Treatment with Terminalia chebula Extract Reduces Insulin Resistance, Hyperglycemia and Improves SIRT1 Expression in Type 2 Diabetic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Preparation of Aqueous Extract
2.4. Quantification of Ellagic Acid and Gallic Acid in the Aqueous Extract by HPTLC Method
2.5. Experimental Animals
2.6. Induction of Diabetes Mellitus
2.7. Pancreatic Tissue Collection and Processing
2.8. Evaluation of Parameters
2.8.1. Body Weight
2.8.2. Estimation of Plasma Glucose and Lipids
2.8.3. Estimation of Liver Enzymes
2.8.4. Estimation of Glycohemoglobin Content
2.8.5. Estimation of Glucose Tolerance and Insulin Resistance
Estimation of Oral Glucose Tolerance Test (OGTT)
Measurement of Plasma Insulin (PI), Insulin Sensitivity Index (ISI), and Homeostatic Model Assessment-Insulin Resistance (HOMA-IR)
2.8.6. Pancreatic Tissue Collection and Processing
Determination of Oxidative Stress Parameters
Histopathology of Pancreatic Tissue
Immunohistochemistry of Pancreatic Tissue
2.9. Statistical Analysis
3. Results
3.1. Identification of Ellagic Acid and Gallic Acid in Aqueous Extract of Fruits of T. chebula by HPTLC Method
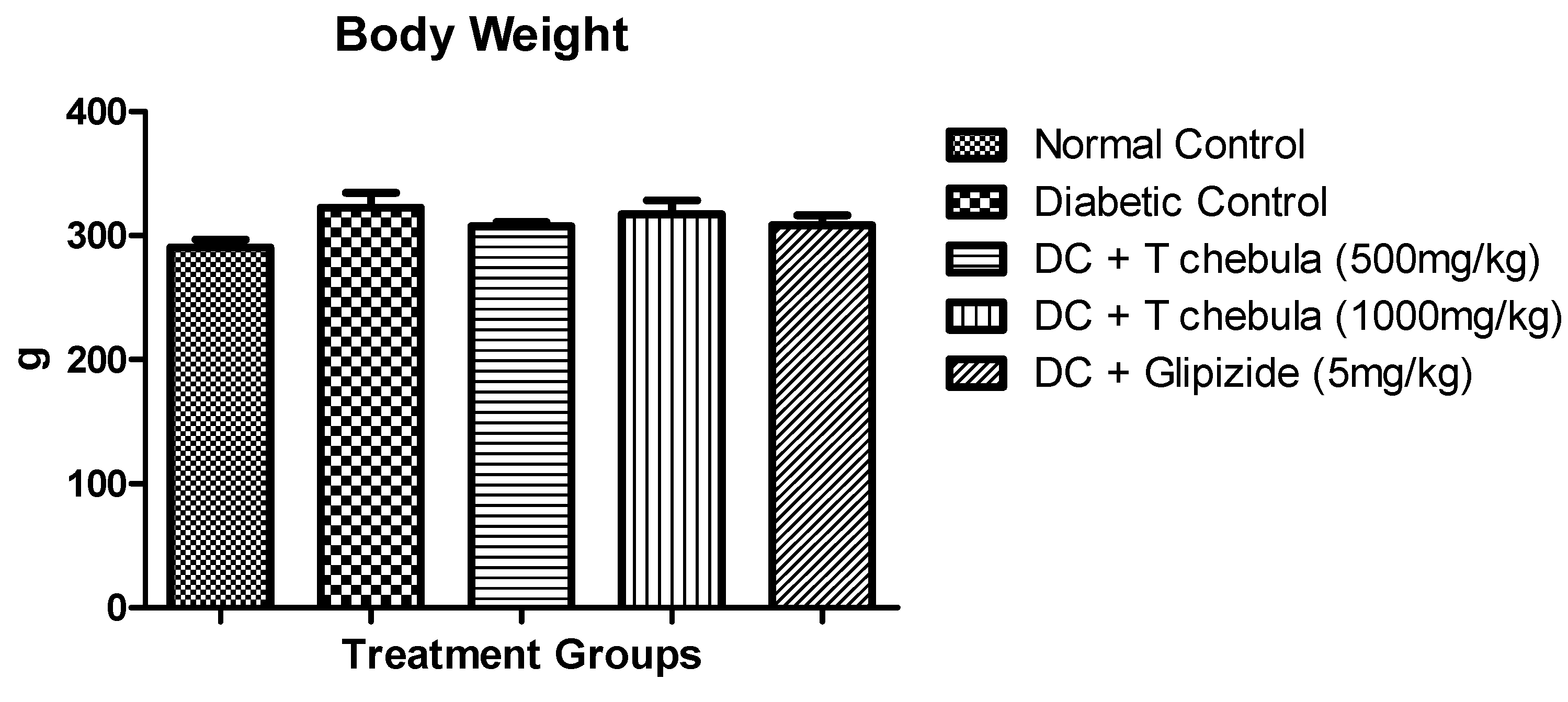
3.2. Body Weight
3.3. Effect of Terminalia chebula Extract on Plasma Glucose and Lipid Parameters in Diabetic Rats
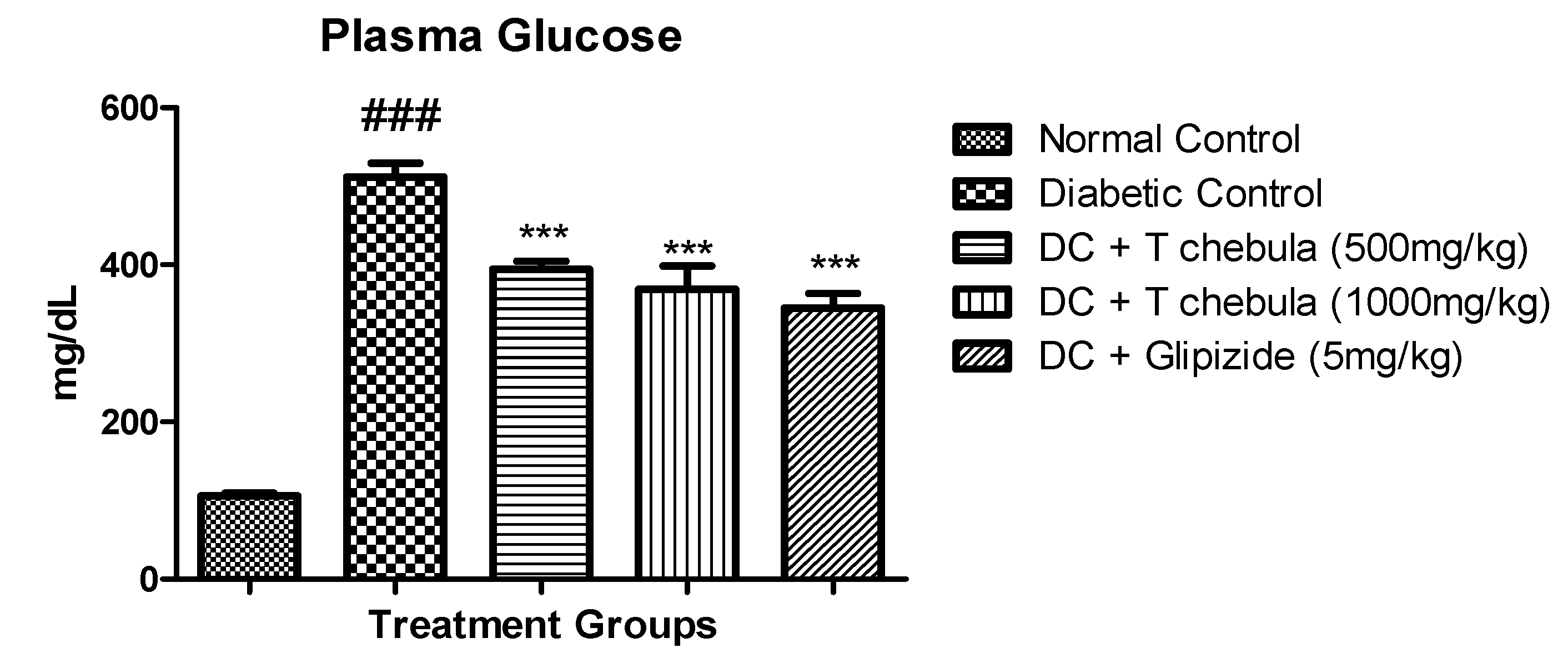
3.3.1. Plasma Glucose
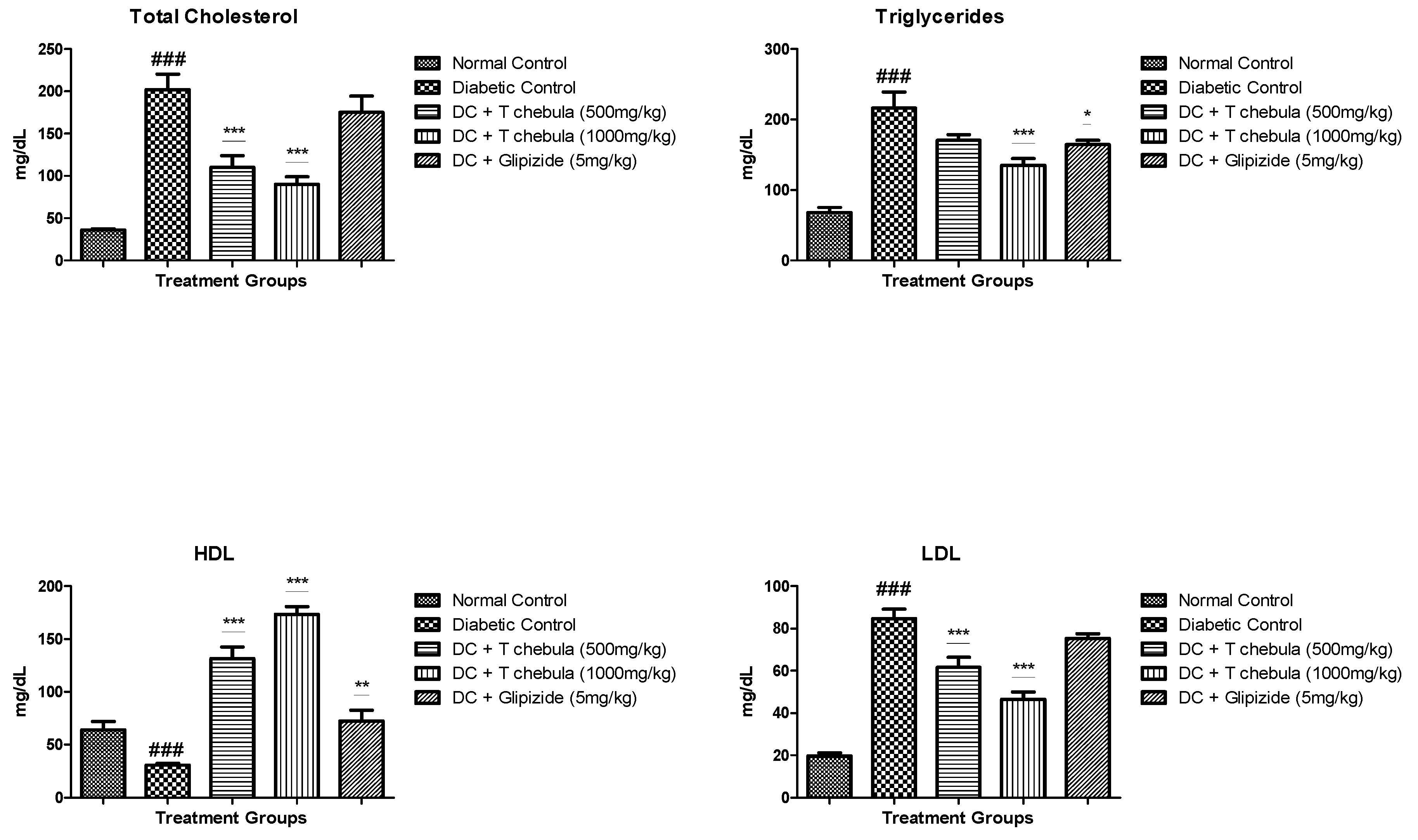
3.3.2. Lipid Parameters
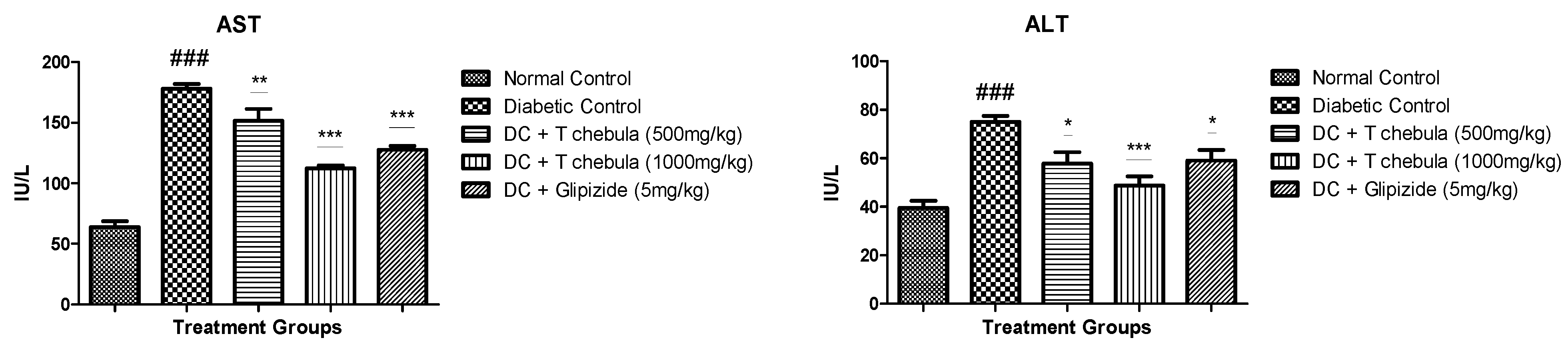
3.4. Effect of T. chebula Extract on Liver Enzymes
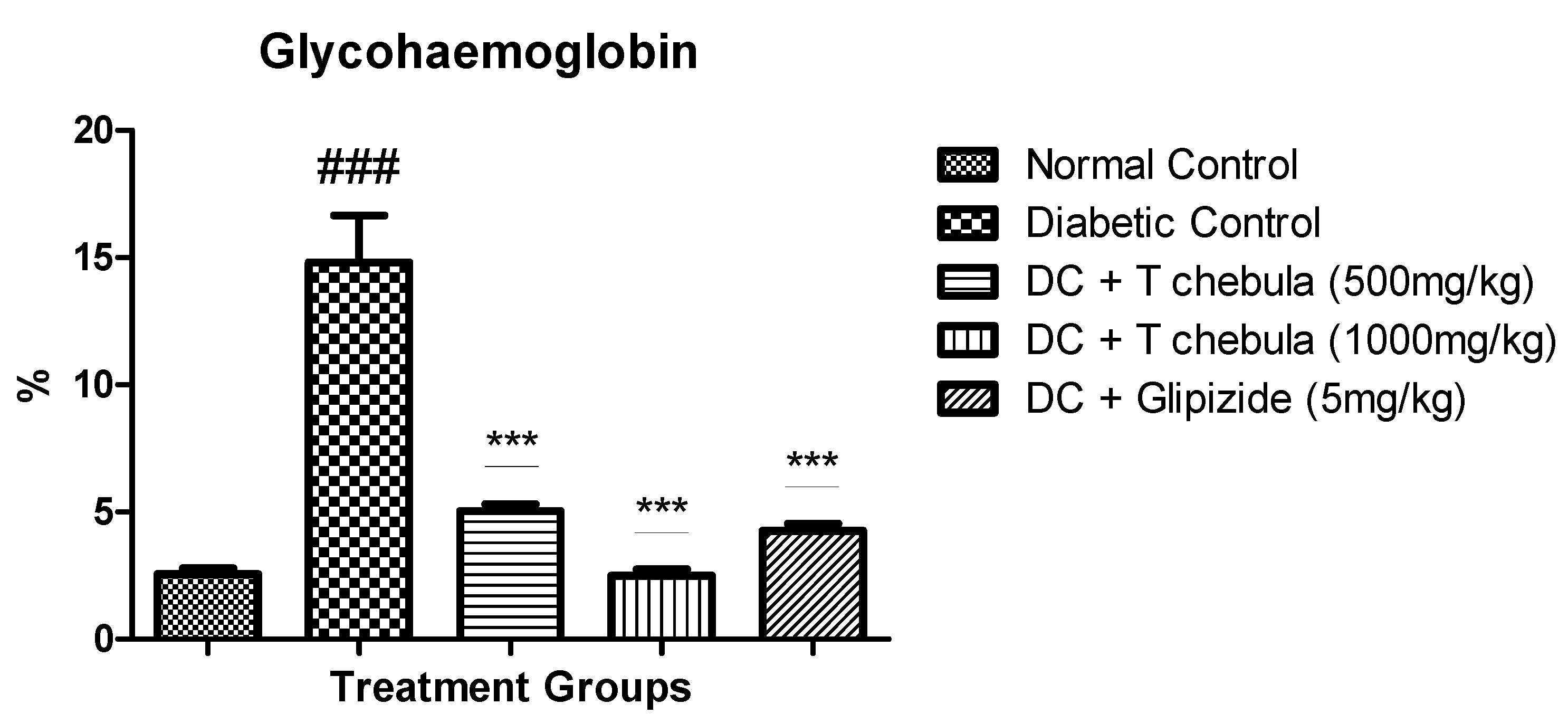
3.5. Effect of T. chebula on Glycohemoglobin
3.6. Glucose Tolerance and Insulin Resistance Measurement
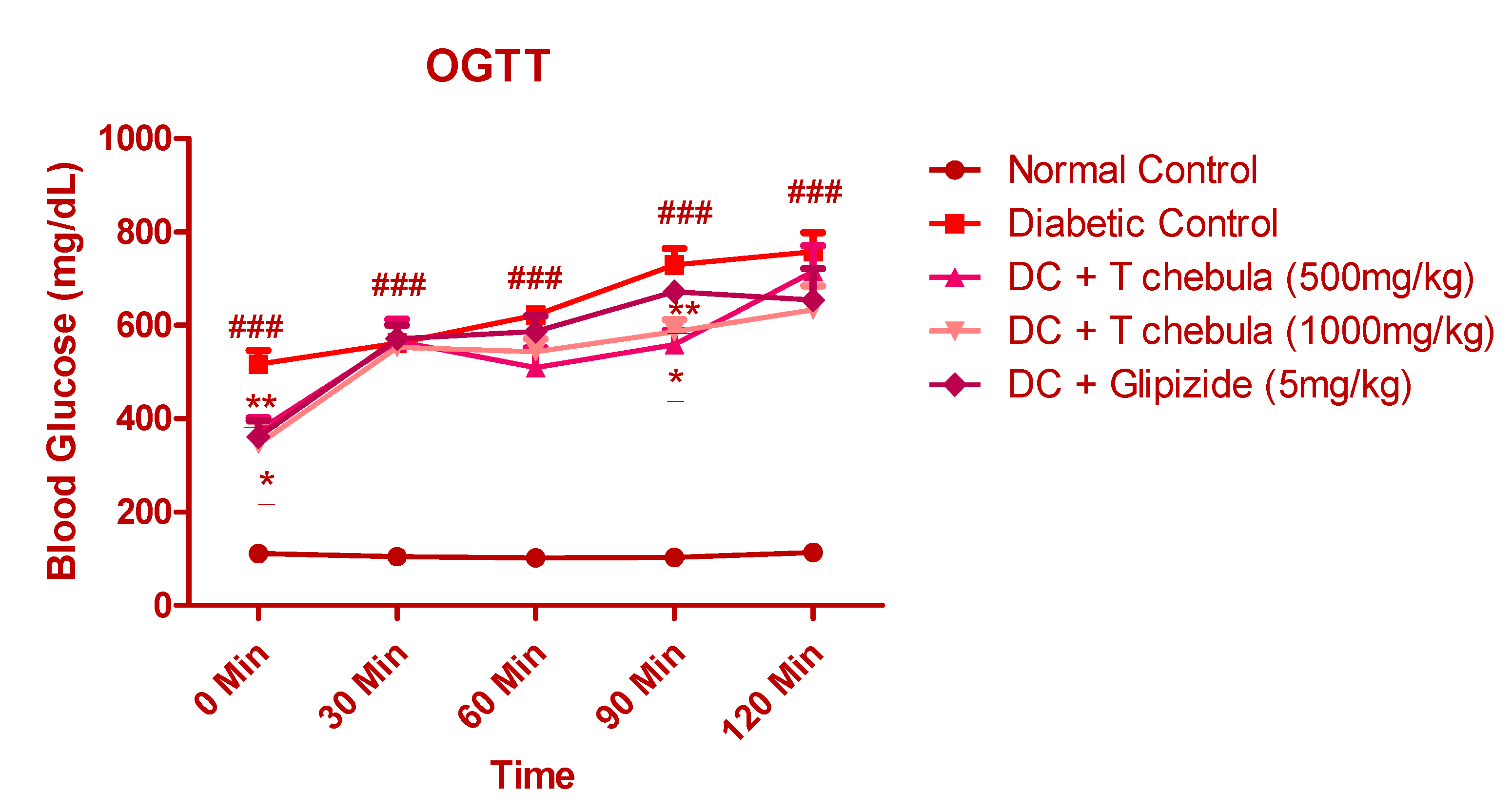
3.6.1. Effect of T. chebula on Oral Glucose Tolerance Test (OGTT)
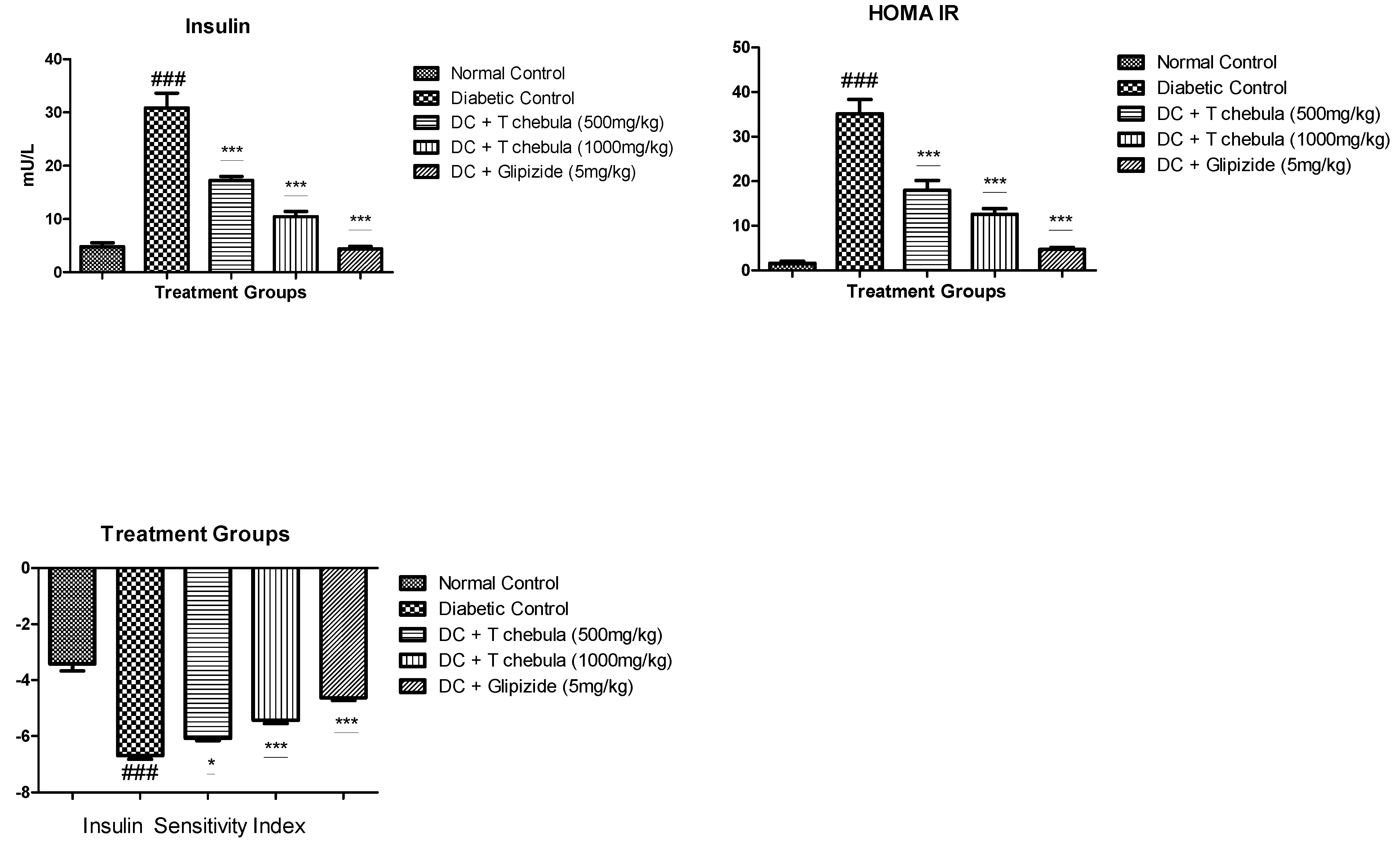
3.6.2. Measurement of Plasma Insulin (PI), Insulin Sensitivity Index (ISI), and Homeostatic Model Assessment-Insulin Resistance (HOMA-IR)
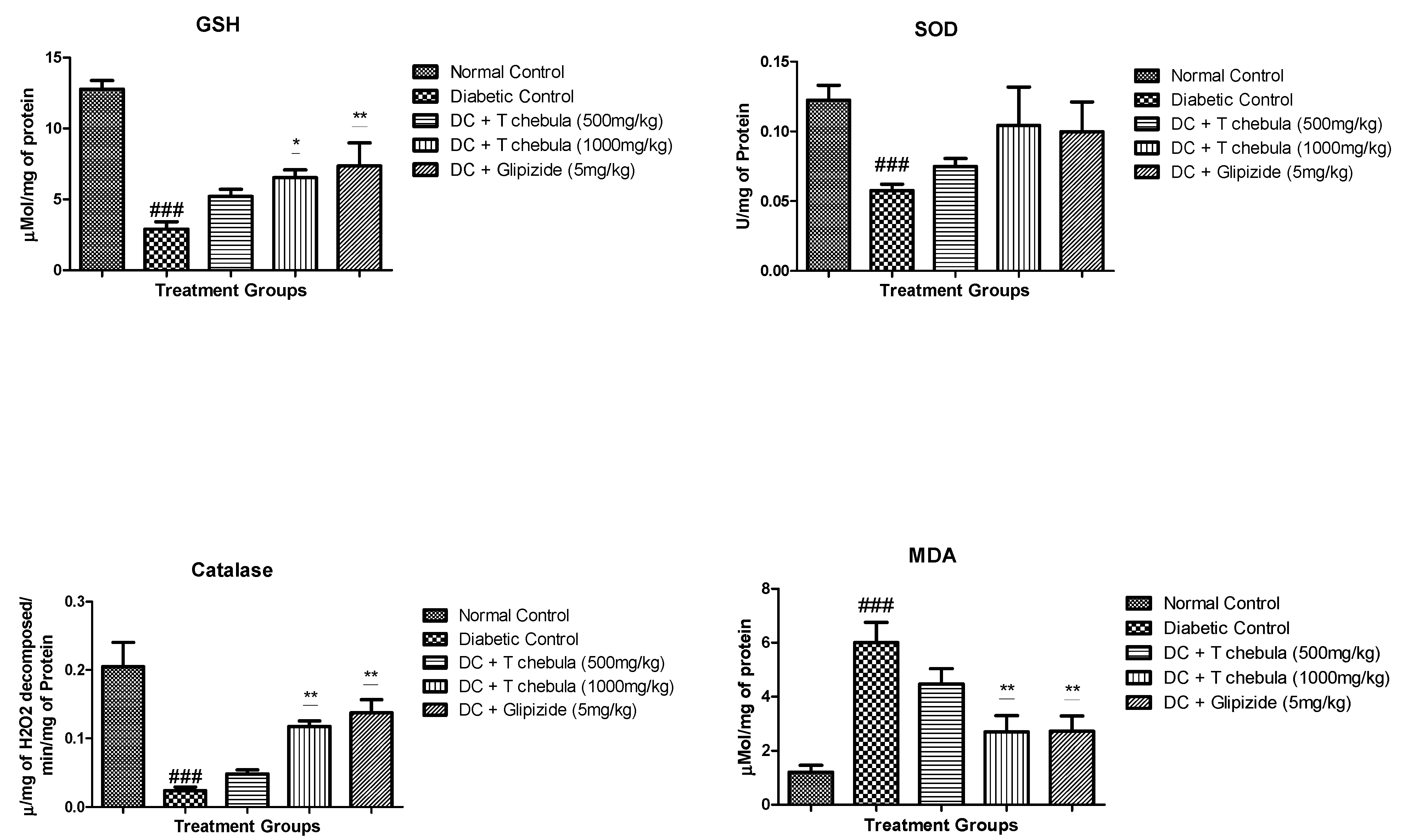
3.7. Effect of T. chebula Treatment on Oxidative Stress Parameters
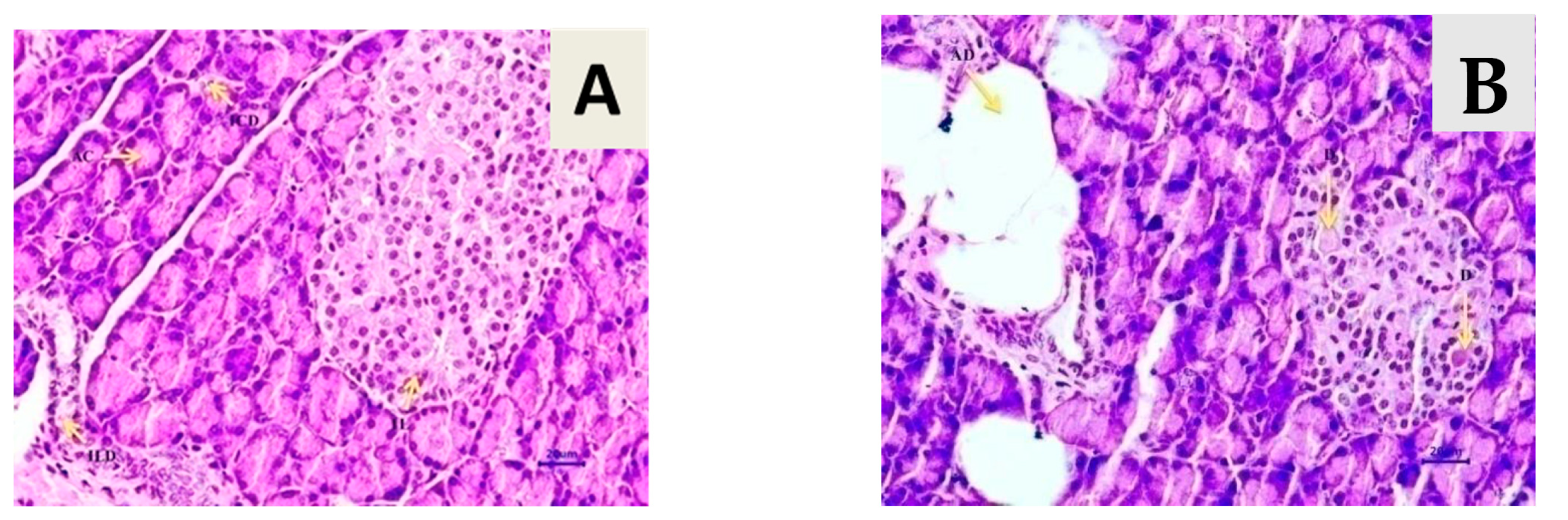
3.8. Effect of T. chebula on Histopathology of Pancreatic Tissue
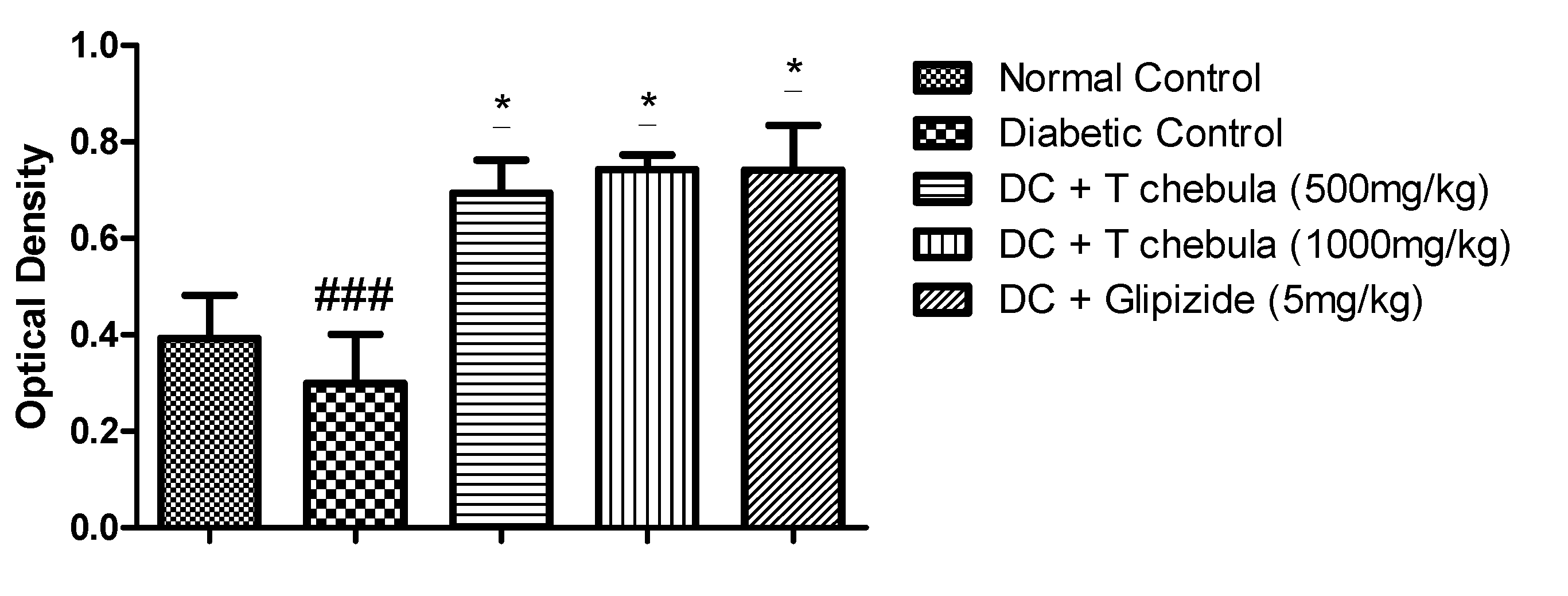
3.9. Effect of T. chebula Aqueous Extract Treatment on SIRT1 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| STZ | Streptozotocin; |
| ISI | Insulin sensitivity index; |
| HOMA-IR | Homeostatic Model Assessment of Insulin Resistance; |
| SIRT1 | Silent Mating Type Information Regulation 2 Homolog; |
| SD rats | Sprague Dawley rats; |
| TC | Total cholesterol; |
| TG | Triglyceride; |
| HDL | High-density lipoprotein; |
| LDL | Low-density lipoprotein; |
| AST | Aspartate Aminotransferase; |
| ALT | Alanine Aminotransferase; |
| H&E | Hematoxylin and Eosin; |
| IHC | Immunohistochemistry; |
| ANOVA | Analysis of variance; |
| NFBG | Non-Fasting Blood Glucose; |
| Na CMC | Sodium Carboxy Methyl Cellulose; |
| HPTLC | High-Performance Thin Layer Chromatography. |
References
- Lecka-Czernik, B. Diabetes, bone and glucose-lowering agents: Basic biology. Diabetologia 2017, 60, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation IDF Diabetes Atlas 9th edition south-east Asia. Int. Diabetes Fed. 2019, 3–4. Available online: http://www.idf.org/diabetesatlas/5e/Update2012 (accessed on 2 April 2022).
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Volume 10, ISBN 9782930229980. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017; Published online 2017. [Google Scholar]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Kiani, R. Dyslipidemia. In Practical Cardiology; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780323809153. [Google Scholar] [CrossRef]
- Jialal, I.; Singh, G. Management of diabetic dyslipidemia: An update. World J. Diabetes 2019, 10, 280–290. [Google Scholar] [CrossRef]
- Berberich, A.J.; Hegele, R.A. A Modern Approach to Dyslipidemia. Endocr. Rev. 2022, 43, 611–653. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Chait, A. Dyslipidemia in Diabetes. In Encyclopedia of Endocrine Diseases, 2nd ed.; Academic Press: Cambridge, MA, USA; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar] [CrossRef]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 diabetes mellitus: A review of current trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef]
- Freeman, J.S. The increasing epidemiology of diabetes and review of current treatment algorithms. J. Am. Osteopat. Assoc. 2010, 110 (Suppl. 7), eS2–eS6. [Google Scholar] [CrossRef]
- Eidi, A.; Eidi, M.; Esmaeili, E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine 2006, 13, 624–629. [Google Scholar] [CrossRef]
- Saikat, A.S.M.; Hossain, R.; Mina, F.B.; Das, S.; Khan, I.N.; Mubarak, M.S.; Islam, M.T. Antidiabetic Effect of Garlic. Rev. Bras. Farmacogn. 2022, 32, 1–11. [Google Scholar] [CrossRef]
- Jana, K.; Bera, T.K.; Ghosh, D. Antidiabetic effects of Eugenia jambolana in the streptozotocin-induced diabetic male albino rat. Biomark. Genom. Med. 2015, 7, 116–124. [Google Scholar] [CrossRef]
- Joseph, B.; Jini, D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop. Dis. 2013, 3, 93–102. [Google Scholar] [CrossRef]
- Sethi, J.; Sood, S.; Seth, S.; Talwar, A. Evaluation of hypoglycemic and antioxidant effect of Ocimum sanctum. Indian J. Clin. Biochem. 2004, 19, 152–155. [Google Scholar] [CrossRef]
- Suanarunsawat, T.; Anantasomboon, G.; Piewbang, C. Anti-diabetic and anti-oxidative activity of fixed oil extracted from Ocimum sanctum L. leaves in diabetic rats. Exp. Ther. Med. 2016, 11, 832–840. [Google Scholar] [CrossRef]
- Adeneye, A.A.; Amole, O.O.; Adeneye, A.K. Hypoglycemic and hypocholesterolemic activities of the aqueous leaf and seed extract of Phyllanthus amarus in mice. Fitoterapia 2006, 77, 511–514. [Google Scholar] [CrossRef]
- Raphael, K.R.; Sabu, M.C.; Kuttan, R. Hypoglycemic effect of methanol extract of Phyllanthus amarus Schum & Thonn on alloxan induced diabetes mellitus in rats and its relation with antioxidant potential. Indian J. Exp. Biol. 2002, 40, 905–909. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12597020 (accessed on 22 April 2020).
- Lawson-Evi, P.; Eklu-Gadeg, K.; Agbonon, A.; Aklikokou, K.; Creppy, E.; Gbeassor, M. Antidiabetic Activity of Phyllanthus amarus Schum and Thonn (Euphorbiaceae) on Alloxan Induced Diabetes in Male Wistar Rats. J. Appl. Sci. 2011, 11, 2968–2973. [Google Scholar] [CrossRef]
- Dhanabal, S.P.; Kokate, C.K.; Ramanathan, M.; Kumar, E.P.; Suresh, B. Hypoglycaemic activity of Pterocarpus marsupium Roxb. Phyther. Res. 2006, 20, 4–8. [Google Scholar] [CrossRef]
- Joladarashi, D.; Chilkunda, N.D.; Salimath, P.V. Glucose uptake-stimulatory activity of Tinospora cordifolia stem extracts in Ehrlich ascites tumor cell model system. J. Food Sci. Technol. 2014, 51, 178–182. [Google Scholar] [CrossRef]
- Geberemeskel, G.A.; Debebe, Y.G.; Nguse, N.A. Antidiabetic Effect of Fenugreek Seed Powder Solution (Trigonella foenum-graecum L.) on Hyperlipidemia in Diabetic Patients. J. Diabetes Res. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Gorelick, J.; Rosenberg, R.; Smotrich, A.; Hanuš, L.; Bernstein, N. Hypoglycemic activity of withanolides and elicitated Withania somnifera. Phytochemistry 2015, 116, 283–289. [Google Scholar] [CrossRef]
- Ali, Z.; Khan, I. Chemical Constituents of Terminalia chebula. Planta Med. 2009, 75, 41. [Google Scholar] [CrossRef]
- Sotoudeh, R.; Hadjzadeh, M.-A.-R.; Gholamnezhad, Z.; Aghaei, A. The anti-diabetic and antioxidant effects of a combination of Commiphora mukul, Commiphora myrrha and Terminalia chebula in diabetic rats. Avicenna J. Phytomed. 2019, 9, 454–464. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31516859 (accessed on 7 April 2020). [PubMed]
- Kumar, G.P.S.; Arulselvan, P.; Kumar, D.S.; Subramanian, S.P. Anti-diabetic activity of fruits of Terminalia chebula on streptozotocin induced diabetic rats. J. Health Sci. 2006, 52, 283–291. [Google Scholar] [CrossRef]
- Sasidharan, I.; Sundaresan, A.; Nisha, V.M.; Kirishna, M.S.; Raghu, K.G.; Jayamurthy, P. Inhibitory effect of Terminalia chebula Retz. fruit extracts on digestive enzyme related to diabetes and oxidative stress. J. Enzyme Inhib. Med. Chem. 2012, 27, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Moradi, B.; Abbaszadeh, S.; Shahsavari, S.; Alizadeh, M.; Beyranvand, F. The most useful medicinal herbs to treat diabetes. Biomed. Res. Ther. 2018, 5, 2538–2551. [Google Scholar] [CrossRef]
- Kim, H.G.; Lee, J.S.; Lee, J.S.; Han, J.M.; Son, C.G. Hepatoprotective and antioxidant effects of Myelophil on restraint stress-induced liver injury in BALB/c mice. J. Ethnopharmacol. 2012, 142, 113–120. [Google Scholar] [CrossRef]
- Choi, M.K.; Kim, H.G.; Han, J.M.; Lee, J.S.; Lee, J.S.; Chung, S.H.; Son, C.G. Hepatoprotective Effect of Terminalia chebula against t-BHP-Induced Acute Liver Injury in C57/BL6 Mice. Evid. Based Complement. Altern. Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Pfundstein, B.; El Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef]
- Yakaew, S.; Itsarasook, K.; Ngoenkam, J.; Jessadayannamaetha, A.; Viyoch, J.; Ungsurungsie, M. Ethanol extract of Terminalia chebula fruit protects against UVB-induced skin damage. Pharm. Biol. 2016, 54, 2701–2707. [Google Scholar] [CrossRef]
- Lee, H.S.; Won, N.H.; Kim, K.H.; Lee, H.; Jun, W.; Lee, K.W. Antioxidant effects of aqueous extract of Terminalia chebula in Vivo and in Vitro. Biol. Pharm. Bull. 2005, 28, 1639–1644. [Google Scholar] [CrossRef]
- Naik, G.H.; Priyadarsini, K.I.; Naik, D.B.; Gangabhagirathi, R.; Mohan, H. Studies on the aqueous extract of Terminalia chebula as a potent antioxidant and a probable radioprotector. Phytomedicine 2004, 11, 530–538. [Google Scholar] [CrossRef]
- Saha, S.; Verma, R.J. Antioxidant activity of polyphenolic extract of Terminalia chebula Retzius fruits. J. Taibah Univ. Sci. 2016, 10, 805–812. [Google Scholar] [CrossRef]
- Chang, C.L.; Lin, C.S. Phytochemical composition, antioxidant activity, and neuroprotective effect of Terminalia chebula Retzius extracts. Evid. Based Complement. Altern. Med. 2012, 2012, 125247. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Lin, T.C.; Yu, K.H.; Yang, C.M.; Lin, C.C. Antioxidant and free radical scavenging activities of Terminalia chebula. Biol. Pharm. Bull. 2003, 26, 1331–1335. [Google Scholar] [CrossRef]
- Sheng, Z.; Zhao, J.; Muhammad, I.; Zhang, Y. Optimization of total phenolic content from Terminalia chebula Retz. fruits using response surface methodology and evaluation of their antioxidant activities. PLoS ONE 2018, 13, e0202368. [Google Scholar] [CrossRef]
- Li, K.; Diao, Y.; Zhang, H.; Wang, S.; Zhang, Z.; Yu, B.; Huang, S.; Yang, H. Tannin extracts from immature fruits of Terminalia chebula Fructus Retz. promote cutaneous wound healing in rats. BMC Complement. Altern. Med. 2011, 11, 86. [Google Scholar] [CrossRef]
- Suguna, L.; Singh, S.; Sivakumar, P.; Sampath, P.; Chandrakasan, G. Influence of Terminalia chebula on dermal wound healing in rats. Phyther. Res. 2002, 16, 227–231. [Google Scholar] [CrossRef]
- Ali, S.K.; Hamed, A.R.; Soltan, M.M.; Hegazy, U.M.; Elgorashi, E.E.; El-Garf, I.A.; Hussein, A.A. In-vitro evaluation of selected Egyptian traditional herbal medicines for treatment of Alzheimer disease. BMC Complement. Altern. Med. 2013, 13, 121. [Google Scholar] [CrossRef]
- Kalra, P.; Karwasra, R.; Gupta, Y.K.; Ray, S.B.; Singh, S. Terminalia chebula supplementation attenuates cisplatin-induced nephrotoxicity in Wistar rats through modulation of apoptotic pathway. Nat. Prod. Res. 2019, 33, 1641–1645. [Google Scholar] [CrossRef]
- Ding, G.; Lu, Y.R.; Ji, C.R.; Liu, Y.Z. Analysis of tannins in Fructus Chebulae and its confusion varieties by HPCE. Yaoxue Xuebao 2001, 36, 294–295. Available online: https://pubmed.ncbi.nlm.nih.gov/12580059/ (accessed on 11 April 2020).
- Juang, L.-J.; Sheu, S.-J.; Lin, T.-C. Determination of hydrolyzable tannins in the fruit of Terminalia chebula Retz. by high-performance liquid chromatography and capillary electrophoresis. J. Sep. Sci. 2004, 27, 718–724. [Google Scholar] [CrossRef]
- Kundu, A.P.; Mahato, S.B. Triterpenoids and their glycosides from Terminalia chebula. Phytochemistry 1993, 32, 999–1002. [Google Scholar] [CrossRef]
- Kulkarni, Y.A.; Panjabi, R.; Patel, V.; Tawade, A.; Gokhale, A. Effect of Gmelina arborea Roxb in experimentally induced infl ammation and nociception. J. Ayurveda Integr. Med. 2013, 4, 152–157. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, Y.A.; Garud, M.S. Bauhinia variegata (Caesalpiniaceae) leaf extract: An effective treatment option in type I and type II diabetes. Biomed. Pharmacother. 2016, 83, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Paoletti, F.; Mocali, A.; Aldinucci, D. Superoxide-driven NAD(P)H oxidation induced by EDTA-manganese complex and mercaptoethanol. Chem. Biol. Interact. 1990, 76, 3–18. [Google Scholar] [CrossRef]
- Garud, M.S.; Kulkarni, Y.A. Eugenol ameliorates renal damage in streptozotocin-induced diabetic rats. Flavour Fragr. J. 2017, 32, 54–62. [Google Scholar] [CrossRef]
- Oza, M.J.; Kulkarni, Y.A. Formononetin treatment in type 2 diabetic rats reduces insulin resistance and hyperglycemia. Front. Pharmacol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef]
- Murata, M.; Takahashi, A.; Saito, I.; Kawanishi, S. Site-specific DNA methylation and apoptosis: Induction by diabetogenic streptozotocin. Biochem. Pharmacol. 1999, 57, 881–887. [Google Scholar] [CrossRef]
- LeDoux, S.P.; Woodley, S.E.; Patton, N.J.; Wilson, G.L. Mechanisms of nitrosourea-induced β-cell damage: Alterations in DNA. Diabetes 1986, 35, 866–872. [Google Scholar] [CrossRef]
- Båvenholm, P.N.; Pigon, J.; Östenson, C.G.; Efendic, S. Insulin sensitivity of suppression of endogenous glucose production is the single most important determinant of glucose tolerance. Diabetes 2001, 50, 1449–1454. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Chen, M.Z.; Olefsky, J.M.; Marth, J.D. Pathway to diabetes through attenuation of pancreatic beta cell glycosylation and glucose transport. Nat. Med. 2011, 17, 1067–1075. [Google Scholar] [CrossRef]
- Gheibi, S.; Kashfi, K.; Ghasemi, A. A practical guide for induction of type-2 diabetes in rat: Incorporating a high-fat diet and streptozotocin. Biomed. Pharmacother. 2017, 95, 605–613. [Google Scholar] [CrossRef]
- Zhang, F.L.; Ye, C.; Li, G.; Ding, W.; Zhou, W.; Zhu, H.; Chen, G.; Luo, T.; Guang, M.; Liu, Y.; et al. The rat model of type 2 diabetic mellitus and its glycometabolism characters. Exp. Anim. 2003, 52, 401–407. [Google Scholar] [CrossRef]
- Reaven, G.M. Insulin resistance, hyperinsulinemia, hypertriglyceridemia, and hypertension: Parallels between human disease and rodent models. Diabetes Care 1991, 14, 195–202. [Google Scholar] [CrossRef]
- Eltimamy, M.; Elshamarka, M.; Aboelsaad, M.; Sayed, M.; Moawad, H. Effects of alcoholic extract of Terminalia chebula dried fruit on blood biochemical profile in diabetic rats. J. Diabetes Metab. Disord. 2022, 21, 159–170. [Google Scholar] [CrossRef]
- Kannan, V.R.; Rajasekar, G.S.; Rajesh, P.; Balasubram, V.; Ramesh, N.; Solomon, E.K.; Nivas, D.; Chandru, S. Anti-diabetic Activity on Ethanolic Extracts of Fruits of Terminalia chebula Retz. Alloxan Induced Diabetic Rats. Am. J. Drug Discov. Dev. 2012, 2, 135–142. [Google Scholar] [CrossRef]
- Hirano, T. Pathophysiology of diabetic dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef]
- Shanik, M.H.; Xu, Y.; Skrha, J.; Dankner, R.; Zick, Y.; Roth, J. Insulin resistance and hyperinsulinemia: Is hyperinsulinemia the cart or the horse? Diabetes Care 2008, 31 (Suppl. 2), S262–S268. [Google Scholar] [CrossRef] [PubMed]
- Krauss, R.M. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 2004, 27, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.; Nazratun Nafizah, A.H.; Zariyantey, A.H.; Budin, S.B. Mechanisms of diabetes-induced liver damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132–e141. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, J.; Wen, J.; Ming, Q.; Zhang, J.; Xu, Y. Correlation of serum alanine aminotransferase and aspartate aminotransferase with coronary heart disease. Int. J. Clin. Exp. Med. 2015, 8, 4399–4404. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4443194/ (accessed on 1 September 2022). [PubMed]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark. Insights 2016, 11, 95–104. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Is there a relationship between mean blood glucose and glycated hemoglobin? J. Diabetes Sci. Technol. 2011, 5, 1572–1583. [Google Scholar] [CrossRef]
- Tsai, K.; Wang, S.S.; Chen, T.S.; Kong, C.W.; Chang, F.Y.; Lee, S.D.; Lu, F.J. Oxidative stress: An important phenomenon with pathogenetic significance in the progression of acute pancreatitis. Gut 1998, 42, 850–855. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Pandey, K.B.; Abidi, A.B.; Rizvi, S.I. Markers of Oxidative Stress during Diabetes Mellitus. J. Biomark. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Schoenberg, M.H.; Büchler, M.; Baczako, K.; Bültmann, B.; Younes, M.; Gasper, M.; Kirchmayr, R.; Beger, H.G. The involvement of oxygen radicals in acute pancreatitis. Klin. Wochenschr. 1991, 69, 1025–1031. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Arigela, C.S.; Gan, S.H.; Salam, S.K.N.; Krishnan, K.T.; Rahman, N.A.; Jeffree, M.S. A review on oxidative stress, diabetic complications, and the roles of honey polyphenols. Oxidative Med. Cell. Longev. 2020, 2020, 8878172. [Google Scholar] [CrossRef]
- Shoba, B.; Lwin, Z.M.; Ling, L.S.; Bay, B.H.; Yip, G.W.; Kumar, S.D. Function of sirtuins in biological tissues. Anat. Rec. 2009, 292, 536–543. [Google Scholar] [CrossRef]
- Pulla, V.K.; Battu, M.B.; Alvala, M.; Sriram, D.; Yogeeswari, P. Can targeting SIRT-1 to treat type 2 diabetes be a good strategy? A review. Expert Opin. Ther. Targets 2012, 16, 819–832. [Google Scholar] [CrossRef]

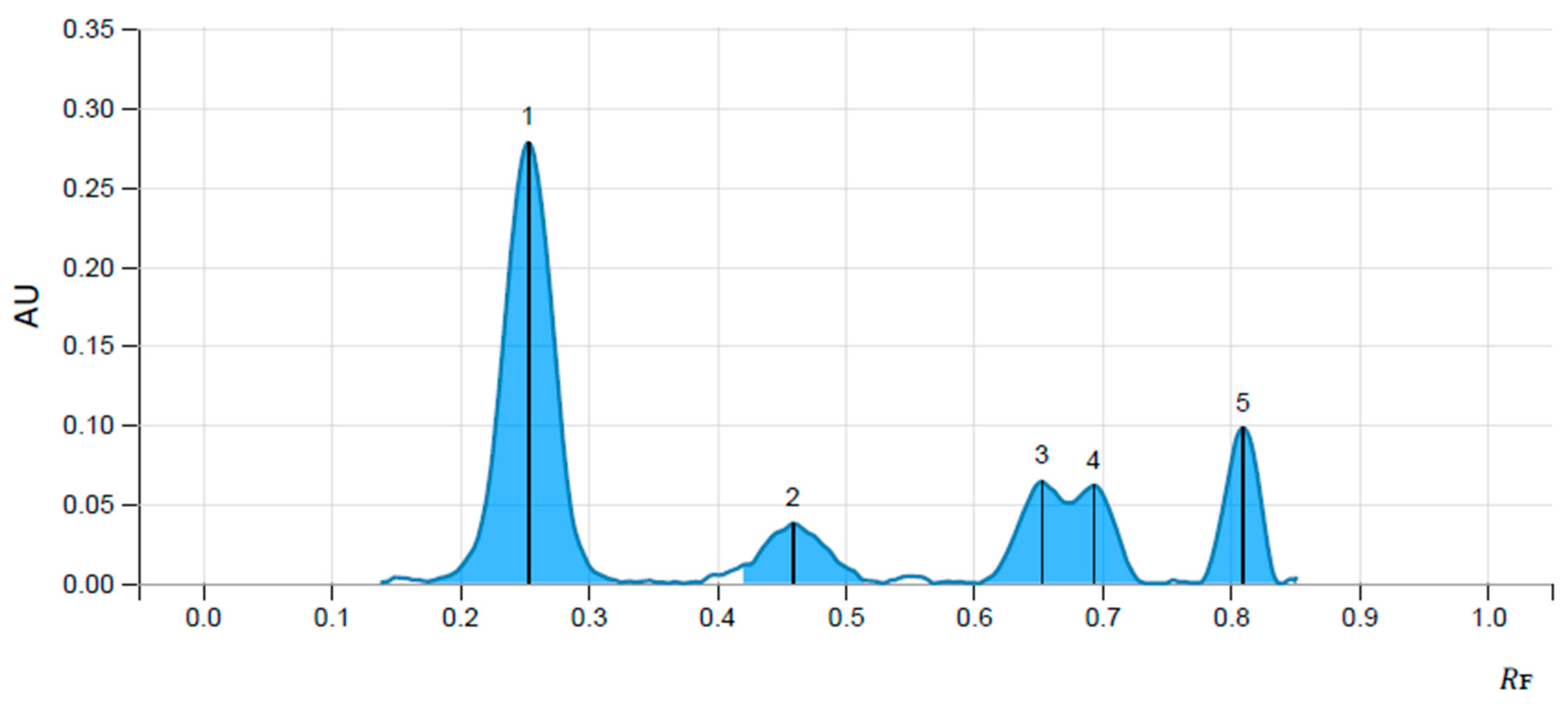


| Name of Marker | Concentration µg/mL | Peak Area | Volume of Solution µL | Concentration |
|---|---|---|---|---|
| Ellagic acid | 100 | 0.01724 | 8 | 0.0018 |
| Gallic acid | 100 | 0.00498 | 8 | 0.0077 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, O.D.; Kulkarni, Y.A. Treatment with Terminalia chebula Extract Reduces Insulin Resistance, Hyperglycemia and Improves SIRT1 Expression in Type 2 Diabetic Rats. Life 2023, 13, 1168. https://doi.org/10.3390/life13051168
Agrawal OD, Kulkarni YA. Treatment with Terminalia chebula Extract Reduces Insulin Resistance, Hyperglycemia and Improves SIRT1 Expression in Type 2 Diabetic Rats. Life. 2023; 13(5):1168. https://doi.org/10.3390/life13051168
Chicago/Turabian StyleAgrawal, Ojaskumar D., and Yogesh A. Kulkarni. 2023. "Treatment with Terminalia chebula Extract Reduces Insulin Resistance, Hyperglycemia and Improves SIRT1 Expression in Type 2 Diabetic Rats" Life 13, no. 5: 1168. https://doi.org/10.3390/life13051168
APA StyleAgrawal, O. D., & Kulkarni, Y. A. (2023). Treatment with Terminalia chebula Extract Reduces Insulin Resistance, Hyperglycemia and Improves SIRT1 Expression in Type 2 Diabetic Rats. Life, 13(5), 1168. https://doi.org/10.3390/life13051168






