Genetic Diversity of Borreliaceae Species Detected in Natural Populations of Ixodes ricinus Ticks in Northern Poland
Abstract
1. Introduction
2. Material and Methods
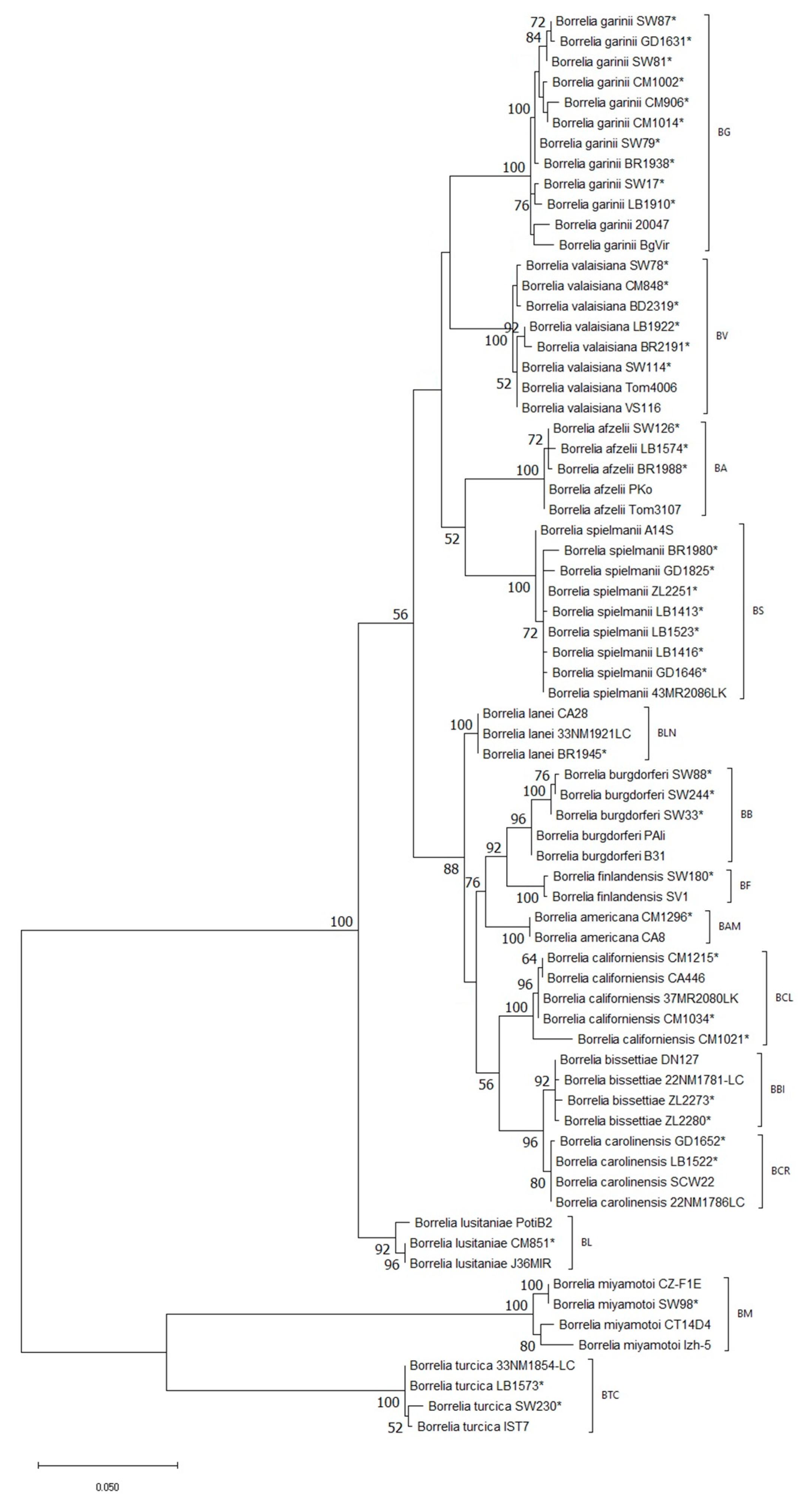
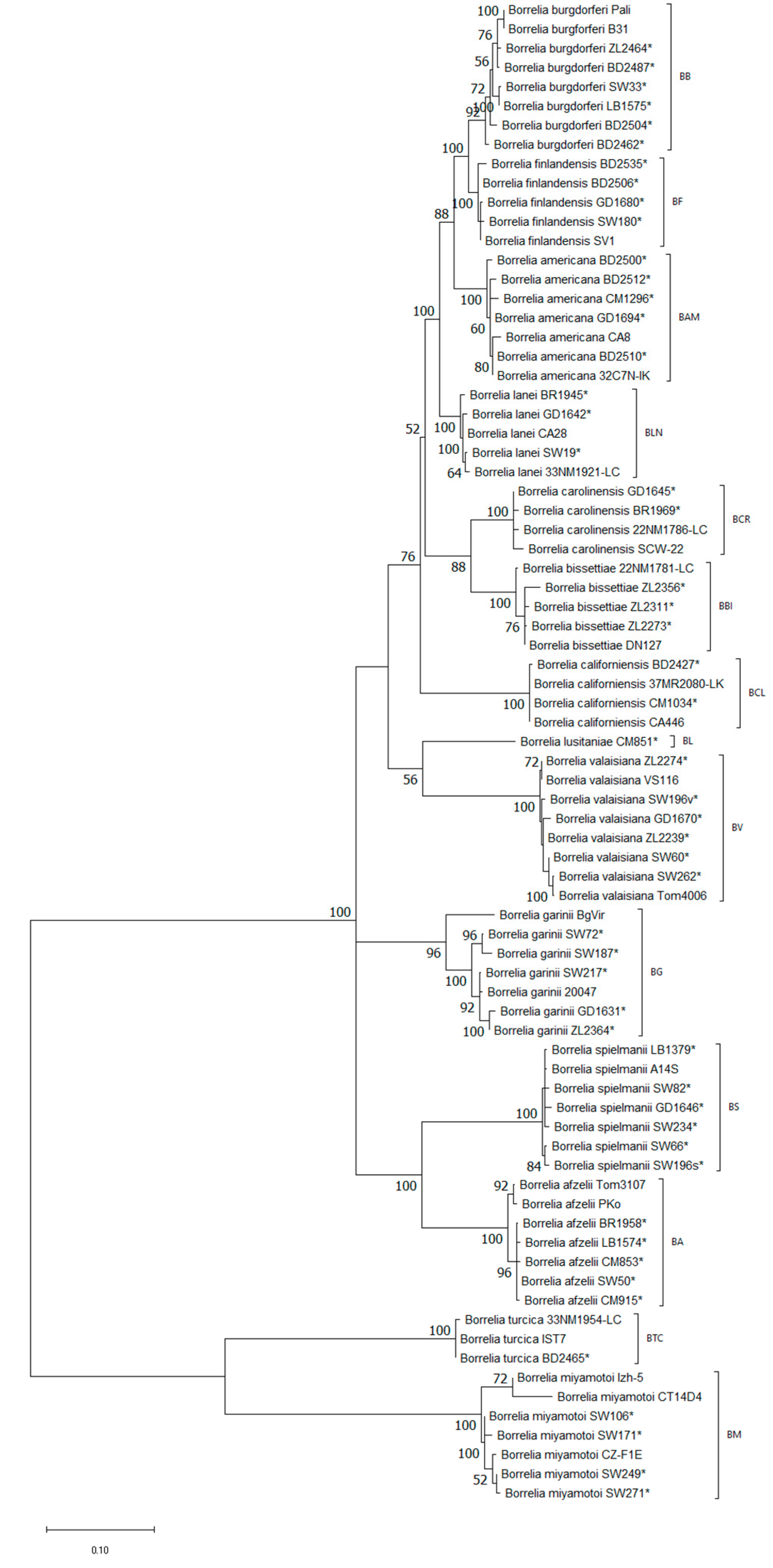
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adeolu, M.; Gupta, R.S. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: The emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie van Leeuwenhoek 2014, 105, 1049–1072. [Google Scholar] [CrossRef] [PubMed]
- Arahal, D.R.; Bull, C.T.; Busse, H.; Christensen, H.; Chuvochina, M.; Dedysh, S.N.; Fournier, P.; Konstantinidis, K.T.; Parker, C.T.; Rossello-Mora, R.; et al. Judicial Opinions 123-127. Int. J. Syst. Evol. Microbiol. 2022, 72, 005708. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Mahmood, S.; Adeolu, M. A phylogenomic and molecular signature based approach for characterization of the phylum Spirochaetes and its major clades: Proposal for a taxonomic revision of the phylum. Front. Microbiol. 2013, 4, 217. [Google Scholar] [CrossRef]
- Sykes, R.A.; Makiello, P. An estimate of Lyme borreliosis incidence in Western Europe. J. Public Health 2017, 39, 74–81. [Google Scholar] [CrossRef]
- Czarkowski, M.P.; Staszewska-Jakubik, E.; Wielgosz, U. Infectious Diseases and Poisonings in Poland in 2020; National Institute of Public Health: Warsaw, Poland, 2021. [Google Scholar]
- Trevisan, G.; Cinco, M.; Trevisini, S.; di Meo, N.; Chersi, K.; Ruscio, M.; Forgione, P.; Bonin, S. Borreliae Part 1: Borrelia Lyme Group and Echidna-Reptile Group. Biology 2021, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Cotté, V.; Bonnet, S.; Cote, M.; Vayssier-Taussat, M. Prevalence of five pathogenic agents in questing Ixodes ricinus ticks from western France. Vector Borne Zoonotic Dis. 2010, 10, 723–730. [Google Scholar] [CrossRef]
- Lommano, E.; Bertaiola, L.; Dupasquier, C.; Gern, L. Infections and coinfections of questing Ixodes ricinus ticks by emerging zoonotic pathogens in Western Switzerland. Appl. Environ. Microbiol. 2012, 78, 4606–4612. [Google Scholar] [CrossRef]
- Heylen, D.; Fonville, M.; van Leeuwen, A.D.; Stroo, A.; Duisterwinke, M.; van Wieren, S.; Diuk-Wasser, M.; de Bruin, A.; Sprong, H. Pathogen communities of songbird-derived ticks in Europe’s low countries. Parasit. Vectors. 2017, 10, 497–508. [Google Scholar] [CrossRef]
- Dunaj, J.; Drewnowska, J.; Moniuszko-Malinowska, A.; Swięcicka, I.; Pancewicz, S. First metagenomic report of Borrelia americana and Borrelia carolinensis in Poland—A preliminary study. Ann. Agric. Environ. Med. 2021, 28, 49–55. [Google Scholar] [CrossRef]
- Gryczyńska, A.; Welc-Falęciak, R. Long-term study of the prevalence of Borrelia burgdorferi s.l. infection in ticks (Ixodes ricinus) feeding on blackbirds (Turdus merula) in NE Poland. Exp. Appl. Acarol. 2016, 70, 381–394. [Google Scholar] [CrossRef]
- Wilhelmsson, P.; Jaenson, T.G.T.; Olsen, B.; Waldenström, J.; Lindgren, P.E. Migratory birds as disseminators of ticks and the tick-borne pathogens Borrelia bacteria and tick-borne encephalitis (TBE) virus: A seasonal study at Ottenby Bird Observatory in South-eastern Sweden. Parasit. Vectors 2020, 13, 607. [Google Scholar] [CrossRef]
- Rudenko, N.; Golovchenko, M.; Grubhoffer, L.; Oliver, J.H., Jr. Borrelia carolinensis sp. nov., a novel species of the Borrelia burgdorferi sensu lato complex isolated from rodents and a tick from the south-eastern USA. Int. J. Syst. Evol. Microbiol. 2011, 61, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Michalik, J.; Wodecka, B.; Liberska, J.; Dabert, M.; Postawa, T.; Piksa, K.; Stańczak, J. Diversity of Lyme borreliosis spirochete species in Ixodes spp. ticks (Acari: Ixodidae) associated with cave-dwelling bats from Poland and Romania. Ticks Tick Borne Dis. 2020, 11, 101300. [Google Scholar] [CrossRef] [PubMed]
- Wodecka, B.; Michalik, J.; Grochowalska, R. Red foxes (Vulpes vulpes) are exposed to high diversity of Borrelia burgdorferi sensu lato species infecting fox-derived Ixodes ticks in west-central Poland. Pathogens 2022, 11, 696. [Google Scholar] [CrossRef]
- Strnad, M.; Hönig, V.; Ružek, D.; Grubhoffer, L.; Rego, R.O.M. Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks. Appl. Environ. Microbiol. 2017, 83, e00609-17. [Google Scholar] [CrossRef] [PubMed]
- Strle, F.; Stanek, G. Clinical manifestations and diagnosis of Lyme borreliosis. Curr. Probl. Dermatol. 2009, 37, 51–110. [Google Scholar] [CrossRef]
- Fraenkel, C.J.; Garpmo, U.; Berglund, J. Determination of novel Borrelia genospecies in Swedish Ixodes ricinus ticks. J. Clin. Microbiol. 2002, 40, 3308–3312. [Google Scholar] [CrossRef]
- Platonov, A.E.; Karan, L.S.; Kolyasnikova, N.M.; Makhneva, N.A.; Toporkova, M.G.; Maleev, V.V.; Fish, D.; Krause, P.J. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis. 2011, 17, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Wodecka, B.; Leońska, A.; Skotarczak, B. A comparative analysis of molecular markers for the detection and identification of Borrelia spirochetes in Ixodes ricinus. J. Med. Microbiol. 2010, 59, 309–314. [Google Scholar] [CrossRef]
- Kalmár, Z.; Sprong, H.; Mihalca, A.D.; Gherman, C.M.; Dumitrache, M.O.; Coipan, E.C.; Fonville, M.; Cozma, V. Borrelia miyamotoi and Candidatus Neoehrlichia mikurensis in Ixodes ricinus ticks, Romania. Emerg. Infect. Dis. 2016, 22, 550–551. [Google Scholar] [CrossRef]
- Hamšíková, Z.; Coipan, C.; Mahríková, L.; Minichová, L.; Sprong, H.; Kazimírová, M. Borrelia miyamotoi and Cco-Iinfection with Borrelia afzelii in Ixodes ricinus ticks and rodents from Slovakia. Microb. Ecol. 2017, 73, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Page, S.; Daschkin, C.; Anniko, S.; Krey, V.; Nicolaus, C.; Maxeiner, H.G. First report of Borrelia miyamotoi in an Ixodes ricinus tick in Augsburg, Germany. Exp. Appl. Acarol. 2018, 74, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, K.; Szczotko, M.; Dmitryjuk, M. Borrelia miyamotoi—An emerging human tick-borne pathogen in Europe. Microorganisms 2021, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Fesler, M.C.; Shah, J.S.; Middelveen, M.J.; Du Cruz, I.; Burrascano, J.J.; Stricker, R.B. Lyme Disease: Diversity of Borrelia species in California and Mexico detected using a novel immunoblot assay. Healthcare 2020, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Casjens, S.R.; Fraser-Liggett, C.M.; Mongodin, E.F.; Qiu, W.G.; Dunn, J.J.; Luft, B.J.; Schutzer, S.E. Whole genome sequence of an unusual Borrelia burgdorferi sensu lato isolate. J. Bacteriol. 2011, 193, 1489–1490. [Google Scholar] [CrossRef]
- Siuda, K. Ticks (Acari: Ixodida) of Poland. Part II: Taxonomy and Distribution; Polskie Towarzystwo Parazytologiczne: Warsaw, Poland, 1993. (In Polish) [Google Scholar]
- Estrada-Peña, A.; Nava, S.; Petney, T. Description of all the stages of Ixodes inopinatus n. sp. (Acari: Ixodidae). Ticks Tick Borne Dis. 2014, 5, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Wodecka, B.; Rymaszewska, A.; Skotarczak, B. Host and pathogen DNA identification in blood meals of nymphal Ixodes ricinus ticks from forest parks and rural forests of Poland. Exp. Appl. Acarol. 2014, 62, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Wodecka, B.; Michalik, J.; Lane, R.S.; Nowak-Chmura, M.; Wierzbicka, A. Differential associations of Borrelia species with European badgers (Meles meles) and raccoon dogs (Nyctereutes procyonoides) in western Poland. Ticks Tick Borne Dis. 2016, 7, 1010–1016. [Google Scholar] [CrossRef]
- Wodecka, B. FlaB gene as a molecular marker for distinct identification of Borrelia species in environmental samples by the PCR-restriction fragment length polymorphism method. Appl. Environ. Microbiol. 2011, 77, 7088–7092. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Chitimia-Dobler, L.; Rieß, R.; Kahl, O.; Wölfel, S.; Dobler, G.; Nava, S.; Estrada-Peña, A. Ixodes inopinatus—Occurring also outside the Mediterranean region. Ticks Tick Borne Dis. 2018, 9, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Chitimia-Dobler, L.; Lemhöfer, G.; Król, N.; Bestehorn, M.; Dobler, G.; Pfeffer, M. Repeated isolation of tick-borne encephalitis virus from adult Dermacentor reticulatus ticks in an endemic area in Germany. Parasit. Vectors 2019, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Hauck, D.; Springer, A.; Pachnicke, S.; Schunack, B.; Fingerle, V.; Strube, C. Ixodes inopinatus in northern Germany: Occurrence and potential vector role for Borrelia spp., Rickettsia spp., and Anaplasma phagocytophilum in comparison with Ixodes ricinus. Parasitol. Res. 2019, 118, 3205–3216. [Google Scholar] [CrossRef] [PubMed]
- Hauck, D.; Springer, A.; Chitimia-Dobler, L.; Strube, C. Two-year monitoring of tick abundance and influencing factors in an urban area (city of Hanover, Germany). Ticks Tick Borne Dis. 2020, 11, 101464. [Google Scholar] [CrossRef] [PubMed]
- Vogelgesang, J.R.; Walter, M.; Kahl, O.; Rubel, F.; Brugger, K. Long-term monitoring of the seasonal density of questing ixodid ticks in Vienna (Austria): Setup and first results. Exp. Appl. Acarol. 2020, 81, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Kahl, O.; Kämmer, D.; Bulling, I.; Komorek, M.; von Eiff, C.; Malerczyk, C. Ticks on the turf: Investigating the presence of ixodid ticks on and around football fields in Germany. Exp. Appl. Acarol. 2021, 84, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Daccord, J.; Takács, N.; Kontschán, J.; Tuska-Szalay, B.; Sándor, A.D.; Szekeres, S.; Meli, M.L.; Hofmann-Lehmann, R. Investigation on haplotypes of ixodid ticks and retrospective finding of Borrelia miyamotoi in bank vole (Myodes glareolus) in Switzerland. Ticks Tick Borne Dis. 2022, 13, 101865. [Google Scholar] [CrossRef]
- Król, N.; Obiegala, A.; Imholt, C.; Arz, C.; Schmidt, E.; Jeske, K.; Ulrich, R.G.; Rentería-Solís, Z.; Jacob, J.; Pfeffer, M. Diversity of Borrelia burgdorferi sensu lato in ticks and small mammals from different habitats. Parasit. Vectors 2022, 15, 195. [Google Scholar] [CrossRef]
- Dwużnik, D.; Mierzejewska, E.J.; Alsarraf, M.; Bajer, A. A new focus of the tick Haemaphysalis concinna in Western Poland. Exp. Appl. Acarol. 2019, 78, 93–112. [Google Scholar] [CrossRef]
- Kiewra, D.; Czułowska, A.; Dyczko, D.; Zieliński, R.; Plewa-Tutaj, K. First record of Haemaphysalis concinna (Acari: Ixodidae) in Lower Silesia, SW Poland. Exp. Appl. Acarol. 2019, 77, 449–454. [Google Scholar] [CrossRef]
- Zięba, P.; Nowakiewicz, A.; Michalski, A.; Wlizło-Skowronek, B.; Gaweł, J.; Niemcewicz, M.; Gnat, S.; Łagowski, D. A new locality of the Haemaphysalis concinna tick (Koch, 1844) in Poland and its role as a potential vector of infectious diseases. Ann. Parasitol. 2019, 65, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Chmura, M.; Siuda, K. Ticks of Poland. Review of contemporary issues and latest research. Ann. Parasitol. 2012, 58, 125–155. [Google Scholar]
- Mysterud, A.; Stigum, V.M.; Jaarsma, R.I.; Sprong, H. Genospecies of Borrelia burgdorferi sensu lato detected in 16 mammal species and questing ticks from northern Europe. Sci. Rep. 2019, 9, 5088. [Google Scholar] [CrossRef] [PubMed]
- Zubriková, D.; Wittmann, M.; Hönig, V.; Švec, P.; Víchová, B.; Essbauer, S.; Dobler, G.; Grubhoffer, L.; Pfister, K. Prevalence of tick-borne encephalitis virus and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks in Lower Bavaria and Upper Palatinate, Germany. Ticks Tick Borne Dis. 2020, 11, 101375. [Google Scholar] [CrossRef]
- Knoll, S.; Springer, A.; Hauck, D.; Schunack, B.; Pachnicke, S.; Fingerle, V.; Strube, C. Distribution of Borrelia burgdorferi s.l. and Borrelia miyamotoi in Ixodes tick populations in Northern Germany, co-infections with Rickettsiales and assessment of potential influencing factors. Med. Vet. Entomol. 2021, 35, 595–606. [Google Scholar] [CrossRef]
- Del Cerro, A.; Oleaga, A.; Somoano, A.; Barandika, J.F.; García-Pérez, A.L.; Espí, A. Molecular identification of tick-borne pathogens (Rickettsia spp., Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, Coxiella burnetii and piroplasms) in questing and feeding hard ticks from North-Western Spain. Ticks Tick Borne Dis. 2022, 13, 101961. [Google Scholar] [CrossRef] [PubMed]
- Dyczko, D.; Kiewra, D.; Kolanek, A.; Błażej, P. The influence of local environmental factors in southwestern Poland on the abundance of Ixodes ricinus and prevalence of infection with Borrelia burgdorferi s.l. and B. miyamotoi. Parasitol. Res. 2022, 121, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Hansford, K.M.; Wheeler, B.W.; Tschirren, B.; Medlock, J.M. Questing Ixodes ricinus ticks and Borrelia spp. in urban green space across Europe: A review. Zoonoses Public Health 2022, 69, 153–166. [Google Scholar] [CrossRef]
- Kubiak, K.; Szymańska, H.; Dmitryjuk, M.; Dzika, E. Abundance of Ixodes ricinus ticks (Acari: Ixodidae) and the diversity of Borrelia species in northeastern Poland. Int. J. Environ. Res. Public Health 2022, 19, 7378. [Google Scholar] [CrossRef]
- Kubiak, K.; Dmitryjuk, M.; Dziekońska-Rynko, J.; Siejwa, P.; Dzika, E. The risk of exposure to ticks and tick-borne pathogens in a spa town in northern Poland. Pathogens 2022, 11, 542. [Google Scholar] [CrossRef]
- Richtrová, E.; Míchalová, P.; Lukavská, A.; Navrátil, J.; Kybicová, K. Borrelia burgdorferi sensu lato infection in Ixodes ricinus ticks in urban green areas in Prague. Ticks Tick Borne Dis. 2022, 13, 102053. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Reiter, M. The expanding Lyme Borrelia complex—Clinical significance of genomic species? Clin. Microbiol. Infect. 2011, 17, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Fish, D.; Narasimhan, S.; Barbour, A.G. Borrelia miyamotoi infection in nature and in humans. Clin. Microbiol. Infect. 2015, 21, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Majerová, K.; Hönig, V.; Houda, M.; Papežík, P.; Fonville, M.; Sprong, H.; Rudenko, N.; Golovchenko, M.; Cerná Bolfíková, B.; Hulva, P.; et al. Hedgehogs, squirrels, and blackbirds as sentinel hosts for active surveillance of Borrelia miyamotoi and Borrelia burgdorferi complex in urban and rural environments. Microorganisms 2020, 8, 1908. [Google Scholar] [CrossRef]
- Guner, E.S.; Watanabe, M.; Hashimoto, N.; Kadosaka, T.; Kawamura, Y.; Ezaki, T.; Kawabata, H.; Imai, Y.; Kaneda, K.; Masuzawa, T. Borrelia turcica sp. nov., isolated from the hard tick Hyalomma aegyptium in Turkey. Int. J. Syst. Evol. Microbiol. 2004, 54, 1649–1652. [Google Scholar] [CrossRef]
- Musilová, L.; Kybicová, K.; Fialová, A.; Richtrová, E.; Kulma, M. First isolation of Borrelia lusitaniae DNA from green lizards (Lacerta viridis) and Ixodes ricinus ticks in the Czech Republic. Ticks Tick Borne Dis. 2022, 13, 101887. [Google Scholar] [CrossRef]
- Schötta, A.M.; Stelzer, T.; Stanek, G.; Stockinger, H.; Wijnveld, M. Bacteria and protozoa with pathogenic potential in Ixodes ricinus ticks in Viennese recreational areas. Wien. Klin. Wochenschr. 2022, 10, 1–8. [Google Scholar] [CrossRef]
- Hansford, K.M.; McGinley, L.; Wheeler, B.W.; Tschirren, B.; Medlock, J.M. Ixodes ricinus density, Borrelia prevalence and the density of infected nymphs along an urban-rural gradient in southern England. Zoonoses Public Health 2023. [Google Scholar] [CrossRef]
- Zhigailov, A.V.; Neupokoyeva, A.S.; Maltseva, E.R.; Perfilyeva, Y.V.; Bissenbay, A.O.; Turebekov, N.A.; Frey, S.; Essbauer, S.; Abdiyeva, K.S.; Ostapchuk, Y.O.; et al. The prevalence of Borrelia in Ixodes persulcatus in southeastern Kazakhstan. Ticks Tick-Borne Dis. 2021, 12, 101716. [Google Scholar] [CrossRef]
- He, Z.; Jiang, B.; Huang, L.; Shao, Z.; Zhang, Y.; Li, Y.; Pu, E.; Duan, X.; Jiang, H.; Wang, J.; et al. High diversity and prevalence of Borrelia burgdorferi sensu lato in wildlife hosts, domestic animals, and ticks in Yunnan province, southwestern China. Front. Microbiol. 2022, 13, 876079. [Google Scholar] [CrossRef]
- Fedorova, N.; Kleinjan, J.E.; James, D.; Hui, L.T.; Peeters, H.; Lane, R.S. Remarkable diversity of tick or mammalian-associated Borreliae in the metropolitan San Francisco Bay Area, California. Ticks Tick Borne Dis. 2014, 5, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Rose, I.; Yoshimizu, M.H.; Bonilla, D.L.; Fedorova, N.; Lane, R.S.; Padgett, K.A. Phylogeography of Borrelia spirochetes in Ixodes pacificus and Ixodes spinipalpis ticks highlights differential acarological risk of tick-borne disease transmission in northern versus southern California. PLoS ONE 2019, 14, e0214726. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.J.; Weinstein, S.B.; O’Connor, K.E.; Swei, A. Circulation of tick-borne spirochetes in tick and small mammal communities in Santa Barbara county, California, USA. J. Med. Entomol. 2020, 57, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Kurtenbach, K.; De Michelis, S.; Etti, S.; Schäfer, S.M.; Sewell, H.S.; Brade, V.; Kraiczy, P. Host association of Borrelia burgdorferi sensu lato—The key role of host complement. Trends Microbiol. 2002, 10, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Pena, A.; Alvarez-Jarreta, J.; Cabezas-Cruz, A. Reservoir and vector evolutionary pressures shaped the adaptation of Borrelia. Infect. Genet. Evol. 2018, 66, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Răileanu, C.; Silaghi, C.; Fingerle, V.; Margos, G.; Thiel, C.; Pfister, K.; Overzier, E. Borrelia burgdorferi sensu lato in questing and engorged ticks from different habitat types in southern Germany. Microorganisms 2021, 9, 1266. [Google Scholar] [CrossRef]

| Specificity | Genetic Marker | Sequence of Primers (5′->3′) | Annealing Temp. (°C) | Length of Amplicons (bp) | Usage | Reference |
|---|---|---|---|---|---|---|
| Ixodidae * | coI | CO1-45F: ACTAACCATAAAGACACATTGG | 44 | 706 | PCR-RFLP, sequencing | This study |
| CO1-1100R: GAATTGGCTAAAATAATTCC | ||||||
| Nested PCR | ||||||
| CO1-375F: GGCAGGAACTGGATGAAC | 47 | |||||
| CO1-1086R: AATTCCTGTTAATCCYCC | ||||||
| Borreliaceae ** | flaB | 132f: TGGTATGGGAGTTTCTGG | 56 | 604 | PCR-RFLP | [24] |
| 905r: TCTGTCATTGTAGCATCTTT | ||||||
| Nested PCR | ||||||
| 220f: CAGACAACAGAGGGAAAT | 54 | |||||
| 823r: TCAAGTCTATTTTGGAAAGCACC | ||||||
| FL84F: AGAAGCTTTCTAGTGGGTACAGA | 57 | |||||
| FL976R: GATTGGCCTGTGCAATCAT | ||||||
| Nested PCR | 789 | sequencing | This study | |||
| FL120F: TGATGATGCTGCTGGGATGG | 56 | |||||
| FL908R: TCATCTGTCATTGTAGCATCTT | ||||||
| mag—trnI | glz199f—GTAAGTTTGCCAGGACCATT | 56 | ||||
| ile20r—TGAACATCCGACCTCAGG | ||||||
| Nested PCR | 309-1183 | sequencing | This study | |||
| glz435f—TAAGCTTCCGTTTCAAC | 58 | |||||
| ile65r—CAGACCTGCGCTCTAACC |
| Borreliaceae Species | Restriction Fragment Size | |||
|---|---|---|---|---|
| HpyF3I | SatI | PsuI | VspI | |
| Bl. burgdorferi | 359, 207, 38 | 241, 200, 112, 51 | ||
| Bl. finlandensis | 359, 207, 38 | 251, 226, 127 | ||
| Bl. americana | 359, 207, 38 | 353, 200, 51 | ||
| Bl. afzelii | 305, 165, 92, 42 * | |||
| Bl. garinii | 388, 135, 72, 9 | 327, 277 | ||
| Bl. bavariensis | 388, 135, 72, 9 | 277, 219, 108 | ||
| Bl. valaisiana | 188, 135, 117, 92, 72 * | |||
| Bl. lusitaniae | 305, 207, 92 * | |||
| Bl. bissettiae | 280, 135, 117, 72 * | |||
| Bl. spielmanii | 397, 207 | 241, 209, 112, 42 | ||
| Bl. lanei | 397, 207 | 353, 200, 51 | ||
| Bl. carolinensis | 280, 135, 117, 45, 27 * | |||
| Bl. californiensis | 280, 207, 117 | 461, 143 | ||
| Bl. turdi | 280, 207, 117 | 352, 143, 109 | ||
| B. miyamotoi | 512, 86 * | |||
| B. turcica | 412, 135, 45, 12 * | |||
| Borreliaceae Species | Strain | Source | Country | Accession Number | |
|---|---|---|---|---|---|
| flaB | mag-trnI | ||||
| B. garinii | 20047 | I. ricinus | France | CP018744 | |
| BgVir | I. persulcatus | Russia | CP003151 | ||
| B. afzelii | PKo | Skin biopsy | Germany | CP002933 | |
| Tom3107 | I. persulcatus | Russia | CP009212 | ||
| B. burgdorferi | PAli | Homo sapiens | Germany | CP019844 | |
| B31 | I. scapularis | USA | AE000783 | ||
| B. valaisiana | VS116 | I. ricinus | Switzerland | ABCY02000001 | |
| Tom4006 | I. persulcatus | Russia | CP009117 | ||
| B. lusitaniae | PotiB2 | I. ricinus | Portugal | D82856 | NA |
| J3-6M-IR | I. ricinus | Poland | KF422805 | NA | |
| Bl. bissettiae | DN127 | I. pacificus | USA | CP002746 | |
| B. spielmanii | A14S | Homo sapiens | Netherlands | ABKB02000003 | ABKB02000009 |
| B. finlandensis | SV1 | I. ricinus | Finland | ABJZ02000005 | |
| B. carolinensis | SCW-22 | I. minor fed on Neotoma floridana | USA | KF422810 | MT119049 |
| B. californiensis | CA446 | Dipodomys californicus | USA | KF422809 | MT119055 |
| B. lanei | CA28 | I. pacificus | USA | KF422812 | MT119061 |
| B. americana | CA8 | I. pacificus | USA | KF422811 | MT119066 |
| B. miyamotoi | CT14D4 | Human blood | USA | CP010308 | |
| Izh-5 | Human blood | Russia | CP024205 | ||
| CZ-F1E | I. ricinus | Czech Republic | CP046389 | ||
| B. turcica | IST7 | Hyalomma aegyptium from tortoise | Turkey | CP028884 | |
| Study Site | No. of Ticks Tested/Infected (%) | ||||
|---|---|---|---|---|---|
| Total | Females | Males | Nymphs | Larvae | |
| Świerznica | 475/116 (24.4) | 14/3 (21.4) | 15/6 (40.0) | 252/101 (40.1) | 194/6 (3.1) |
| Ciemnik | 509/42 (8.3) | 20/4 (20.0) | 29/1 (3.4) | 276/24 (8.7) | 184/13 (7.1) |
| Lubieszyn | 306/40 (13.1) | 16/1 (6.3) | 26/3 (11.5) | 249/28 (11.2) | 15/8 (53.3) |
| Gdańsk | 266/63 (23.7) | 11/3 (27.3) | 13/7 (53.8) | 237/53 (22.4) | 5/0 (0) |
| Bartoszewo | 185/35 (18.9) | 17/2 (11.8) | 19/9 (47.4) | 148/24 (16.2) | 1/0 (0) |
| Zielonczyn | 302/72 (23.8) | 2/0 (0) | 3/0 (0) | 260/69 (26.5) | 37/3 (8.1) |
| Bełdany Lake | 144/47 (32.6) | 16/8 (50) | 27/8 (29.6) | 101/31 (30.7) | |
| Total | 2187/415 (19.0) | 96/21 (21.9) | 132/34 (25.8) | 1523/330 (21.7) | 436/30 (6.9) |
| Borreliaceae Species | Study Site (PCR+ [F/M/N/L]) | |||||||
|---|---|---|---|---|---|---|---|---|
| Lubieszyn | Bartoszewo | Zielonczyn | Ciemnik | Świerznica | Gdańsk | Bełdany Lake | Total (%) | |
| Bl. garinii | 9 [1/3/5/0] | 8 [2/3/3/0] | 4 [0/0/4/0] | 7 [0/1/5/1] | 35 [1/1/27/6] | 5 [0/2/3/0] | 3 [1/0/2/0] | 71 [5/10/49/7] (17.1) |
| Bl. afzelii | 5 [0/0/3/2] | 7 [0/0/7/0] | 30 [0/0/28/2] | 10 [1/0/8/1] | 38 [1/2/35/0] | 3 [1/0/2/0] | 10 [2/2/6/0] | 103 [5/4/89/5] (24.8) |
| Bl. burgdorferi | 1 [0/0/1/0] | 3 [0/0/3/0] | 1 [0/0/1/0] | 7 [0/0/7/0] | 5 [1/0/4/0] | 17 [1/0/16/0] (4.1) | ||
| Bl. valaisiana | 5 [0/0/4/1] | 7 [0/2/5/0] | 4 [0/0/4/0] | 1 [1/0/0/0] | 9 [1/1/7/0] | 9 [0/0/9/0] | 35 [2/3/29/1] (8.4) | |
| Bl. lusitaniae | 1 [0/0/1/0] | 2 [2/0/0/0] | 2 [0/0/2/0] | 5 [2/0/3/0] (1.2) | ||||
| Bl. spielmanii | 12 [0/0/8/4] | 4 [0/1/3/0] | 1 [0/0/1/0] | 3 [0/0/1/2] | 3 [0/0/3/0] | 22 [2/3/17/0] | 45 [2/4/33/6] (10.8) | |
| Bl. bissettiae | 4 [0/0/4/0] | 4 [0/0/4/0] (1.0) | ||||||
| Bl. finlandensis | 1 [0/0/1/0] | 2 [0/1/1/0] | 3 [0/1/2/0] (0.7) | |||||
| Bl. californiensis | 1 [0/0/1/0] | 1 [0/1/0/0] | 8 [0/0/7/1] | 4 [0/0/2/2] | 13 [1/3/9/0] | 27 [1/4/19/3] (6.5) | ||
| Bl. carolinensis | 2 [0/0/2/0] | 4 [0/0/4/0] | 6 [0/0/6/0] | 12 [0/0/12/0] (2.9) | ||||
| Bl. lanei | 3 [0/0/3/0] | 1 [0/1/0/0] | 5 [0/0/2/3] | 2 [0/0/2/0] | 14 [0/1/13/0] | 25 [0/2/20/3] (6.0) | ||
| Bl. americana | 2 [0/0/2/0] | 1 [0/0/0/1] | 1 [0/0/1/0] | 4 [1/2/1/0] | 8 [1/2/4/1] (1.9) | |||
| B. miyamotoi | 2 [0/1/1/0] | 8 [0/0/8/0] | 3 [0/0/3/0] | 9 [0/2/7/0] | 3 [0/0/3/0] | 25 [0/3/22/0] (6.0) | ||
| B. turcica | 1 [0/0/0/1] | 2 [0/0/2/0] | 1 [0/0/1/0] | 4 [0/0/3/1] (1.0) | ||||
| Bl. garinii/Bl. afzelii | 5 [0/0/5/0] | 3 [0/0/3/0] | 8 [0/0/8/0] (1.9) | |||||
| Bl. garinii/Bl. burgdorferi | 1 [0/0/1/0] | 1 [0/0/1/0] (0.2) | ||||||
| Bl. garinii/Bl. lanei | 1 [0/1/0/0] | 1 [0/1/0/0] (0.2) | ||||||
| Bl. garinii/Bl. lusitaniae | 1 [0/0/1/0] | 1 [0/0/1/0] (0.2) | ||||||
| Bl. garinii/Bl. valaisiana | 1 [0/0/1/0] | 1 [0/0/1/0] (0.2) | ||||||
| Bl. afzelii/Bl. burgdorferi | 2 [0/0/2/0] | 1 [0/0/1/0] | 3 [0/0/3/0] (0.7) | |||||
| Bl. afzelii/Bl. californiensis | 1 [0/0/1/0] | 1 [1/0/0/0] | 2 [1/0/1/0] (0.5) | |||||
| Bl. afzelii/Bl. carolinensis | 1 [0/0/1/0] | 1 [0/0/1/0] (0.2) | ||||||
| Bl. afzelii/Bl. lanei | 2 [0/0/0/2] | 1 [0/0/1/0] | 3 [0/0/1/2] (0.7) | |||||
| Bl. afzelii/B. turcica | 1 [0/0/1/0] | 1 [0/0/1/0] (0.2) | ||||||
| Bl. burgdorferi/Bl. valaisiana | 1 [0/0/1/0] | 1 [1/0/0/0] | 2 [1/0/1/0] (0.5) | |||||
| Bl. burgdorferi/Bl. californiensis | 1 [0/0/1/0] | 1 [0/0/1/0] (0.2) | ||||||
| Bl. burgdorferi/Bl. finlandensis | 1 [0/0/1/0] | 1 [0/0/1/0] (0.2) | ||||||
| Bl. valaisiana/Bl. spielmanii | 1 [0/0/1/0] | 1 [0/0/1/0] (0.2) | ||||||
| Bl. spielmanii/Bl. lanei | 1 [0/0/1/0] | 1 [0/0/1/0] (0.2) | ||||||
| Bl. californiensis/B. turcica | 1 [0/0/1/0] | 1 [0/0/1/0] (0.2) | ||||||
| Bl. lanei/B. miyamotoi | 1 [0/0/0/1] | 1 [0/0/0/1] (0.2) | ||||||
| Bl. afzelii/Bl. lanei/B. turcica | 1 [0/0/1/0] | 1 [0/0/1/0] (0.2) | ||||||
| Total | 40 [1/3/28/8] | 35 [2/9/24/0] | 72 [0/0/69/3] | 42 [4/1/24/13] | 116 [3/6/101/6] | 63 [3/7/53/0] | 47 [8/8/31/0] | 415 [21/34/330/30] (100.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wodecka, B.; Kolomiiets, V. Genetic Diversity of Borreliaceae Species Detected in Natural Populations of Ixodes ricinus Ticks in Northern Poland. Life 2023, 13, 972. https://doi.org/10.3390/life13040972
Wodecka B, Kolomiiets V. Genetic Diversity of Borreliaceae Species Detected in Natural Populations of Ixodes ricinus Ticks in Northern Poland. Life. 2023; 13(4):972. https://doi.org/10.3390/life13040972
Chicago/Turabian StyleWodecka, Beata, and Valentyna Kolomiiets. 2023. "Genetic Diversity of Borreliaceae Species Detected in Natural Populations of Ixodes ricinus Ticks in Northern Poland" Life 13, no. 4: 972. https://doi.org/10.3390/life13040972
APA StyleWodecka, B., & Kolomiiets, V. (2023). Genetic Diversity of Borreliaceae Species Detected in Natural Populations of Ixodes ricinus Ticks in Northern Poland. Life, 13(4), 972. https://doi.org/10.3390/life13040972







