Application of Lactobacillus reuteri B1/1 (Limosilactobacillus reuteri) Improves Immunological Profile of the Non-Carcinogenic Porcine-Derived Enterocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.1.1. Growth and Maintenance
2.1.2. Bacterial Strain Growth and Maintenance
2.2. Viability of the Cell Line CLAB after Exposure to Probiotic Bacteria (MTT Assay)
2.3. Adhesion Assay of L. reuteri B1/1 and L. rhamnosus GG (LGG) to CLAB Cells
2.4. Design of the Experiment
2.5. Measurement of Gene Expression
2.5.1. Isolation of Total RNA from Cell Culture
2.5.2. Quantitative Real-Time PCR
2.6. Statistical Analyses
2.6.1. Viability of Cell Line CLAB after Exposure to Probiotic Bacteria (MTT Assay)
2.6.2. Relative Gene Expression
3. Results
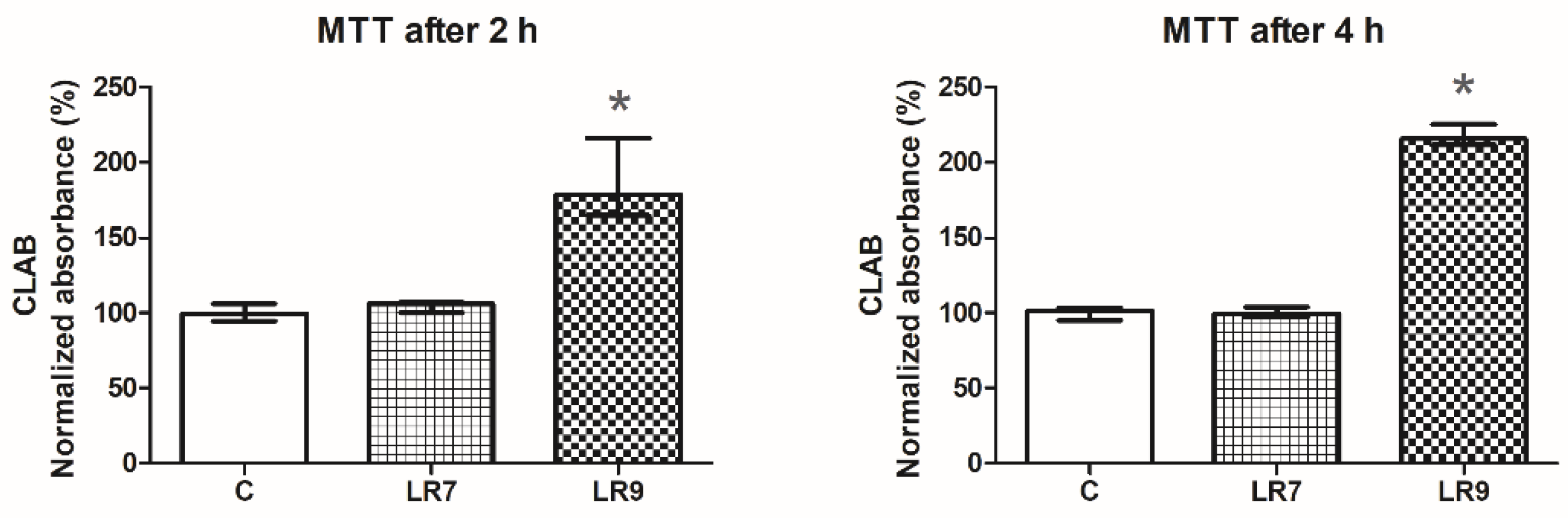
3.1. Viability of the Cell Line CLAB after Exposure to Probiotic Bacteria (MTT Assay)
3.2. Adhesion Assay
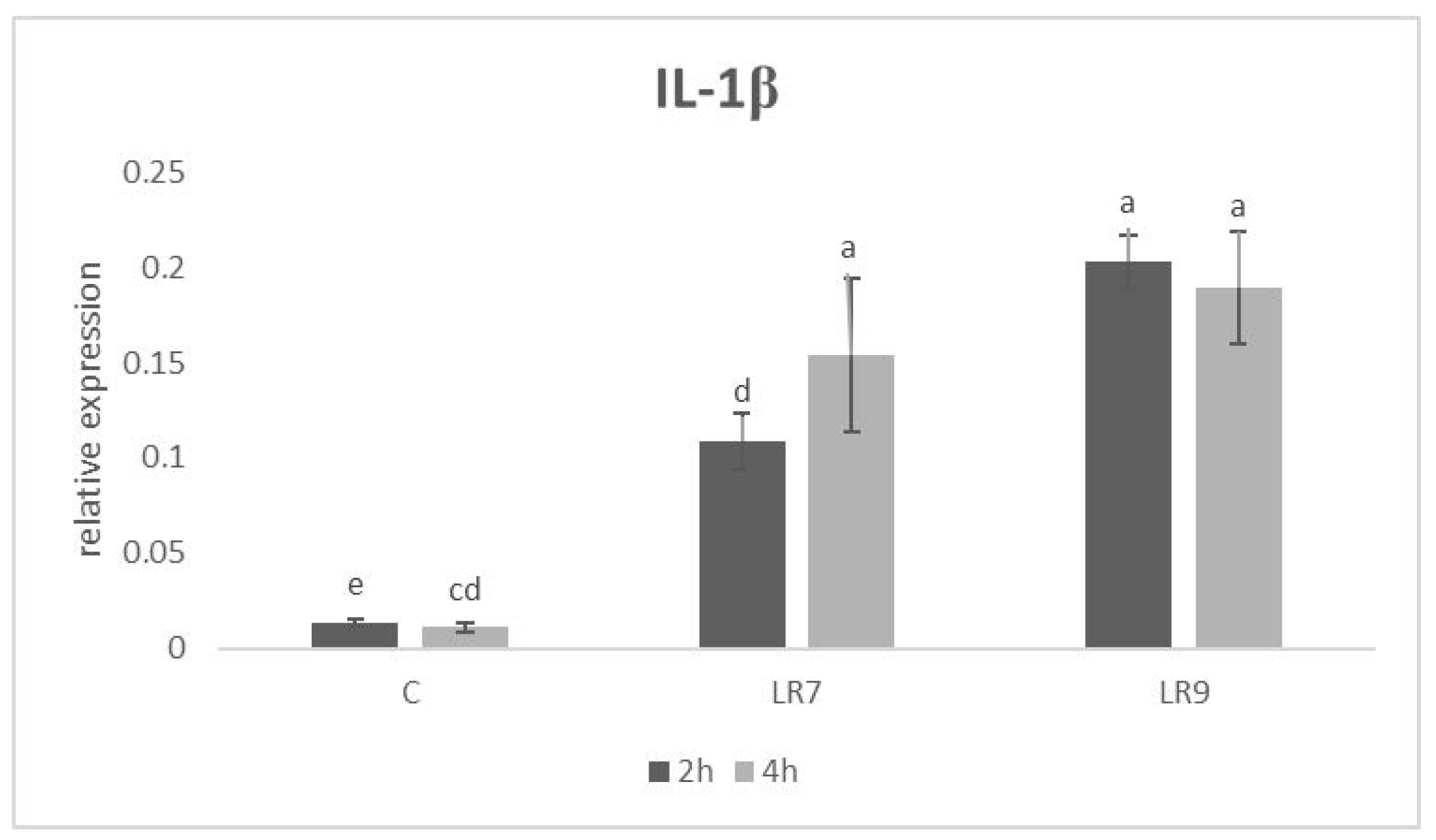
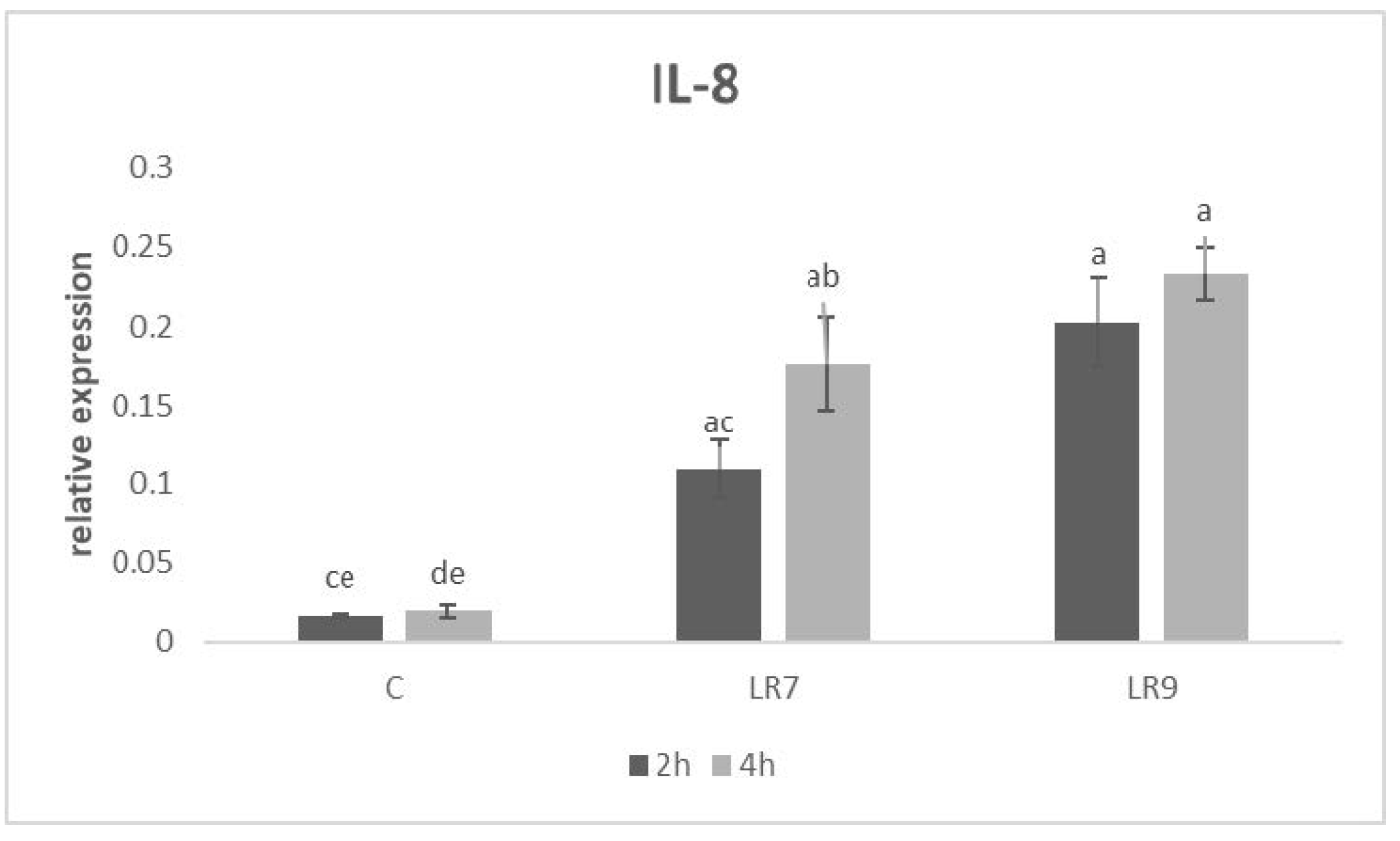
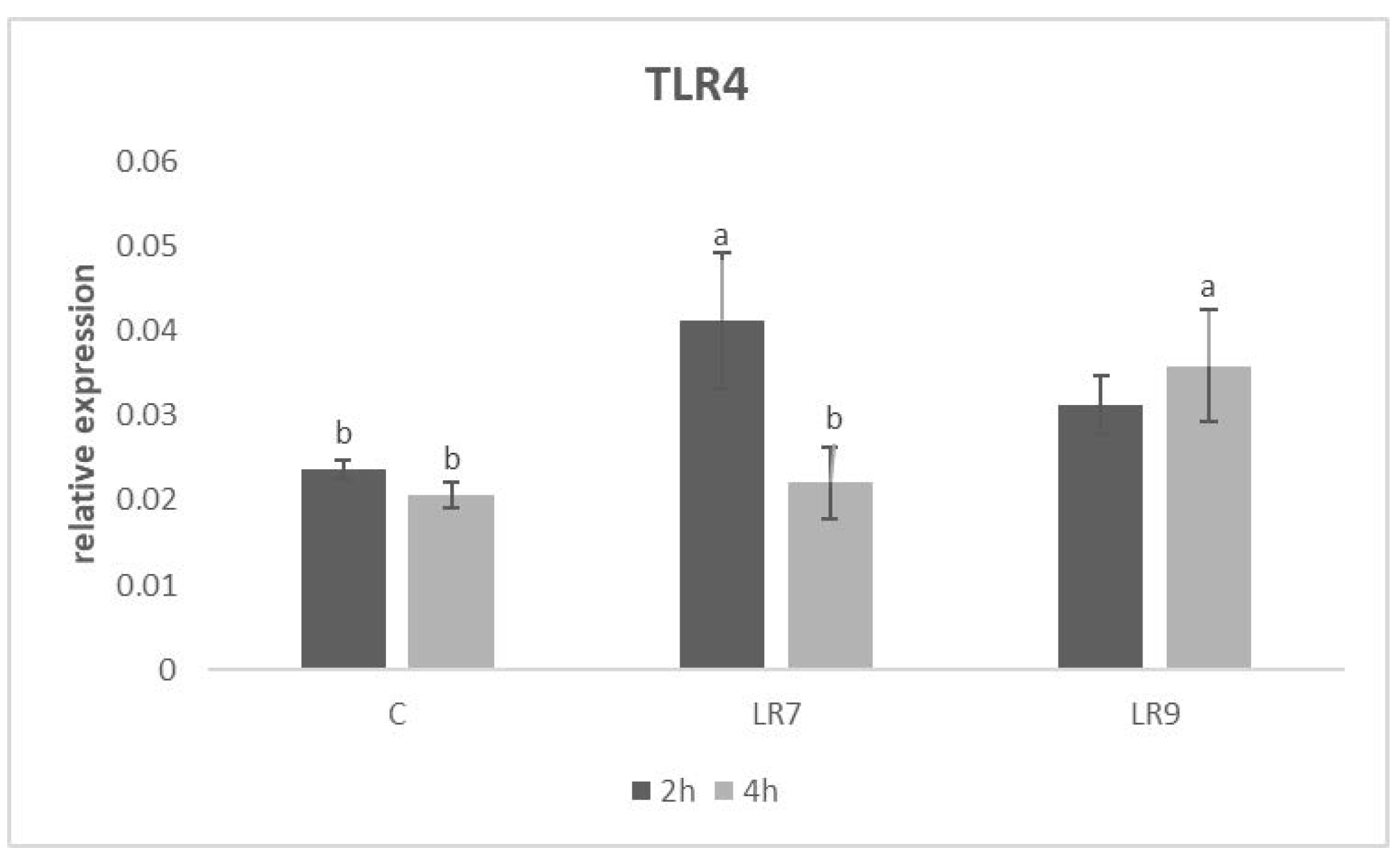
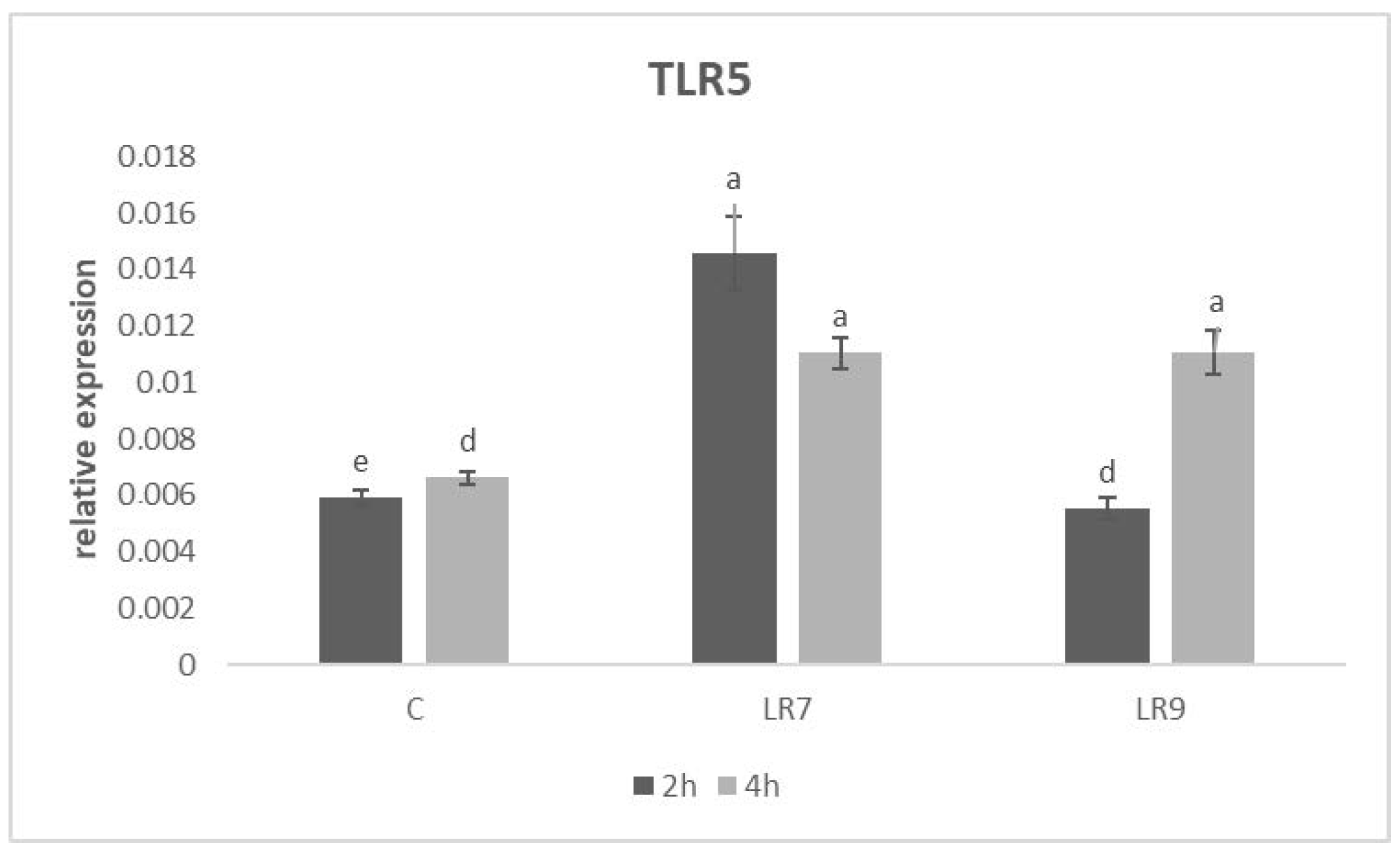
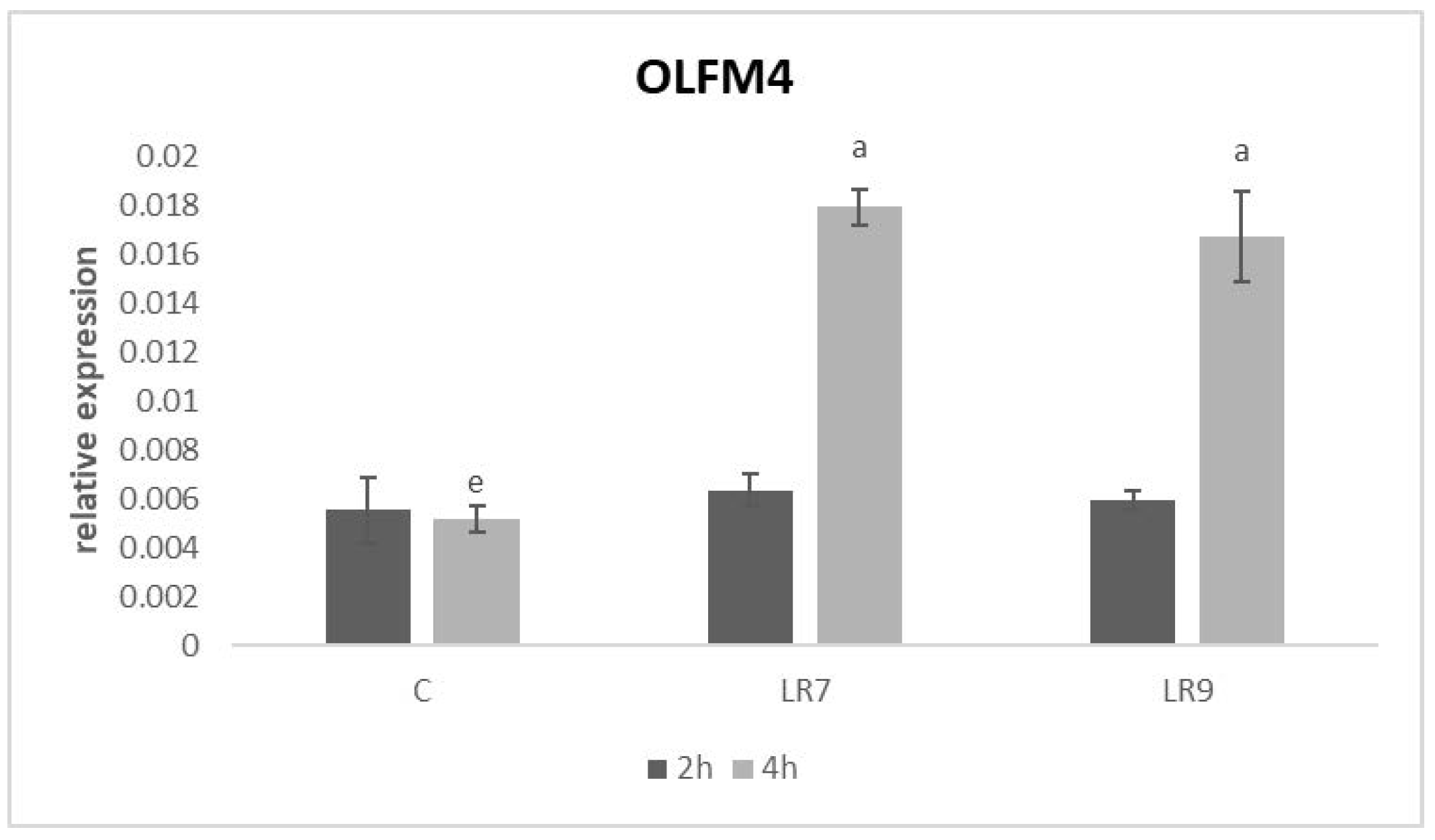
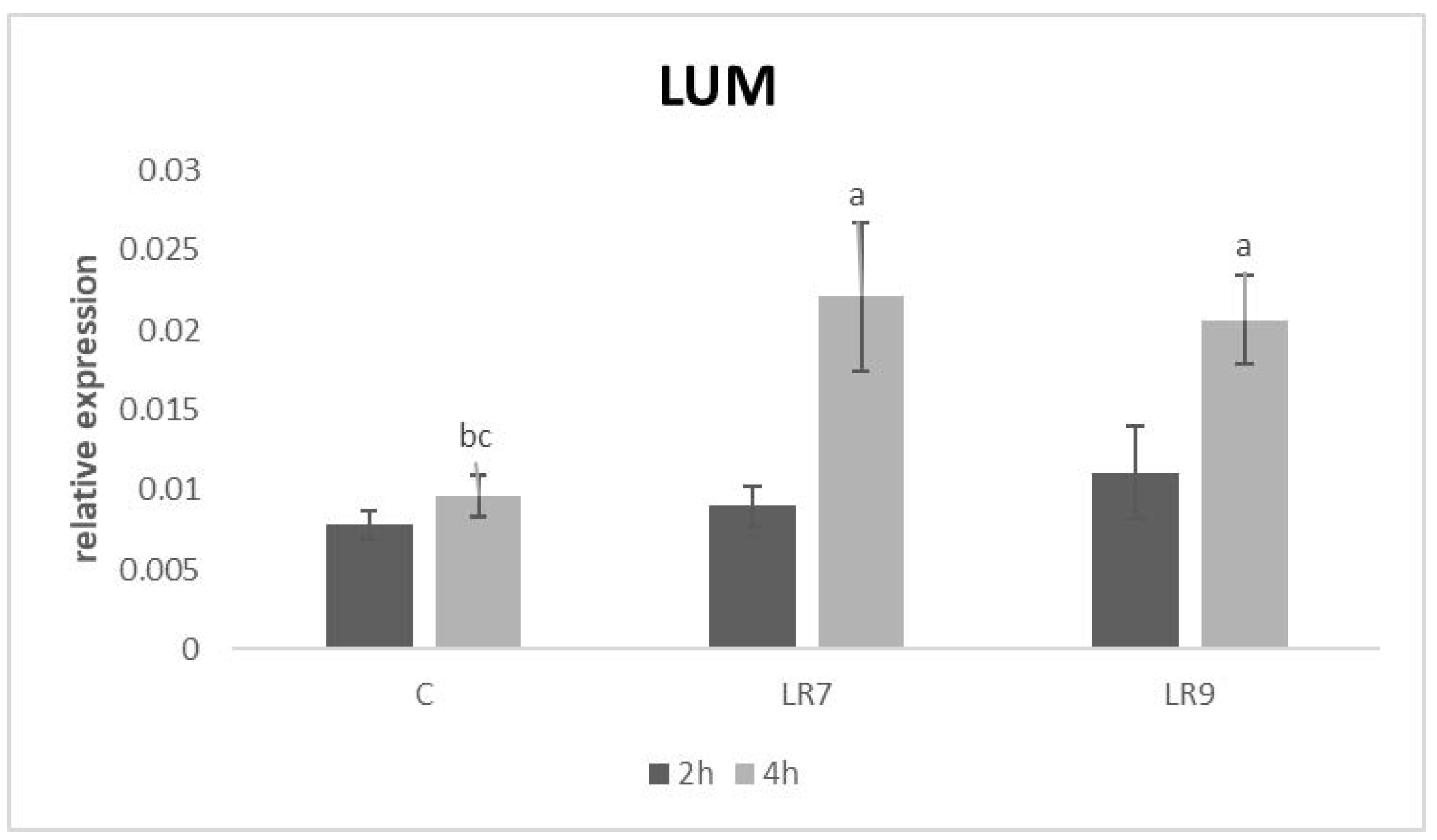
3.3. Relative Expression for Selected Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Várhidi, Z.; Máté, M.; Ózsvári, L. The use of probiotics in nutrition and herd health management in large hungarian dairy cattle farms. Front. Vet. Sci. 2022, 9, 957935. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, M.A.; Rama, G.R.; de Souza, C.F.V.; Granada, C.E. Acid lactic lactobacilli as a biotechnological toll to improve food quality and human health. Biotechnol. Prog. 2019, 36, e2937. [Google Scholar] [CrossRef]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for gastrointestinal health: Current and future perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Plavec, T.V.; Berlec, A. Safety aspects of genetically modified lactic acid bacteria. Microorganisms 2020, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Saviano, A.; Brigida, M.; Migneco, A.; Gunawardena, G.; Zanza, C.; Candelli, M.; Franceschi, F.; Ojetti, V. Lactobacillus reuteri DSM 17938 (Limosilactobacillus reuteri) in diarrhea and constipation: Two sides of the same Coin? Medicina 2021, 57, 643. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Hou, C.; Zeng, X.; Yang, F.; Liu, H.; Qiao, S. Study and use of the probiotic Lactobacillus reuteri in pigs: A review. J. Anim. Sci. Biotechnol. 2015, 6, 14. [Google Scholar] [CrossRef]
- Shi, S.; Zhou, D.; Xu, Y.; Dong, J.; Han, Y.; He, G.; Li, W.; Hu, J.; Liu, Y.; Zhao, K. Effect of Lactobacillus reuteri S5 Intervention on intestinal microbiota composition of chickens challenged with Salmonella enteritidis. Animals 2022, 12, 2528. [Google Scholar] [CrossRef]
- Karaffová, V.; Revajová, V.; Koščová, J.; Gancarčíková, S.; Nemcová, R.; Ševčíková, Z.; Herich, R.; Levkut, M., Sr. Local intestinal immune response including NLRP3 inflammasome in broiler chicken infected with Campylobacter jejuni after administration of Lactobacillus reuteri B1/1. Food Agric. Immunol. 2020, 31, 937–949. [Google Scholar] [CrossRef]
- Karaffová, V.; Revajová, V.; Nemcová, R.; Ševčíková, Z.; Levkutová, M.; Levkut, M. In vitro study of immune properties of new lactobacilli isolates from pheasant gut. Folia Vet. 2020, 64, 39–47. [Google Scholar] [CrossRef]
- Vijay-Kumar, M.; Aitken, J.D.; Kumar, A.; Neish, A.S.; Uematsu, S.; Akira, S.; Gewirtz, A.T. Toll-like receptor 5-deficient mice have dysregulated intestinal gene expression and nonspecific resistance to Salmonella-induced typhoid-like disease. Infect. Immun. 2008, 76, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Rodgers, G.P. Olfactomedin 4 expression and functions in innate immunity, inflammation, and cancer. Cancer Metastasis Rev. 2016, 35, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Dong, Q.; Lu, Y.; Dong, K.; Wang, R.; Liang, Z. Lumican inhibits immune escape and carcinogenic pathways in colorectal adenocarcinoma. Aging 2021, 13, 4388–4408. [Google Scholar] [CrossRef]
- Feuillet, V.; Medjane, S.; Mondor, I.; Demaria, O.; Pagni, P.P.; Galán, J.E.; Flavell, R.A.; Alexopoulou, L. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 12487–12492. [Google Scholar] [CrossRef]
- Yang, J.; Yan, H. TLR5: Beyond the recognition of flagellin. Cell Mol. Immunol. 2017, 14, 1017–1019. [Google Scholar] [CrossRef]
- Cencič, A.; Langerholc, T. Functional cell models of the gut and their applications in food microbiology—A review. Int. J. Food Microbiol. 2010, 141, S4–S14. [Google Scholar] [CrossRef]
- Ryznerova, D. The Study of the Properties of the Probiotic Bacteria in Terms of Their Biological Effects and Applications. Dissertation, The University of Veterinary Medicine and Pharmacy, Košice, Slovakia, 2013. [Google Scholar]
- Lebeer, S.; Claes, I.; Tytgat, H.L.; Verhoeven, T.L.; Marien, E.; von Ossowski, I.; Reunanen, J.; Palva, A.; Vos, W.M.; Keersmaecker, S.C.; et al. Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 2012, 78, 185–193. [Google Scholar] [CrossRef]
- Karaffová, V.; Marcinková, E.; Bobíková, K.; Herich, R.; Revajová, V.; Stašová, D.; Kavuľová, A.; Levkutová, M.; Levkut, M., Jr.; Lauková, A.; et al. TLR4 and TLR21 expression, MIF, IFN-β, MD-2, CD14 activation, and sIgA production in chickens administered with EFAL41 strain challenged with Campylobacter jejuni. Folia Microbiol. 2017, 62, 89–97. [Google Scholar]
- Whyte, J.J.; Meyer, A.E.; Spate, L.D.; Benne, J.A.; Cecil, R.; Samuel, M.S.; Murphy, C.N.; Prather, R.S.; Geisert, R.D. Inactivation of porcine interleukin-1β results in failure of rapid conceptus elongation. Proc. Natl. Acad. Sci. USA 2018, 115, 307–312. [Google Scholar] [CrossRef]
- Dang, Y.; Lachance, C.; Wang, Y.; Gagnon, C.A.; Savard, C.; Segura, M.; Grenier, D.; Gottschalk, M. Transcriptional approach to study porcine tracheal epithelial cells individually or dually infected with swine influenza virus and Streptococcus suis. BMC Vet. Res. 2014, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Moue, M.; Tohno, M.; Shimazu, T.; Kido, T.; Aso, H.; Saito, T.; Kitazawa, H. Toll-like receptor 4 and cytokine expression involved in functional immune response in an originally established porcine intestinal epitheliocyte cell line. Biochem. Biophys. Acta 2008, 1780, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Cinar, M.U.; Islam, M.A.; Uddin, M.J.; Tholen, E.; Tesfaye, D.; Looft, C.; Schellander, K. Evaluation of suitable reference genes for gene expression studies in porcine alveolar macrophages in response to LPS and LTA. BMC Res. Notes 2012, 5, 107. [Google Scholar] [CrossRef]
- Sazawal, S.; Hiremath, G.; Dhingra, U.; Malik, P.; Deb, S.; Black, R.E. Efficacy of probiotics in prevention of acute diarrhoea: A meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect. Dis. 2006, 6, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, Y.; Xia, Y.; Song, X.; Ai, L. Characteristics of probiotic preparations and their applications. Foods 2022, 11, 2472. [Google Scholar] [CrossRef] [PubMed]
- Castiblanco, G.; Yucel-Lindberg, T.; Roos, S.; Twetman, S. Effect of Lactobacillus reuteri on cell viability and PGE2 production in human gingival fibroblasts. Probiotics Antimicrob. Proteins 2017, 9, 278–283. [Google Scholar] [CrossRef]
- Guan, C.; Chen, X.; Jiang, X.; Zhao, R.; Yuan, Y.; Chen, D.; Zhang, C.; Lu, M.; Lu, Z.; Gu, R. In vitro studies of adhesion properties of six lactic acid bacteria isolated from the longevous population of China. RSC Adv. 2020, 10, 24234–24240. [Google Scholar] [CrossRef]
- Šikić Pogačar, M.; Langerholc, T.; Mičetić-Turk, D.; Mičetić-Turk, D.; Možina, S.S.; Klančnik, A. Effect of Lactobacillus spp. on adhesion, invasion, and translocation of Campylobacter jejuni in chicken and pig small-intestinal epithelial cell lines. BMC Vet. Res. 2020, 16, 34. [Google Scholar] [CrossRef]
- Schaefer, T.M.; Desouza, K.; Fahey, J.V.; Beagley, K.W.; Wira, C.R. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology 2004, 112, 428–436. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Ihim, S.A.; Abubakar, S.D.; Zian, Z.; Sasaki, T.; Saffarioun, M.; Maleknia, S.; Azizi, G. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: Biological role in induction, regulation, and treatment. Front. Immunol. 2022, 13, 919973. [Google Scholar] [CrossRef] [PubMed]
- Cesta, M.C.; Zippoli, M.; Marsiglia, C.; Gavioli, E.M.; Mantelli, F.; Allegretti, M.; Balk, R.A. The Role of interleukin-8 in lung inflammation and injury: Implications for the management of COVID-19 and hyperinflammatory acute respiratory distress syndrome. Front. Pharmacol. 2022, 12, 808797. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Lillehoj, H.S. Immunity, immunomodulation, and antibiotic alternatives to maximize the genetic potential of poultry for growth and disease response. Anim. Feed Sci. Technol. 2019, 250, 41–50. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 6, a001651. [Google Scholar]
- Akhtar, M.; Watson, J.L.; Nazli, A.; McKay, D.M. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-kappaB-independent pathway. FASEB J. 2003, 17, 1319–1321. [Google Scholar] [CrossRef]
- Terada, T.; Nii, T.; Isobe, N.; Yoshimura, Y. Effects of probiotics Lactobacillus reuteri and Clostridium butyricum on the expression of Toll-like receptors, pro- and anti-inflammatory cytokines, and antimicrobial peptides in broiler chick intestine. J. Poult. Sci. 2020, 57, 310–318. [Google Scholar] [CrossRef]
- Alizadeh, M.; Bavananthasivam, J.; Shojadoost, B.; Astill, J.; Taha-Abdelaziz, K.; Alqazlan, N.; Boodhoo, N.; Shoja Doost, J.; Sharif, S. In ovo and oral administration of probiotic lactobacilli modulate cell- and antibody-mediated immune responses in newly hatched chicks. Front. Immunol. 2021, 12, 664387. [Google Scholar] [CrossRef]
- Karamanou, K.; Perrot, G.; Maquart, F.X.; Brézillon, S. Lumican as a multivalent effector in wound healing. Adv. Drug Deliv. Rev. 2018, 129, 344–351. [Google Scholar] [CrossRef]
- Wang, X.Y.; Chen, S.H.; Zhang, Y.N.; Xu, C.F. Olfactomedin-4 in digestive diseases: A mini-review. World J. Gastroenterol. 2018, 24, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef]
- Shin, D.; Chang, S.Y.; Bogere, P.; Won, K.; Choi, J.Y.; Choi, Y.J.; Lee, H.K.; Hur, J.; Park, B.Y.; Kim, Y.; et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS ONE 2019, 14, e0220843. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xie, S.; Miao, J.; Li, Y.; Wang, Z.; Wang, M.; Yu, Q. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes 2020, 11, 997–1014. [Google Scholar] [CrossRef] [PubMed]


| Primer | Sequence 5′–3′ | References |
|---|---|---|
| IL-1β Fw | GAAGTGATGGCTAACTACGGTGAC | [21] |
| IL-1β Rev | ACCTGGACCTTGGTTCTCTGAGA | |
| IL-6 Fw | TGGGTTCAATCAGGAGACCT | [22] |
| IL-6 Rev | CAGCCTCGACATTTCCCTTA | |
| IL-8 Fw | TTATCGGAGGCCACAATAAG | |
| IL-8 Rev | TGGAATAGTAGATGGAGCCA | |
| IL-18 Fw | CTGCTGAACCGGAAGACAAT | [23] |
| IL-18 Rev | TCCGATTCCAGGTCTTCATC | |
| TLR4 Fw | CTCTGCCTTCACTACAGAGA | |
| TLR4 Rev | CTGAGTCGTCTCCAGAAGAT | |
| TLR5 Fw | TTTCTGGCAATGGCTGGACA | |
| TLR5 Rev | TGGAGGTTGTCAAGTCCATG | |
| OLFM4 Fw | GGTGATTTACGCAACTGAAG | In this study |
| OLFM4 Rev | GTTTGTACTGCTTGGTATGC | |
| LUM Fw | ACCTGCGTTTGTCTCATAAT | |
| LUM Rev | ATTGTAGGAGAGATCCAGCT | |
| HPRT Fw | AACCTTGCTTTCCTTGGTCA | [24] |
| HPRT Rev | TCAAGGGCATAGCCTACCAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaffová, V.; Teleky, J.; Pintarič, M.; Langerholc, T.; Mudroňová, D.; Hudec, E.; Ševčíková, Z. Application of Lactobacillus reuteri B1/1 (Limosilactobacillus reuteri) Improves Immunological Profile of the Non-Carcinogenic Porcine-Derived Enterocytes. Life 2023, 13, 1090. https://doi.org/10.3390/life13051090
Karaffová V, Teleky J, Pintarič M, Langerholc T, Mudroňová D, Hudec E, Ševčíková Z. Application of Lactobacillus reuteri B1/1 (Limosilactobacillus reuteri) Improves Immunological Profile of the Non-Carcinogenic Porcine-Derived Enterocytes. Life. 2023; 13(5):1090. https://doi.org/10.3390/life13051090
Chicago/Turabian StyleKaraffová, Viera, Jana Teleky, Maša Pintarič, Tomaž Langerholc, Dagmar Mudroňová, Erik Hudec, and Zuzana Ševčíková. 2023. "Application of Lactobacillus reuteri B1/1 (Limosilactobacillus reuteri) Improves Immunological Profile of the Non-Carcinogenic Porcine-Derived Enterocytes" Life 13, no. 5: 1090. https://doi.org/10.3390/life13051090
APA StyleKaraffová, V., Teleky, J., Pintarič, M., Langerholc, T., Mudroňová, D., Hudec, E., & Ševčíková, Z. (2023). Application of Lactobacillus reuteri B1/1 (Limosilactobacillus reuteri) Improves Immunological Profile of the Non-Carcinogenic Porcine-Derived Enterocytes. Life, 13(5), 1090. https://doi.org/10.3390/life13051090








