Degree of Actinic Elastosis Is a Surrogate of Exposure to Chronic Ultraviolet Radiation and Correlates More Strongly with Cutaneous Squamous Cell Carcinoma than Basal Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics and Inclusion Criteria
2.2. Histopathological Assessment
2.3. Statistical Analysis
2.4. Microscope and Digital Photography
3. Results
3.1. Patient Characteristics
3.1.1. Clinical Characteristics of KC Patients in Regensburg/South-Eastern Bavaria over the Course of 20 Years
3.1.2. Clinical Characteristics of Patients Included in the Histopathological Analysis
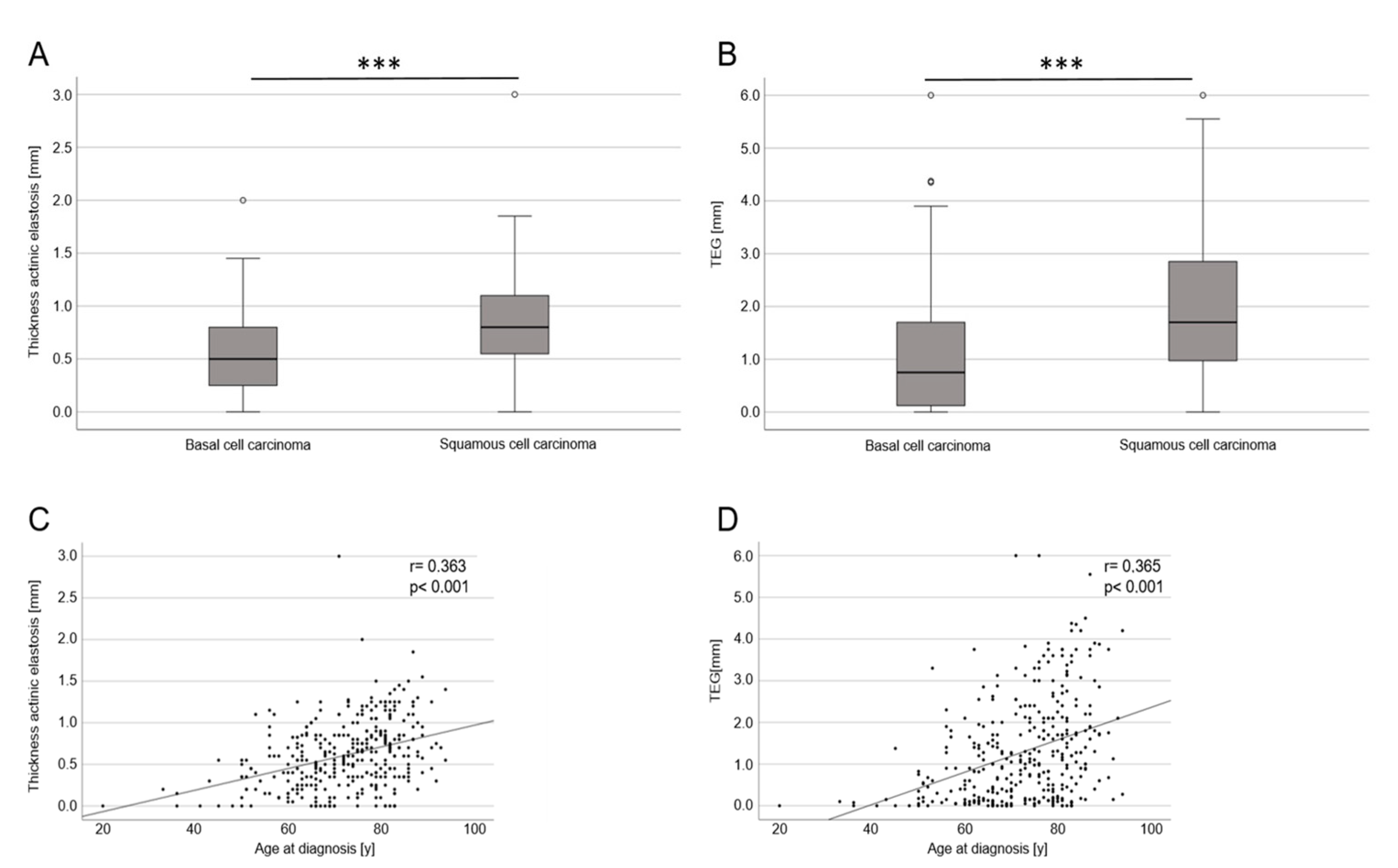
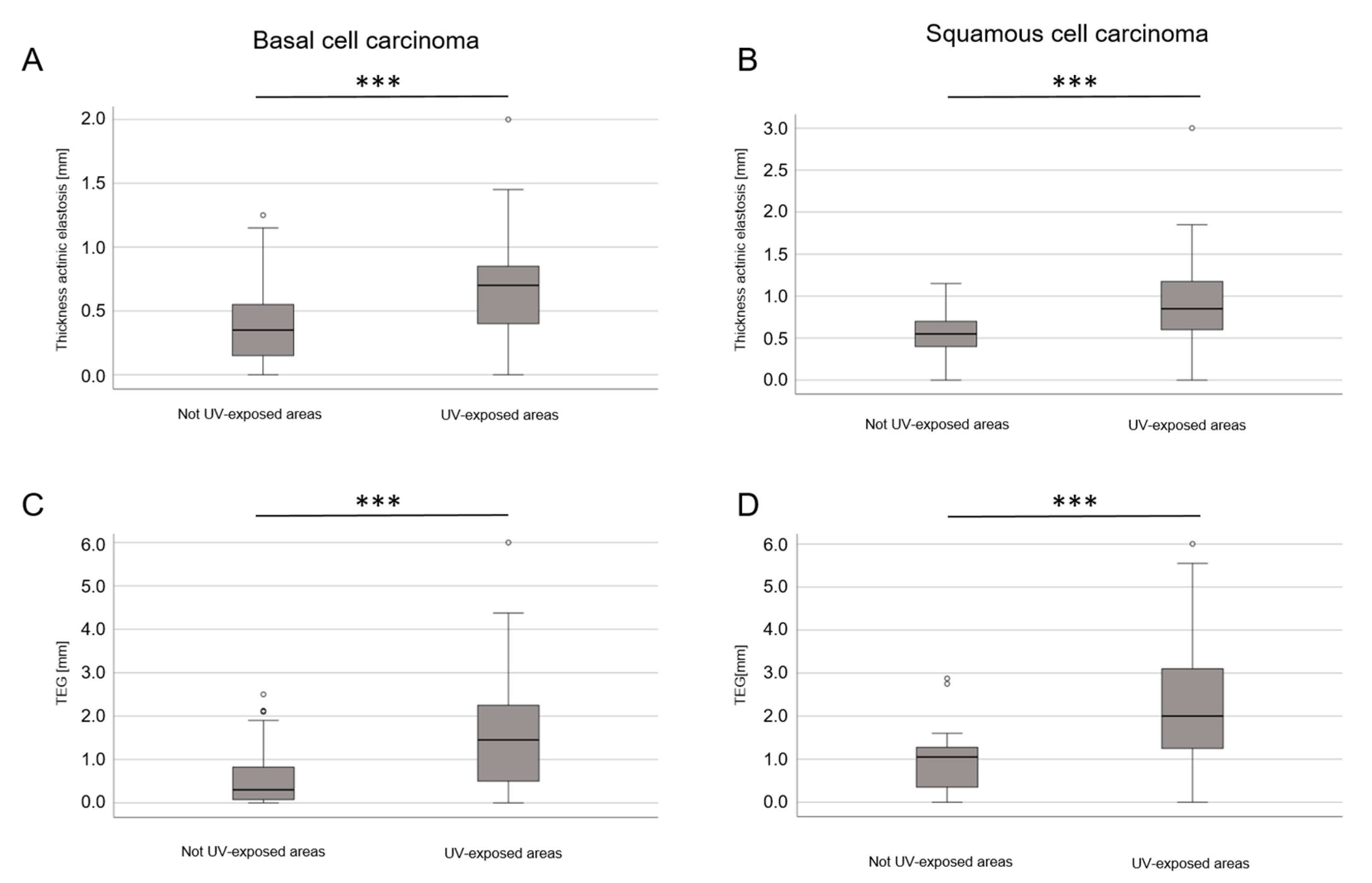
3.2. Histopathological Results—Thickness/Width of Actinic Elastosis in Regard to Age, Sex, Body Site and Tumor Type
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurz, B.; Berneburg, M.; Singer, S. Sonnenschutz der menschlichen Haut: Grundlagen. Hautarzt 2022, 73, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Calderone, D.C.; Fenske, N.A. The clinical spectrum of actinic elastosis. J. Am. Acad. Dermatol. 1995, 32, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.W. Weedon’s Skin Pathology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780702075827. [Google Scholar]
- Krutmann, J.; Berneburg, M. Lichtalterung (Photoaging) der Haut: Was gibt es Neues? Hautarzt 2021, 72, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Bastiaens, M.; Hoefnagel, J.; Westendorp, R.; Vermeer, B.-J.; Bouwes Bavinck, J.N. Solar lentigines are strongly related to sun exposure in contrast to ephelides. Pigment Cell Res. 2004, 17, 225–229. [Google Scholar] [CrossRef]
- Armstrong, B.K.; Kricker, A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B 2001, 63, 8–18. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Katiyar, S.K. UV-induced immune suppression and photocarcinogenesis: Chemoprevention by dietary botanical agents. Cancer Lett. 2007, 255, 1–11. [Google Scholar] [CrossRef]
- Bonkevitch, F.; Souza, P.R.M. Cutis rhomboidalis protects skin from malignant epithelial tumors. Med. Hypotheses 2014, 82, 652–653. [Google Scholar] [CrossRef]
- Kricker, A.; Armstrong, B.K.; English, D.R.; Heenan, P.J. Pigmentary and cutaneous risk factors for non-melanocytic skin cancer--a case-control study. Int. J. Cancer 1991, 48, 650–662. [Google Scholar] [CrossRef]
- Rosso, S.; Zanetti, R.; Martinez, C.; Tormo, M.J.; Schraub, S.; Sancho-Garnier, H.; Franceschi, S.; Gafà, L.; Perea, E.; Navarro, C.; et al. The multicentre south European study ‘Helios’. II: Different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. Br. J. Cancer 1996, 73, 1447–1454. [Google Scholar] [CrossRef]
- Diepgen, T.L.; Brandenburg, S.; Aberer, W.; Bauer, A.; Drexler, H.; Fartasch, M.; John, S.M.; Krohn, S.; Palfner, S.; Römer, W.; et al. Skin cancer induced by natural UV-radiation as an occupational disease—Requirements for its notification and recognition. J. Dtsch. Dermatol. Ges. 2014, 12, 1102–1106. [Google Scholar] [CrossRef]
- Schmitt, J.; Haufe, E.; Trautmann, F.; Schulze, H.-J.; Elsner, P.; Drexler, H.; Bauer, A.; Letzel, S.; John, S.M.; Fartasch, M.; et al. Is ultraviolet exposure acquired at work the most important risk factor for cutaneous squamous cell carcinoma? Results of the population-based case-control study FB-181. Br. J. Dermatol. 2018, 178, 462–472. [Google Scholar] [CrossRef]
- Lindelöf, B.; Lapins, J.; Dal, H. Shift in Occupational Risk for Basal Cell Carcinoma from Outdoor to Indoor Workers: A Large Population-based Case-control Register Study from Sweden. Acta Derm. Venereol. 2017, 97, 830–833. [Google Scholar] [CrossRef]
- Kaskel, P.; Lange, U.; Sander, S.; Huber, M.A.; Utikal, J.; Leiter, U.; Krähn, G.; Meurer, M.; Kron, M. Ultraviolet exposure and risk of melanoma and basal cell carcinoma in Ulm and Dresden, Germany. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 134–142. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Watt, P.; Purdie, D.M.; Hughes, M.C.; Hayward, N.K.; Green, A.C. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J. Natl. Cancer Inst. 2003, 95, 806–812. [Google Scholar] [CrossRef]
- Gefeller, O.; Tarantino, J.; Lederer, P.; Uter, W.; Pfahlberg, A.B. The relation between patterns of vacation sun exposure and the development of acquired melanocytic nevi in German children 6–7 years of age. Am. J. Epidemiol. 2007, 165, 1162–1169. [Google Scholar] [CrossRef]
- Khalesi, M.; Whiteman, D.C.; Doi, S.A.R.; Clark, J.; Kimlin, M.G.; Neale, R.E. Cutaneous markers of photo-damage and risk of Basal cell carcinoma of the skin: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1483–1489. [Google Scholar] [CrossRef]
- Bauer, A.; Haufe, E.; Heinrich, L.; Seidler, A.; Schulze, H.J.; Elsner, P.; Drexler, H.; Letzel, S.; John, S.M.; Fartasch, M.; et al. Basal cell carcinoma risk and solar UV exposure in occupationally relevant anatomic sites: Do histological subtype, tumor localization and Fitzpatrick phototype play a role? A population-based case-control study. J. Occup. Med. Toxicol. 2020, 15, 28. [Google Scholar] [CrossRef]
- Schmitt, J.; Haufe, E.; Trautmann, F.; Schulze, H.-J.; Elsner, P.; Drexler, H.; Bauer, A.; Letzel, S.; John, S.M.; Fartasch, M.; et al. Occupational UV-Exposure is a Major Risk Factor for Basal Cell Carcinoma: Results of the Population-Based Case-Control Study FB-181. J. Occup. Environ. Med. 2018, 60, 36–43. [Google Scholar] [CrossRef]
- Thomas, N.E.; Kricker, A.; From, L.; Busam, K.; Millikan, R.C.; Ritchey, M.E.; Armstrong, B.K.; Lee-Taylor, J.; Marrett, L.D.; Anton-Culver, H.; et al. Associations of cumulative sun exposure and phenotypic characteristics with histologic solar elastosis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2932–2941. [Google Scholar] [CrossRef]
- Riegler, M.J.; Mannweiler, S.; Sturm, A.; Donnerer, A.; Darok, M.; Kopera, D. Thickness of actinic elastosis a surrogate marker of chronic UV-damage: A post mortem analysis of 41 cases. Dermatol. Ther. 2020, 33, e14037. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Oh, C.H. Solar damage in skin tumors: Quantification of elastotic material. Dermatology 2001, 202, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, M.M.; Moon, T.E.; Bozzo, P.D. Chronic solar ultraviolet damage associated with malignant melanoma of the skin. J. Am. Acad. Dermatol. 1984, 10, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.W.; Drury, P.; Heenan, P.J. Atypical fibroxanthoma: A histological and immunohistochemical review of 171 cases. Am. J. Dermatopathol. 2010, 32, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Drexler, K.; Drexler, H.; Geissler, E.K.; Berneburg, M.; Haferkamp, S.; Apfelbacher, C. Incidence and Mortality of Malignant Melanoma in Relation to Dermatologist Density in Bavaria. Adv. Ther. 2021, 38, 5548–5556. [Google Scholar] [CrossRef]
- Bundeinstitut für Bevölkerungsforschung. Durchschnittsalter der Bevölkerung in Deutschland (1871–2021). Available online: https://www.bib.bund.de/Permalink.html?cms_permaid=1217910 (accessed on 27 February 2023).
- Ciążyńska, M.; Kamińska-Winciorek, G.; Lange, D.; Lewandowski, B.; Reich, A.; Sławińska, M.; Pabianek, M.; Szczepaniak, K.; Hankiewicz, A.; Ułańska, M.; et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci. Rep. 2021, 11, 4337. [Google Scholar] [CrossRef]
- Almaani, N.; Juweid, M.E.; Alduraidi, H.; Ganem, N.; Abu-Tayeh, F.A.; Alrawi, R.; Hawwari, T. Incidence Trends of Melanoma and Nonmelanoma Skin Cancers in Jordan From 2000 to 2016. JCO Glob. Oncol. 2023, 9, e2200338. [Google Scholar] [CrossRef]
- Delfino, S.; Innocenzi, D.; Di Lorenzo, G.; Scalvenzi, M.; Montesarchio, V.; Feroce, F.; Baldi, A.; Persichetti, P. An increase in basal cell carcinoma among the young: An epidemiological study in a middle-south Italian population. Anticancer Res. 2006, 26, 4979–4983. [Google Scholar]
- McCormack, C.J.; Kelly, J.W.; Dorevitch, A.P. Differences in age and body site distribution of the histological subtypes of basal cell carcinoma. A possible indicator of differing causes. Arch. Dermatol. 1997, 133, 593–596. [Google Scholar] [CrossRef]
- Betti, R.; Radaelli, G.; Mussino, F.; Menni, S.; Crosti, C. Anatomic location and histopathologic subtype of basal cell carcinomas in adults younger than 40 or 90 and older: Any difference? Dermatol. Surg. 2009, 35, 201–206. [Google Scholar] [CrossRef]
- Pelucchi, C.; Di Landro, A.; Naldi, L.; La Vecchia, C. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: An italian case-control study. J. Investig. Dermatol. 2007, 127, 935–944. [Google Scholar] [CrossRef]
- Kvaskoff, M.; Pandeya, N.; Green, A.C.; Perry, S.; Baxter, C.; Davis, M.B.; Mortimore, R.; Westacott, L.; Wood, D.; Triscott, J.; et al. Solar elastosis and cutaneous melanoma: A site-specific analysis. Int. J. Cancer 2015, 136, 2900–2911. [Google Scholar] [CrossRef]
- Vollmer, R.T. Solar elastosis in cutaneous melanoma. Am. J. Clin. Pathol. 2007, 128, 260–264. [Google Scholar] [CrossRef]
- Titus, L.; Barnhill, R.L.; Lott, J.P.; Piepkorn, M.W.; Elder, D.E.; Frederick, P.D.; Nelson, H.D.; Carney, P.A.; Knezevich, S.R.; Weinstock, M.A.; et al. The influence of tumor regression, solar elastosis, and patient age on pathologists’ interpretation of melanocytic skin lesions. Lab. Investig. 2017, 97, 187–193. [Google Scholar] [CrossRef]
- Phan, A.; Touzet, S.; Dalle, S.; Ronger-Savlé, S.; Balme, B.; Thomas, L. Acral lentiginous melanoma: A clinicoprognostic study of 126 cases. Br. J. Dermatol. 2006, 155, 561–569. [Google Scholar] [CrossRef]
- Luca, E.V.d.; Perino, F.; Di Stefani, A.; Coco, V.; Fossati, B.; Peris, K. Lentigo maligna: Diagnosis and treatment. G. Ital. Dermatol. Venereol. 2020, 155, 179–189. [Google Scholar] [CrossRef]
- Karagas, M.R.; Waterboer, T.; Li, Z.; Nelson, H.H.; Michael, K.M.; Bavinck, J.N.B.; Perry, A.E.; Spencer, S.K.; Daling, J.; Green, A.C.; et al. Genus beta human papillomaviruses and incidence of basal cell and squamous cell carcinomas of skin: Population based case-control study. BMJ 2010, 341, c2986. [Google Scholar] [CrossRef]
- Kütting, B.; Drexler, H. UV-induced skin cancer at workplace and evidence-based prevention. Int. Arch. Occup. Environ. Health 2010, 83, 843–854. [Google Scholar] [CrossRef]
- Drexler, H.; Diepgen, T.L.; Schmitt, J.; Schwarz, T.; Letzel, S. Arbeitsbedingte UV-Exposition und Malignome der Haut. Überlegungen zu einer neuen Berufskrankheit: UV-induzierter Hautkrebs. DB 2012, 60, 48–55. [Google Scholar] [CrossRef]
- Gemeinsames Ministerialblatt des Auswärtigen Amtes/des Bundesministerium des Innern/des Bundesministerium der Finanzen des Bundesministeriums für Wirtschaft und Technologie/des Bundesministeriums für Arbeit und Soziales des Bundesministeriums für Ernährung, Landwirtschaft und Verbraucherschutz/des Bundesministeriums der Verteidigung des Bundesministeriums für Familie, Senioren, Frauen und Jugend/des Bundesministeriums für Gesundheit des Bundesministeriums für Verkehr, Bau und Stadtentwicklung/des Bundesministeriums für Umwelt, Naturschutz und Reaktorsicherheit des Bundesministeriums für Bildung und Forschung/des Bundesministeriums für wirtschaftliche Zusammenarbeit und Entwicklung/des Beauftragten der Bundesregierung für Kultur und Medien, herausgegeben vom Bundesministerium des Inneren, Seite 669 64. Jahrgang ISSN 0939-4729 Berlin, den 12. August 2013 Nr. 35. Available online: https://www.econbiz.de/Record/gemeinsames-ministerialblatt-ausw%C3%A4rtigen-amtes-bundesministeriums-innern-bundesministeriums-finanzen-bundesministeriums-wirtschaft-technologie/10003773951 (accessed on 5 February 2023).
- Bundesministerium für Arbeit und Soziales. Ärztlicher Sachverständigenbeirat Berufskrankheiten. Available online: https://www.bmas.de/DE/Soziales/Gesetzliche-Unfallversicherung/Aerztlicher-Sachverstaendigenbeirat/aerztliche-sachverstaendigenbeirat.html#docc133d534-e257-4ab0-b5c9-e8cfe4be9efdbodyText11 (accessed on 5 February 2023).
- Krutmann, J.; Berking, C.; Berneburg, M.; Diepgen, T.L.; Dirschka, T.; Szeimies, M. New Strategies in the Prevention of Actinic Keratosis: A Critical Review. Skin Pharmacol. Physiol. 2015, 28, 281–289. [Google Scholar] [CrossRef]
- Kütting, B.; Drexler, H. Evaluation of skin-protective means against acute and chronic effects of ultraviolet radiation from sunlight. Curr. Probl. Dermatol. 2007, 34, 87–97. [Google Scholar] [CrossRef] [PubMed]


| 1999 | 2009 | 2019 | |
|---|---|---|---|
| Total number of specimen | 18,121 | 32,191 | 34,588 |
| Total number of BCC | 1656 | 3240 | 4477 |
| Diagnosis of BCC (%) | 9.14 | 10.06 | 12.94 |
| Age at diagnosis BCC (y) | 66.1 (±13.3) | 67.8 (±13.0) | 70.9 (±12.4) |
| Age at diagnosis BCC (100 youngest) (y) | 40.2 (±6.4) | 37.1 (±6.6) | 40.2 (±6.0) |
| Total number of SCC | 220 | 352 | 853 |
| Diagnosis of SCC (%) | 1.21 | 1.09 | 2.47 |
| Age at diagnosis SCC (y) | 72.3 (±14.0) | 75.7 (±10.8) | 77.9 (±9.5) |
| BCC | SCC | p | |
|---|---|---|---|
| Sex | male = 145 (60.2%) female = 96 (39.8%) | male = 60 (69.0%) female = 27 (31.0%) | |
| Age at diagnosis (y) | 69.7 (±11.6) | 78.1 (±9.0) | ≤0.001 |
| UV-exposed body site | 121 (50.2%) | 65 (74.7%) | |
| Mean thickness of actinic elastosis (µm) | 0.53 (±0.37) | 0.81 (±0.45) | ≤0.001 |
| TEG (µm) | 1.05 (±1.09) | 1.91 (±1.33) | ≤0.001 |
| Thickness of Actinic Elastosis | TEG | |||||
|---|---|---|---|---|---|---|
| Unstandardized Coefficients | Unstandardized Coefficients | |||||
| B | Std. Error | p | B | Std. Error | p | |
| BCC vs. cSCC | 0.128 | 0.046 | 0.006 | 0.446 | 0.133 | <0.001 |
| Age at diagnosis | 0.009 | 0.002 | <0.001 | 0.024 | 0.005 | <0.001 |
| UV-exposed body site | 0.266 | 0.040 | <0.001 | 0.968 | 0.115 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drexler, K.; Drexler, H.; Karrer, S.; Landthaler, M.; Haferkamp, S.; Zeman, F.; Berneburg, M.; Niebel, D. Degree of Actinic Elastosis Is a Surrogate of Exposure to Chronic Ultraviolet Radiation and Correlates More Strongly with Cutaneous Squamous Cell Carcinoma than Basal Cell Carcinoma. Life 2023, 13, 811. https://doi.org/10.3390/life13030811
Drexler K, Drexler H, Karrer S, Landthaler M, Haferkamp S, Zeman F, Berneburg M, Niebel D. Degree of Actinic Elastosis Is a Surrogate of Exposure to Chronic Ultraviolet Radiation and Correlates More Strongly with Cutaneous Squamous Cell Carcinoma than Basal Cell Carcinoma. Life. 2023; 13(3):811. https://doi.org/10.3390/life13030811
Chicago/Turabian StyleDrexler, Konstantin, Hans Drexler, Sigrid Karrer, Michael Landthaler, Sebastian Haferkamp, Florian Zeman, Mark Berneburg, and Dennis Niebel. 2023. "Degree of Actinic Elastosis Is a Surrogate of Exposure to Chronic Ultraviolet Radiation and Correlates More Strongly with Cutaneous Squamous Cell Carcinoma than Basal Cell Carcinoma" Life 13, no. 3: 811. https://doi.org/10.3390/life13030811
APA StyleDrexler, K., Drexler, H., Karrer, S., Landthaler, M., Haferkamp, S., Zeman, F., Berneburg, M., & Niebel, D. (2023). Degree of Actinic Elastosis Is a Surrogate of Exposure to Chronic Ultraviolet Radiation and Correlates More Strongly with Cutaneous Squamous Cell Carcinoma than Basal Cell Carcinoma. Life, 13(3), 811. https://doi.org/10.3390/life13030811









