 77.14 | 0.809 | 77.8 | 84.4 |
| P100 amplitude (1°), μv (PVEP) | 0.798 | (0.688, 0.908) | 10.75 | 78.79 | 71.43 | 0.778 | 72.2 | 78.1 |
| P100 amplitude (0.25°), μv (PVEP) | 0.742 | (0.620, 0.864) | 10.57 | 63.64 | 82.86 | 0.722 | 77.8 | 70.7 |
| P100 latency (1°), ms (PVEP) | 0.582 | (0.431, 0.732) | 109 | 39.39 | 85.71 | 0.485 | 72.2 | 60 |
| Parameter of Comparison | Value | 95%CI | p | |
|---|---|---|---|---|
| VF PSD vs. icVEP SNR | ∆AUC | 0.009 | (−0.101, 0.118) | 0.877 |
| IDI | 0.066 | (−0.102, 0.235) | 0.439 | |
| NRI | −0.007 | (−0.271, 0.257) | 0.96 | |
| VF MD vs. icVEP SNR | ∆AUC | −0.009 | (−0.126, 0.108) | 0.879 |
| IDI | 0.013 | (−0.15, 0.176) | 0.874 | |
| NRI | −0.036 | (−0.319, 0.248) | 0.809 | |
| PVEP P100 latency (0.25°) vs. ic VEP SNR | ∆AUC | −0.072 | (−0.211, 0.067) | 0.311 |
| IDI | −0.105 | (−0.268, 0.058) | 0.208 | |
| NRI | −0.005 | (−0.286, 0.275) | 0.971 | |
| PVEP P100 amplitude (1°) vs. icVEP SNR | ∆AUC | −0.088 | (−0.209, 0.0324) | 0.152 |
| IDI | −0.197 | (−0.336, 0.059) | 0.005 | |
| NRI | −0.152 | (−0.422, 0.119) | 0.28 | |
| PVEP P100 amplitude (0.25°) vs. icVEP SNR | ∆AUC | −0.145 | (−0.262, 0.028) | 0.015 |
| IDI | −0.277 | (−0.398, 0.156) | <0.001 | |
| NRI | −0.268 | (−0.533, 0.002) | 0.056 | |
| PVEP P100 latency (1°) vs. icVEP SNR | ∆AUC | −0.305 | (−0.47, 0.139) | <0.001 |
| IDI | −0.368 | (−0.494, 0.242) | <0.001 | |
| NRI | −0.278 | (−0.563, 0.007) | 0.081 |
| Parameters | Sensitivity (%) | Specificity (%) |
|---|---|---|
| icVEP | 72.73 | 82.86 |
| VF | 75.76 | 80 |
| P100 latency (0.25°) | 78.79 | 80 |
| P100 amplitude (0.25°) | 36.36 | 97.14 |
| P100 latency (1°) | 39.39 | 100 |
| P100 amplitude (0.25°) | 51.52 | 97.14 |
| VF | McNemar p | PVEP (P100 Latency of 0.25°) | McNemar p | ||||
|---|---|---|---|---|---|---|---|
| + | - | + | - | ||||
| icVEP | + | 18 | 12 | 0.845 | 21 | 9 | 0.523 |
| - | 14 | 24 | 13 | 25 | |||
| PVEP(P100 latency, 0.25°) | + | 21 | 12 | 0.839 | NA | NA | NA |
| - | 11 | 23 | NA | NA | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Fang, Y.; Li, R.; Pan, Y. Diagnostic Capability of Isolated-Check Visual Evoked Potential for Early to Moderate Primary Open-Angle Glaucoma. Life 2023, 13, 1257. https://doi.org/10.3390/life13061257
Wang X, Fang Y, Li R, Pan Y. Diagnostic Capability of Isolated-Check Visual Evoked Potential for Early to Moderate Primary Open-Angle Glaucoma. Life. 2023; 13(6):1257. https://doi.org/10.3390/life13061257
Chicago/Turabian StyleWang, Xia, Yuan Fang, Ruoshi Li, and Yingzi Pan. 2023. "Diagnostic Capability of Isolated-Check Visual Evoked Potential for Early to Moderate Primary Open-Angle Glaucoma" Life 13, no. 6: 1257. https://doi.org/10.3390/life13061257
APA StyleWang, X., Fang, Y., Li, R., & Pan, Y. (2023). Diagnostic Capability of Isolated-Check Visual Evoked Potential for Early to Moderate Primary Open-Angle Glaucoma. Life, 13(6), 1257. https://doi.org/10.3390/life13061257







