Neuromorphological Atlas of Human Prenatal Brain Development: White Paper
Abstract
1. Introduction
- Brain maturation is a complex morphogenetical process. A detailed study of the early development of the human brain is necessary to understand the normal function of the mature human brain and the causes, manifestation, and progression of pathological processes. Currently, there is a great lack in the fundamental knowledge of human developmental neurology. There is no consistent periodization of the development of fetal brain structures or integrative gyrification hypothesis. This deficiency is related to the fact that the majority of studies in the field of neurobiology are designed as model experiments.
- Studies on human autopsy material are limited due to the complex reasons. Furthermore, intrauterine fetal death often leads to poor condition of the material even for a routine pathology examination. There are also other limitations, both legally established and in the field of individual and professional ethics. Thus, there are relatively few research groups over the world dealing with fetal human material. Only the printed Bayer and Altman Atlas [82,83,84,85] sequentially provides data on human fetal brain development.
- Most of the current publications on the development of the human brain are based on non-invasive research materials (neurosonography, MRI, and X-ray CT), which provide information mainly at the macromorphological level. Histological research materials cover in more detail the embryonic and pre-fetal periods of development, while data on the fetal period are presented mainly by macro-preparations and schemes [82,83,84,85,86]. Published data on human brain development at different fetal stages are not systematized; they relate to individual aspects of development or individual brain structures and are often controversial.
- Another consequence of the small number of scientific groups dealing with fetal autopsy material is the lack of research performed at the modern methodological level. Current advanced research is concentrated mainly on the features of transcriptome functioning in the developing brain [3,51,56]. Besides the well-known studies of the group of PaskoRakic and his students and colleagues, which have already become classic, immunohistochemical studies of the proteome of the developing human brain are episodic and unsystematic. At the same time, proteins are the main functional molecules in cells. Gene transcription products do not always result in translation, and, therefore, it is not always possible to judge the functioning of a protein product in a cell on the basis of transcriptional activity. Studying the translational mechanisms in human brain development is a relevant problem in current neurobiology.
- Worldwide, there is a growing interest in the accumulation of large-scale scientific and reference data into databases available for general use. Morphology, including immunomorphological tissue research, generates large amounts of of digital images, which provide valuable information. However, data are mostly provided only as quantity tables or conclusions and do not fully contribute to publishing data in original research papers. Complete and detailed data are not available to a wide range of experts, including neuroscientists and neurologists.
2. Materials and Methods
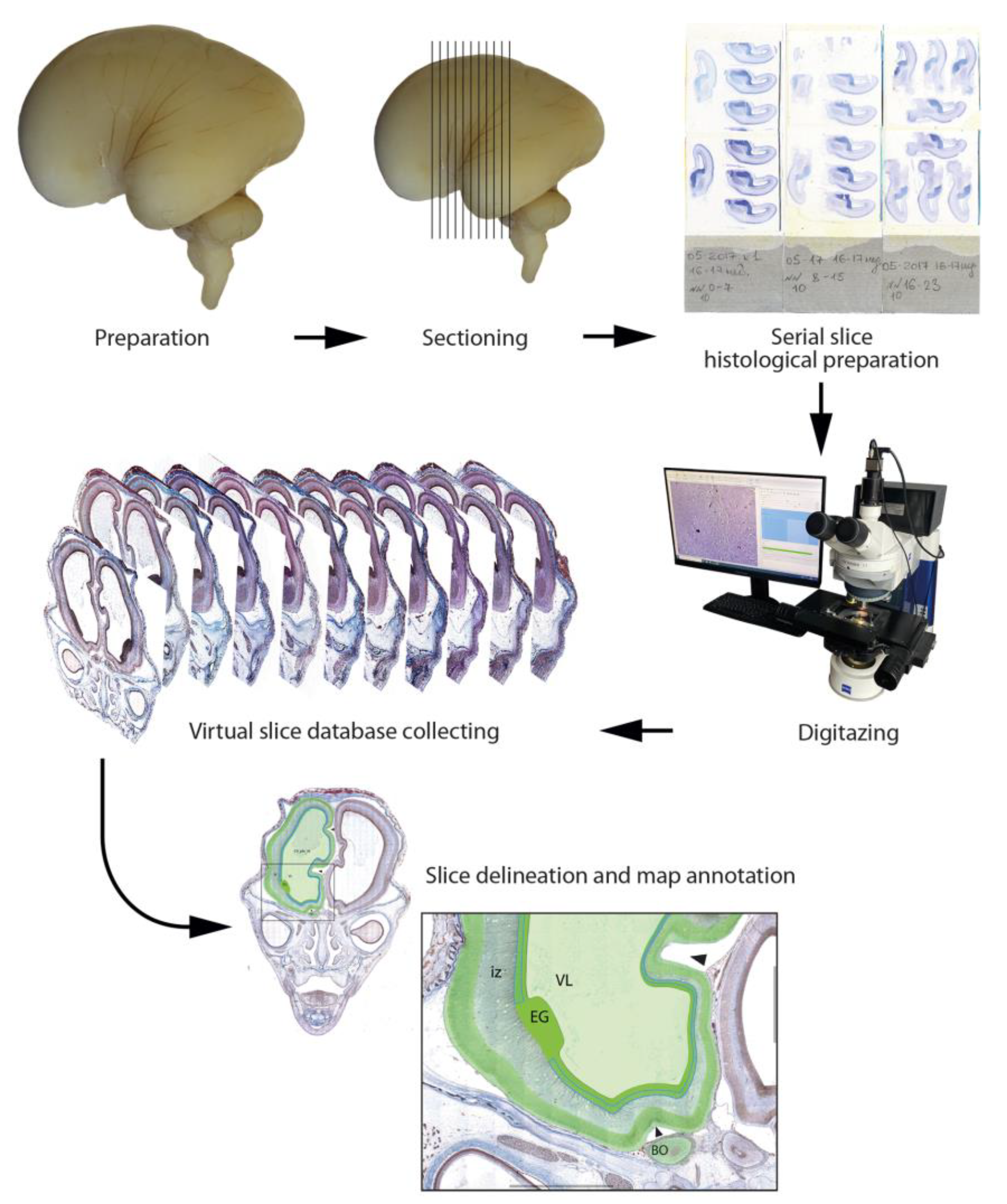
2.1. Tissue Sources and Processing
2.2. Histological Preparation
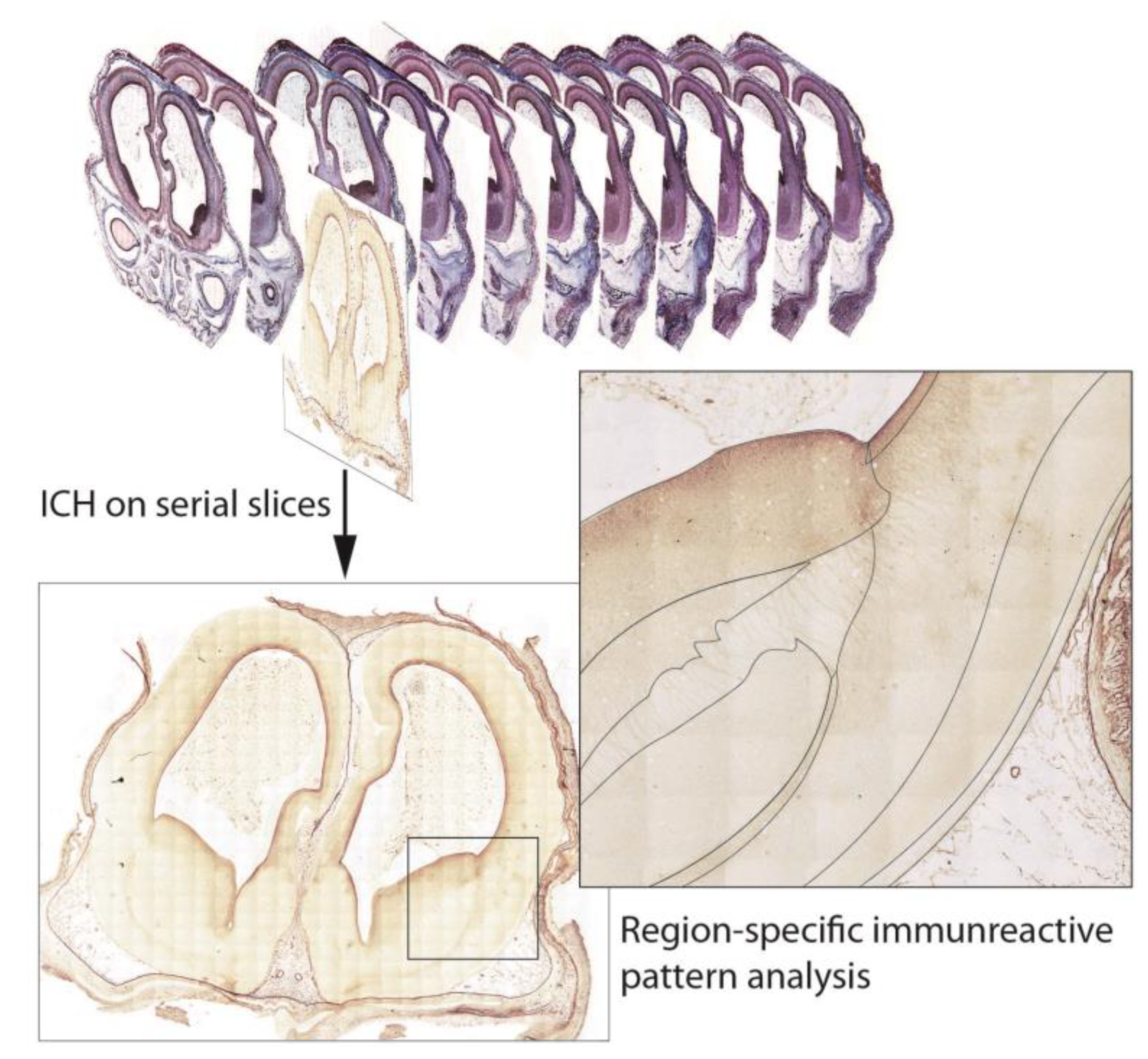
2.3. Immunohistochemistry (ICH)
2.4. Human Brain Imaging
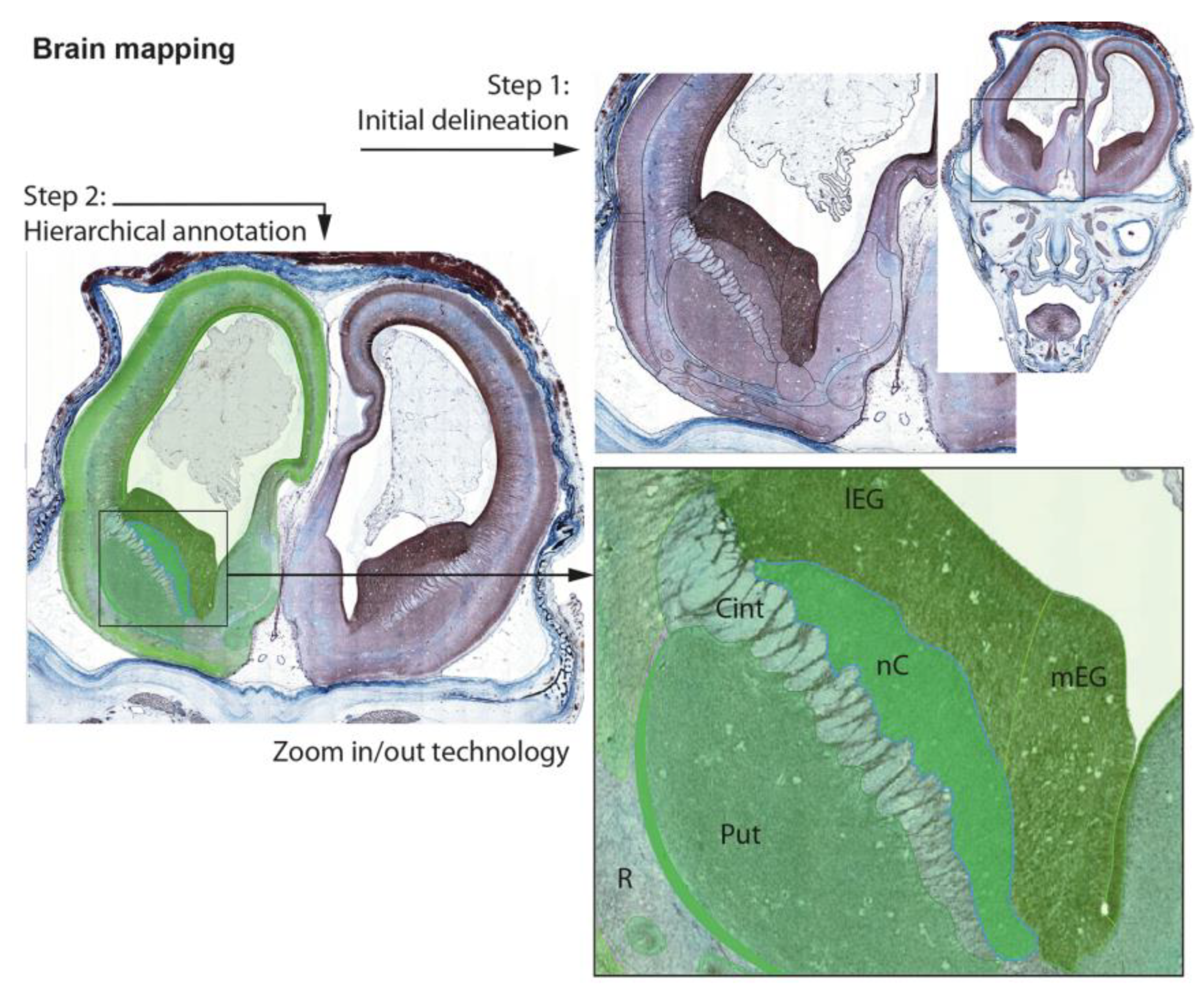
2.5. Data Organization, Neuroanatomical Parcellation, Annotation of 2-D Sections
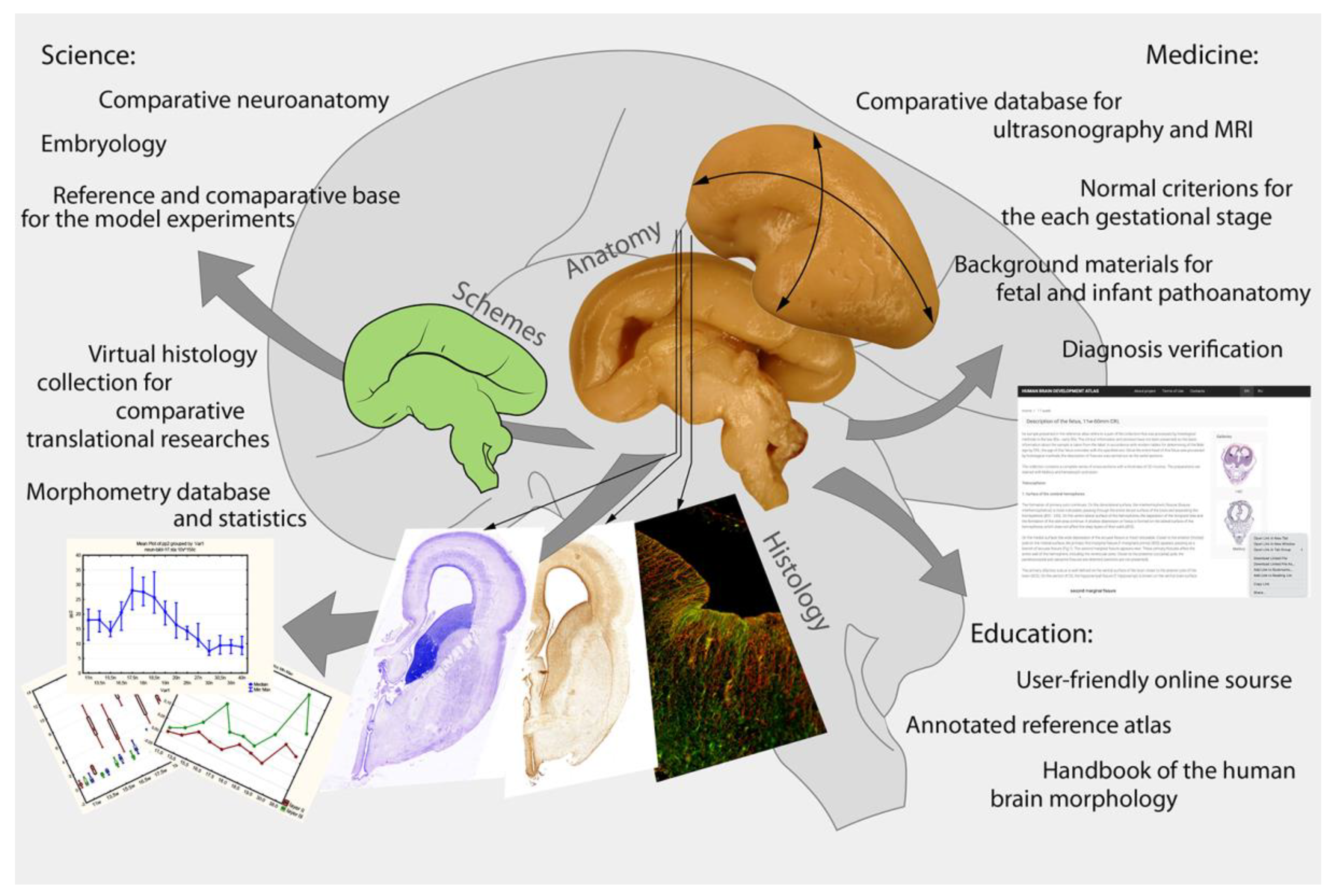
3. Discussion, Highlights, and Perspectives
- A reference database for the developmental research of different human brain structures. These materials are unique, which makes it possible for them to be in demand for many years.
- Comparative material for general neurology—non-human mammal, cultural, and other experimental studies.
- A reference database for the comparative analysis of non-invasive (ultrasonography, MRI, CT) study results, newly spatial genomic sequence technology, and high-resolution 3D-visualization of the native tissue samples with phase-contrast CT.
- (a)
- in obstetrics practice—for the comparison of ultrasonography and MRI/CT results;
- (b)
- in neonatology—for brain maturity criteria;
- (c)
- in prenatal and perinatal pathoanatomy—for the intrauterine and neonatal death cause analysis and searching for pathological changes in the fetal brain.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amunts, K.; Hawrylycz, M.J.; Van Essen, D.C.; Van Horn, J.D.; Harel, N.; Poline, J.B.; De Martino, F.; Bjaalie, J.G.; Dehaene-Lambertz, G.; Dehaene, S.; et al. Interoperable atlases of the human brain. Neuroimage 2014, 99, 525–532. [Google Scholar] [CrossRef]
- Nowinski, W.L. Evolution of human brain atlases in terms of content, applications, functionality, and availability. Neuroinformatics 2021, 19, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.; Royall, J.J.; Lesnar, P.; Facer, B.A.C.; Smith, K.A.; Wei, Y.; Brouner, K.; Dalley, R.A.; Dee, N.; Dolbeare, T.A.; et al. Cellular resolution anatomical and molecular atlases for prenatal human brains. J. Comp. Neurol. 2022, 530, 6–503. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, W.L. Human brain atlasing: Past, present and future. Neuroradiol. J. 2017, 30, 504–519. [Google Scholar] [CrossRef] [PubMed]
- Sunkin, S.M.; Ng, L.; Lau, C.; Dolbeare, T.; Gilbert, T.L.; Thompson, C.L.; Hawrylycz, M.; Dang, C. Allen Brain Atlas: An integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res. 2013, 41, D996–D1008. [Google Scholar] [CrossRef] [PubMed]
- Bjaalie, J. Advances in computational neuroanatomy. Anat. Embryol. 2001, 204, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Toga, A.W.; Thompson, P.M.; Mega, M.S.; Narr, K.L.; Blanton, R.E. Probabilistic approaches for atlasing normal and disease-specific brain variability. Anat. Embryol. 2001, 204, 267–282. [Google Scholar] [CrossRef]
- Zaborszky, L. Brain Structure and Function: The first 15 years-a retrospective. Brain Struct.Funct. 2021, 226, 2467–2475. [Google Scholar] [CrossRef]
- Gilbert, T.L. The Allen Brain Atlas as a Resource for Teaching Undergraduate Neuroscience. J. Undergrad. Neurosci. Educ. 2018, 16, A261–A267. [Google Scholar]
- Campbell, A.W. Histological Studies on the Localization of Cerebral Function; University Press: Cambridge, UK, 1905. [Google Scholar]
- Smith, G.E. A New Topographical Survey of the Human Cerebral Cortex, being an Account of the Distribution of the Anatomically Distinct Cortical Areas and their Relationship to the Cerebral Sulci. J. Anat. Physiol. 1907, 41, 237–254. [Google Scholar]
- Von Economo, C.F.; Koskinas, G.N. Die Cytoarchitektonik der Hirnrinde des Erwachsenen Menschen; Springer: Berlin/Heidelberg, Germany, 1925. [Google Scholar]
- Brodmann, K. Vergleichende Lokalisationslehre der Grosshirnrinde: In Ihren Principien Dargestelltauf Grund des Zellenbaues; Johann Ambrosius Barth Verlag: Leipzig, Germany, 1909. [Google Scholar]
- Vogt, O. Die myeloarchitektonische Felderung des menschlichen Stirnhirns. J. Psychol. Neurol. 1910, 15, 221–232. [Google Scholar]
- Vogt, C.; Vogt, O. Allgemeinere Ergebnisseunserer Hirnforschung. J. Psychol. Neurol. 1919, 25, 292–398. [Google Scholar]
- Sarkissov, S.A.; Filiminoff, I.N.; Kononova, E.P.; Peobraschenskaya, I.S.; Kukuev, L.A. Atlas of the Cytoarchitectonics of the Human Cerebral Cortex; Medgiz: Moscow, Russia, 1955. (In Russian) [Google Scholar]
- Dean, D.C., 3rd; Planalp, E.M.; Wooten, W.; Schmidt, C.K.; Kecskemeti, S.R.; Frye, C.; Schmidt, N.L.; Goldsmith, H.H.; Alexander, A.L.; Davidson, R.J. Investigation of brain structure in the 1-month infant. Brain Struct. Funct. 2018, 223, 1953–1970. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Chang, L.; Huang, H. Baby brain atlases. Neuroimage 2019, 185, 865–880. [Google Scholar] [CrossRef]
- Miller, J.A.; Ding, S.L.; Sunkin, S.M.; Smith, K.A.; Ng, L.; Szafer, A.; Ebbert, A.; Riley, Z.L.; Royall, J.J.; Aiona, K.; et al. Transcriptional landscape of the prenatal human brain. Nature 2014, 508, 199–206. [Google Scholar] [CrossRef]
- Molnár, Z.; Clowry, G.J.; Šestan, N.; Alzu’bi, A.; Bakken, T.; Hevner, R.F.; Hüppi, P.S.; Kostović, I.; Rakic, P.; Anton, E.S.; et al. New insights into the development of the human cerebral cortex. J. Anat. 2019, 235, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Caneja, C.M.; State, M.W.; Hagerman, R.J.; Jacquemont, S.; Marín, O.; Bagni, C.; Umbricht, D.; Simonoff, E.; de Andrés-Trelles, F.; Kaale, A.; et al. A white paper on a neurodevelopmental framework for drug discovery in autism and other neurodevelopmental disorders. Eur. Neuropsychopharmacol. 2021, 48, 49–88. [Google Scholar] [CrossRef]
- Habas, P.A.; Kim, K.; Corbett-Detig, J.M.; Rousseau, F.; Glenn, O.A.; Barkovich, A.J.; Studholme, C. A spatiotemporal atlas of MR intensity, tissue probability and shape of the fetal brain with application to segmentation. Neuroimage 2010, 53, 460–470. [Google Scholar] [CrossRef]
- Huang, H.; Xue, R.; Zhang, J.; Ren, T.; Richards, L.J.; Yarowsky, P.; Miller, M.I.; Mori, S. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J.Neurosci. 2009, 29, 4263–4273. [Google Scholar] [CrossRef]
- Toga, A.W.; Thompson, P.M.; Sowell, E.R. Mapping brain maturation. Trends Neurosci. 2006, 29, 148–159. [Google Scholar] [CrossRef]
- Zhan, J.; Dinov, I.D.; Li, J.; Zhang, Z.; Hobel, S.; Shi, Y.; Lin, X.; Zamanyan, A.; Feng, L.; Teng, G.; et al. Spatial-temporal atlas of human fetal brain development during the early second trimester. Neuroimage 2013, 82, 115–126. [Google Scholar] [CrossRef]
- Rutherford, M.; Jiang, S.; Allsop, J.; Perkins, L.; Srinivasan, L.; Hayat, T.; Kumar, S.; Hajnal, J. MR imaging methods for assessing fetal brain development. Dev.Neurobiol. 2008, 68, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Prayer, D.; Kasprian, G.; Krampl, E.; Ulm, B.; Witzani, L.; Prayer, L.; Brugger, P.C. MRI of normal fetal brain development. Eur. J.Radiol. 2006, 57, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Turk, E.; van den Heuvel, M.I.; Benders, M.J.; de Heus, R.; Franx, A.; Manning, J.H.; Hect, J.L.; Hernandez-Andrade, E.; Hassan, S.S.; Romero, R.; et al. Functional connectome of the fetal brain. J.Neurosci. 2019, 39, 9716–9724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hou, Z.; Lin, X.; Teng, G.; Meng, H.; Zang, F.; Fang, F.; Liu, S. Development of the fetal cerebral cortex in the second trimester: Assessment with 7T postmortem MR imaging. AJNR Am. J.Neuroradiol. 2013, 34, 1462–1467. [Google Scholar] [CrossRef]
- Wright, R.; Kyriakopoulou, V.; Ledig, C.; Rutherford, M.A.; Hajnal, J.V.; Rueckert, D.; Aljabar, P. Automatic quantification of normal cortical folding patterns from fetal brain MRI. Neuroimage 2014, 91, 21–32. [Google Scholar] [CrossRef]
- Malinger, G.; Ginath, S.; Lerman-Sagie, T.; Watemberg, N.; Lev, D.; Glezerman, M. The fetal cerebellar vermis: Normal development as shown by transvaginal ultrasound. Prenat. Diagn. 2001, 21, 687–692. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Z.; Lin, X.; Teng, G.; Meng, H.; Yu, T.; Fang, F.; Zang, F.; Li, Z.; Liu, S. Development of the human fetal cerebellum in the second trimester: A post mortem magnetic resonance imaging evaluation. J. Anat. 2011, 219, 582–588. [Google Scholar] [CrossRef]
- Hata, T.; Kuno, A.; Dai, S.Y.; Inubashiri, E.; Hanaoka, U.; Kanenishi, K.; Yamashiro, C.; Tanaka, H.; Yanagihara, T. Three-dimensional sonographic volume measurement of the fetal cerebellum. J. Med. Ultrason. 2007, 34, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.A.; Hamzelou, K.S.; Rajagopalan, V.; Habas, P.A.; Kim, K.; Barkovich, A.J.; Glenn, O.A.; Studholme, C. 3D morphometric analysis of human fetal cerebellar development. Cerebellum 2012, 11, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Poretti, A.; Boltshauser, E.; Huisman, T.A. Prenatal Cerebellar Disruptions: Neuroimaging Spectrum of Findings in Correlation with Likely Mechanisms and Etiologies of Injury. Neuroimaging Clin. N. Am. 2016, 26, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ge, X.; Shi, Y.; Zhang, Z.; Tang, Y.; Lin, X.; Teng, G.; Zang, F.; Gao, N.; Liu, H.; et al. Morphometric development of the human fetal cerebellum during the early second trimester. Neuroimage 2020, 207, 116372. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Shi, Y.; Li, J.; Zhang, Z.; Lin, X.; Zhan, J.; Ge, H.; Xu, J.; Yu, Q.; Leng, Y.; et al. Development of the human fetal hippocampal formation during early second trimester. Neuroimage 2015, 119, 33–43. [Google Scholar] [CrossRef]
- Dittrich, E.; Kasprian, G.; Prayer, D.; Langs, G. Atlas learning in fetal brain development. Top. Magn. Reason. Imaging 2011, 22, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Vasung, L.; Marami, B.; Rollins, C.K.; Afacan, O.; Ortinau, C.M.; Yang, E.; Warfield, S.K.; Gholipour, A. Fetal brain growth portrayed by a spatiotemporal diffusion tensor MRI atlas computed from in utero images. Neuroimage 2019, 185, 593–608. [Google Scholar] [CrossRef]
- Kuklisova-Murgasova, M.; Aljabar, P.; Srinivasan, L.; Counsell, S.J.; Doria, V.; Serag, A.; Gousias, I.S.; Boardman, J.P.; Rutherford, M.A.; Edwards, A.D.; et al. A dynamic 4D probabilistic atlas of the developing brain. Neuroimage 2011, 54, 2750–2763. [Google Scholar] [CrossRef]
- Lim, I.A.; Faria, A.V.; Li, X.; Hsu, J.T.; Airan, R.D.; Mori, S.; van Zijl, P.C. Human brain atlas for automated region of interest selection in quantitative susceptibility mapping: Application to determine iron content in deep gray matter structures. Neuroimage 2013, 82, 449–469. [Google Scholar] [CrossRef]
- Xia, J.; Wang, F.; Benkarim, O.M.; Sanroma, G.; Piella, G.; González Ballester, M.A.; Hahner, N.; Eixarch, E.; Zhang, C.; Shen, D.; et al. Fetal cortical surface atlas parcellation based on growth patterns. Hum. Brain Mapp. 2019, 40, 3881–3899. [Google Scholar] [CrossRef]
- Glenn, O.A.; Barkovich, A.J. Magnetic resonance imaging of the fetal brain and spine: An increasingly important tool in prenatal diagnosis, part 1. AJNR Am. J. Neuroradiol. 2006, 27, 1604–1611. [Google Scholar]
- Li, H.; Yan, G.; Luo, W.; Liu, T.; Wang, Y.; Liu, R.; Zheng, W.; Zhang, Y.; Li, K.; Zhao, L.; et al. Mapping fetal brain development based on automated segmentation and 4D brain atlasing. Brain Struct.Funct. 2021, 226, 1961–1972. [Google Scholar] [CrossRef]
- Bendersky, M.; Musolino, P.L.; Rugilo, C.; Schuster, G.; Sica, R.E. Normal anatomy of the developing fetal brain. Ex vivo anatomical-magnetic resonance imaging correlation. J. Neurol. Sci. 2006, 250, 20–26. [Google Scholar] [CrossRef]
- Lhuaire, M.; Martinez, A.; Kaplan, H.; Nuzillard, J.M.; Renard, Y.; Tonnelet, R.; Braun, M.; Avisse, C.; Labrousse, M. Human developmental anatomy: Microscopic magnetic resonance imaging (μMRI) of four human embryos (from Carnegie Stage 10 to 20). Ann. Anat. 2014, 196, 402–409. [Google Scholar] [CrossRef]
- Rados, M.; Judas, M.; Kostović, I. In vitro MRI of brain development. Eur. J.Radiol. 2006, 57, 187–198. [Google Scholar] [CrossRef]
- Wang, R.; Dai, G.; Takahashi, E. High resolution MRI reveals detailed layer structures in early human fetal stages: In vitro study with histologic correlation. Front. Neuroanat. 2015, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Bloy, L.; Ingalhalikar, M.; Eavani, H.; Schultz, R.T.; Roberts, T.P.; Verma, R. White matter atlas generation using HARDI based automated parcellation. Neuroimage 2012, 59, 4055–4063. [Google Scholar] [CrossRef]
- Mahfouz, A.; Huisman, S.M.H.; Lelieveldt, B.P.F.; Reinders, M.J.T. Brain transcriptome atlases: A computational perspective. Brain Struct.Funct. 2017, 222, 1557–1580. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, H.; Zhuo, J.; Zhang, Y.; Wang, J.; Chen, L.; Yang, Z.; Chu, C.; Xie, S.; Laird, A.R.; et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb. Cortex 2016, 26, 3508–3526. [Google Scholar] [CrossRef]
- Kriegstein, A.; Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef]
- Nowakowski, T.J.; Pollen, A.A.; Sandoval-Espinosa, C.; Kriegstein, A.R. Transformation of the radial glia scaffold demarcates two stages of human cerebral cortex development. Neuron 2016, 91, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Velmeshev, D.; Herranz-Pérez, V.; Sandoval, K.; Mayer, S.; Chang, E.F.; Insausti, R.; Kriegstein, A.R.; Rubenstein, J.L.; et al. Immature excitatory neurons develop during adolescence in the human amygdala. Nat. Commun. 2019, 10, 2748. [Google Scholar] [CrossRef] [PubMed]
- Eze, U.C.; Bhaduri, A.; Haeussler, M.; Nowakowski, T.J.; Kriegstein, A.R. Single-cell atlas of early human brain development highlights heterogeneity of human neuroepithelial cells and early radial glia. Nat. Neurosci. 2021, 24, 584–594. [Google Scholar] [CrossRef]
- Rakic, P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol. 1972, 145, 61–83. [Google Scholar] [CrossRef]
- Kornack, D.R.; Rakic, P. Changes in cell-cycle kinetics during the development and evolution of primate neocortex. Proc. Natl. Acad. Sci. USA 1998, 95, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Letinic, K.; Zoncu, R.; Rakic, P. Origin of GABAergic neurons in the human neocortex. Nature 2002, 417, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.S.; Angelucci, A.; Bressloff, P.C. Anatomical substrates for functional columns in macaque monkey primary visual cortex. Cereb. Cortex 2003, 13, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Rakic, P.; Nowakowski, R.S. The time of origin of neurons in the hippocampal region of the rhesus monkey. J. Comp. Neurol. 1981, 196, 99–128. [Google Scholar] [CrossRef]
- Nowakowski, R.S.; Rakic, P. The mode of migration of neurons to the hippocampus: A Golgi and electron microscopic analysis in foetal rhesus monkey. J.Neurocytol. 1979, 8, 697–718. [Google Scholar] [CrossRef]
- Nowakowski, R.S.; Rakic, P. The site of origin and route and rate of migration of neurons to the hippocampal region of the rhesus monkey. J. Comp. Neurol. 1981, 196, 129–154. [Google Scholar] [CrossRef]
- Eckenhoff, M.F.; Rakic, P. Radial organization of the hippocampal dentate gyrus: A Golgi, ultrastructural, and immunocytochemical analysis in the developing rhesus monkey. J. Comp. Neurol. 1984, 223, 1–21. [Google Scholar] [CrossRef]
- Arnold, S.E.; Trojanowski, J.Q. Human fetal hippocampal development: II. The neuronal cytoskeleton. J. Comp. Neurol. 1996, 367, 293–307. [Google Scholar] [CrossRef]
- Ostrem, B.E.; Lui, J.H.; Gertz, C.C.; Kriegstein, A.R. Control of outer radial glial stem cell mitosis in the human brain. Cell Rep. 2014, 8, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, J.; Fang, A.; Li, R.; Bai, Y.; Kriegstein, A.R.; Wang, X. The dynamics of neuronal migration. Adv. Exp. Med. Biol. 2014, 800, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Pollen, A.A.; Nowakowski, T.J.; Chen, J.; Retallack, H.; Sandoval-Espinosa, C.; Nicholas, C.R.; Shuga, J.; Liu, S.J.; Oldham, M.C.; Diaz, A.; et al. Molecular identity of human outer radial glia during cortical development. Cell 2015, 163, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Sarnat, H.B.; Nochlin, D.; Born, D.E. Neuronal nuclear antigen (NeuN): A marker of neuronal maturation in early human fetal nervous system. Brain Dev. 1998, 20, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Verney, C.; Milosevic, A.; Alvarez, C.; Berger, B. Immunocytochemical evidence of well-developed dopaminergic and noradrenergic innervations in the frontal cerebral cortex of human fetuses at midgestation. J. Comp. Neurol. 1993, 336, 331–344. [Google Scholar] [CrossRef]
- Cho, K.H.; Rodríguez-Vázquez, J.F.; Kim, J.H.; Abe, H.; Murakami, G.; Cho, B.H. Early fetal development of the human cerebellum. Surg. Radiol. Anat. 2011, 33, 523–530. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, J.; Shi, H.; Zhang, J.; Xu, X.; Xiao, X.; Liu, Y. Developmental profile of neurogenesis in prenatal human hippocampus: An immunohistochemical study. Int. J. Dev. Neurosci. 2014, 38, 1–9. [Google Scholar] [CrossRef]
- Kharlamova, A.S.; Barabanov, V.; Saveliev, S.V. Development of human olfactory bulbs in prenatal ontogenesis: An immunochistochemical study with markers of presynaptic terminals (anti-SNAP-25, synapsin-I, and synaptophysin). Russ. J. Dev. Biol. 2015, 46, 137–147. [Google Scholar] [CrossRef]
- Sarnat, H.B.; Yu, W. Maturation and Dysgenesis of the Human Olfactory Bulb. Brain Pathol. 2016, 26, 301–318. [Google Scholar] [CrossRef]
- Rakic, S.; Zecevic, N. Early oligodendrocyte progenitor cells in the human fetal telencephalon. Glia 2003, 41, 117–127. [Google Scholar] [CrossRef]
- Alzu’bi, A.; Lindsay, S.J.; Harkin, L.F.; McIntyre, J.; Lisgo, S.N.; Clowry, G.J. The transcription factors COUP-TFI and COUP-TFII have distinct roles in arealisation and GABAergic interneuron specification in the early human fetal telencephalon. Cereb. Cortex 2017, 27, 4971–4987. [Google Scholar] [CrossRef]
- Alzu’bi, A.; Lindsay, S.; Kerwin, J.; Looi, S.J.; Khalil, F.; Clowry, G.J. Distinct cortical and sub-cortical neurogenic domains for GABAergic interneuron precursor transcription factors NKX2.1, OLIG2 and COUP-TFII in early fetal human telencephalon. Brain Struct. Funct. 2017, 222, 2309–2328. [Google Scholar] [CrossRef]
- Kharlamova, A.S.; Godovalova, O.S.; Junemann, O.I.; Saveliev, S.V. Developmental dynamics of prepiriform cortex in prenatal human ontogenesis. J. Chem. Neuroanat. 2018, 92, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Björling, E.; Agaton, C.; Szigyarto, C.A.; Amini, B.; Andersen, E.; Andersson, A.C.; Angelidou, P.; Asplund, A.; Asplund, C.; et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell. Proteom. 2005, 4, 1920–1932. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Milovanov, A.P.; Saveliev, S.V. (Eds.) Rational periodization and methodical aspects of embryology. In Prenatal Human Development; MDV: Moscow, Russia, 2006; pp. 21–32. (In Russian) [Google Scholar]
- Bayer, S.A.; Altman, J. The Human Brain during the Third Trimester; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar] [CrossRef]
- Bayer, S.A.; Altman, J. The Human Brain during the Second Trimester; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Bayer, S.A.; Altman, J. The Human Brain during the Late First Trimesterr; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Bayer, S.A.; Altman, J. The Human Brain during the Early First Trimesterr; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- O’Rahilly, R.; Muller, F. The Embryonic Human Brain: An Atlas of Developmental Stages, 3rd ed.; Wiley-Liss: New York, NY, USA, 2006. [Google Scholar]
- Sarnat, H.B.; Born, D.E. Synaptophysin immunocytochemistry withthermal intensification: A marker of terminal axonal maturation in the human fetal nervous system. Brain Dev. 1999, 21, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Jakovcevski, I.; Zecevic, N. Sequence of oligodendrocyte development in the human fetal telencephalon. Glia 2005, 49, 480–491. [Google Scholar] [CrossRef]
- Vinci, L.; Ravarino, A.; Fanos, V.; Naccarato, A.G.; Senes, G.; Gerosa, C.; Bevilacqua, G.; Faa, G.; Ambu, R. Immunohistochemical markers of neural progenitor cells in the early embryonic human cerebral cortex. Eur. J. Histochem. 2016, 60, 2563. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Kee, N.; Sivalingam, S.; Boonstra, R.; Wojtowicz, J.M. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J.Neurosci. Methods 2002, 115, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Francis, F.; Koulakoff, A.; Boucher, D.; Chafey, P.; Schaar, B.; Vinet, M.C.; Friocourt, G.; McDonnell, N.; Reiner, O.; Kahn, A.; et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 1999, 23, 247–256. [Google Scholar] [CrossRef]
- Nacher, J.; Crespo, C.; McEwen, B.S. Doublecortin expression in the adult rat telencephalon. Eur. J. Neurosci. 2001, 14, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xiong, K.; Chu, Y.; Luo, D.W.; Luo, X.G.; Yuan, X.Y.; Struble, R.G.; Clough, R.W.; Spencer, D.D.; Williamson, A.; et al. Doublecortin expression in adult cat and primate cerebral cortex relates to immature neurons that develop into GABAergic subgroups. Exp. Neurol. 2009, 216, 342–356. [Google Scholar] [CrossRef]
- Zhang, X.M.; Cai, Y.; Chu, Y.; Chen, E.Y.; Feng, J.C.; Luo, X.G.; Xiong, K.; Struble, R.G.; Clough, R.W.; Patrylo, P.R.; et al. Doublecortin-expressing cells persist in the associative cerebral cortex and amygdala in aged nonhuman primates. Front. Neuroanat. 2009, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Verwer, R.W.; Sluiter, A.A.; Balesar, R.A.; Baayen, J.C.; Noske, D.P.; Dirven, C.M.; Wouda, J.; van Dam, A.M.; Lucassen, P.J.; Swaab, D.F. Mature astrocytes in the adult human neocortex express the early neuronal marker doublecortin. Brain 2007, 130 Pt 12, 3321–3335. [Google Scholar] [CrossRef]
- Wegner, M. Expression of transcription factors during oligodendroglial development. Microsc. Res. Tech. 2001, 52, 746–752. [Google Scholar] [CrossRef]
- Tatsumi, K.; Isonishi, A.; Yamasaki, M.; Kawabe, Y.; Morita-Takemura, S.; Nakahara, K.; Terada, Y.; Shinjo, T.; Okuda, H.; Tanaka, T.; et al. Olig2-Lineage Astrocytes: A Distinct Subtype of Astrocytes That Differs from GFAP Astrocytes. Front. Neuroanat. 2018, 12, 8. [Google Scholar] [CrossRef]
- Voigt, T.; De Lima, A.D.; Beckmann, M. Synaptophysin immunohistochemistry reveals inside-out pattern of early synaptogenesis in ferret cerebral cortex. J. Comp. Neurol. 1993, 330, 48. [Google Scholar] [CrossRef] [PubMed]
- Grabs, D.; Bergmann, M.; Schuster, T.; Fox, P.A.; Brich, M.; Gratz, V. Differential expression of synaptophysin and synaptoporin during pre- and postnatal development of the rat hippocampal network. Eur. J. Neurosci. 1994, 6, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Biranowska, J.; Dziewiatkowski, J.; Ludkiewicz, B.; Morys, J. Developmental changes of synaptic proteins expression within the hippocampal formation of the rat. J. Anat. Embryol. 2002, 206, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Takashima, S.; Takeshita, K.; Tanaka, J. Developmental changes of neuron-specific enolase in human brain: An immunohistochemical study. Brain Dev. 1985, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gasse, H.; Meyer, W. Neuron-specific enolase as a marker of hypothalamoneurohypophyseal development in postnatal Monodelphis domestica (Marsupialia). Neurosci. Lett. 1995, 189, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuys, R.; Voogd, J.; van Huijzen, C. The Human Central Nervous System, 4th ed.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Federative International Programme for Anatomical Terminology (FIPAT). Terminologia Neuroanatomica. Federative International Programme for Anatomical Terminology. 2017. Available online: https://fipat.library.dal.ca/TNA/ (accessed on 25 March 2023).
- BrainSpan Atlas of the Developing Human Brain. Available online: http://www.brainspan.org/static/atlas (accessed on 25 March 2023).
- Jin, P.P.; Xia, F.; Ma, B.F.; Li, Z.; Zhang, G.F.; Deng, Y.C.; Tu, Z.L.; Zhang, X.X.; Hou, S.X. Spatiotemporal expression of NDRG2 in the human fetal brain. Ann. Anat. 2019, 221, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Dong, J.; Zhong, S.; Wei, Y.; Wu, Q.; Yan, L.; Yong, J.; Sun, L.; Wang, X.; Zhao, Y.; et al. Spatial transcriptomic survey of human embryonic cerebral cortex by single-cell RNA-seq analysis. Cell Res. 2018, 28, 730–745. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, T.J.; Bhaduri, A.; Pollen, A.A.; Alvarado, B.; Mostajo-Radji, M.A.; Di Lullo, E.; Haeussler, M.; Sandoval-Espinosa, C.; Liu, S.J.; Velmeshev, D.; et al. Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex. Science 2017, 358, 1318–1323. [Google Scholar] [CrossRef]
- Bhaduri, A.; Sandoval-Espinosa, C.; Otero-Garcia, M.; Oh, I.; Yin, R.; Eze, U.C.; Nowakowski, T.J.; Kriegstein, A.R. An atlas of cortical arealization identifies dynamic molecular signatures. Nature 2021, 598, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Olczak, M.; Poniatowski, Ł.A.; Niderla-Bielińska, J.; Kwiatkowska, M.; Chutorański, D.; Tarka, S.; Wierzba-Bobrowicz, T. Concentration of microtubule associated protein tau (MAPT) in urine and saliva as a potential biomarker of traumatic brain injury in relationship with blood-brain barrier disruption in postmortem examination. Forensic Sci. Int. 2019, 301, 28–36. [Google Scholar] [CrossRef]
- Leibovitz, Z.; Lerman-Sagie, T.; Haddad, L. Fetal Brain Development: Regulating Processes and Related Malformations. Life 2022, 12, 809. [Google Scholar] [CrossRef]
- Flierman, S.; Tijsterman, M.; Rousian, M.; de Bakker, B.S. Discrepancies in Embryonic Staging: Towards a Gold Standard. Life 2023, 13, 1084. [Google Scholar] [CrossRef]
- Scheffer, L.K.; Meinertzhagen, I.A. The Fly Brain Atlas. Annu. Rev. Cell Dev. Biol. 2019, 35, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Court, R.; Costa, M.; Pilgrim, C.; Millburn, G.; Holmes, A.; McLachlan, A.; Larkin, A.; Matentzoglu, N.; Kir, H.; Parkinson, H.; et al. Virtual Fly Brain—An interactive atlas of the Drosophila nervous system. Front. Physiol. 2023, 14, 1076533. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proshchina, A.; Kharlamova, A.; Krivova, Y.; Godovalova, O.; Otlyga, D.; Gulimova, V.; Otlyga, E.; Junemann, O.; Sonin, G.; Saveliev, S. Neuromorphological Atlas of Human Prenatal Brain Development: White Paper. Life 2023, 13, 1182. https://doi.org/10.3390/life13051182
Proshchina A, Kharlamova A, Krivova Y, Godovalova O, Otlyga D, Gulimova V, Otlyga E, Junemann O, Sonin G, Saveliev S. Neuromorphological Atlas of Human Prenatal Brain Development: White Paper. Life. 2023; 13(5):1182. https://doi.org/10.3390/life13051182
Chicago/Turabian StyleProshchina, Alexandra, Anastasia Kharlamova, Yuliya Krivova, Olga Godovalova, Dmitriy Otlyga, Victoria Gulimova, Ekaterina Otlyga, Olga Junemann, Gleb Sonin, and Sergey Saveliev. 2023. "Neuromorphological Atlas of Human Prenatal Brain Development: White Paper" Life 13, no. 5: 1182. https://doi.org/10.3390/life13051182
APA StyleProshchina, A., Kharlamova, A., Krivova, Y., Godovalova, O., Otlyga, D., Gulimova, V., Otlyga, E., Junemann, O., Sonin, G., & Saveliev, S. (2023). Neuromorphological Atlas of Human Prenatal Brain Development: White Paper. Life, 13(5), 1182. https://doi.org/10.3390/life13051182






