In Vitro Effects of Photon Beam and Carbon Ion Radiotherapy on the Perineural Invasion of Two Cell Lines of Neurotropic Tumours
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. Irradiations
2.3. Cell Viability
2.4. Cell Proliferation
2.5. Cell Migration
2.6. Statistical Analysis
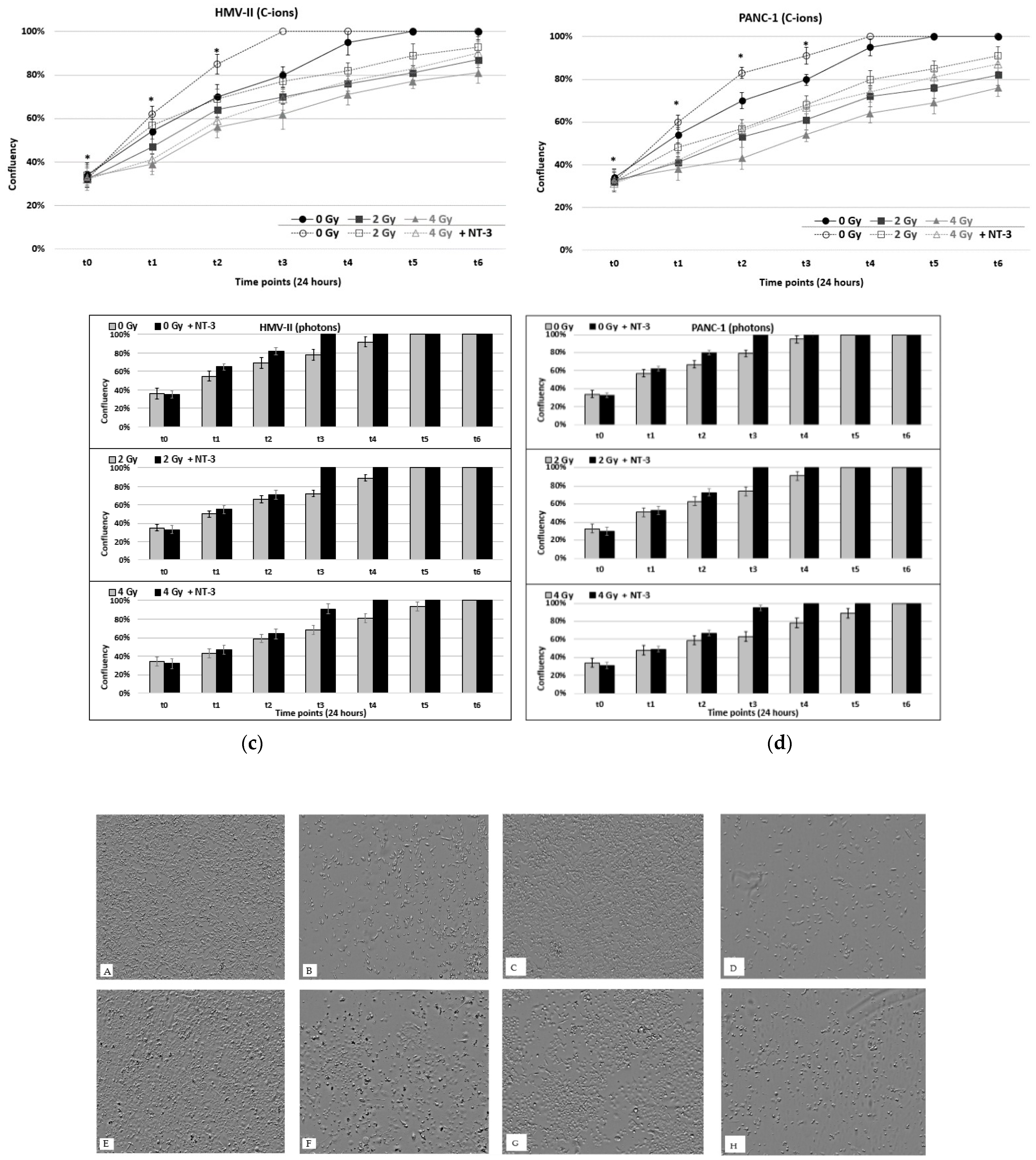
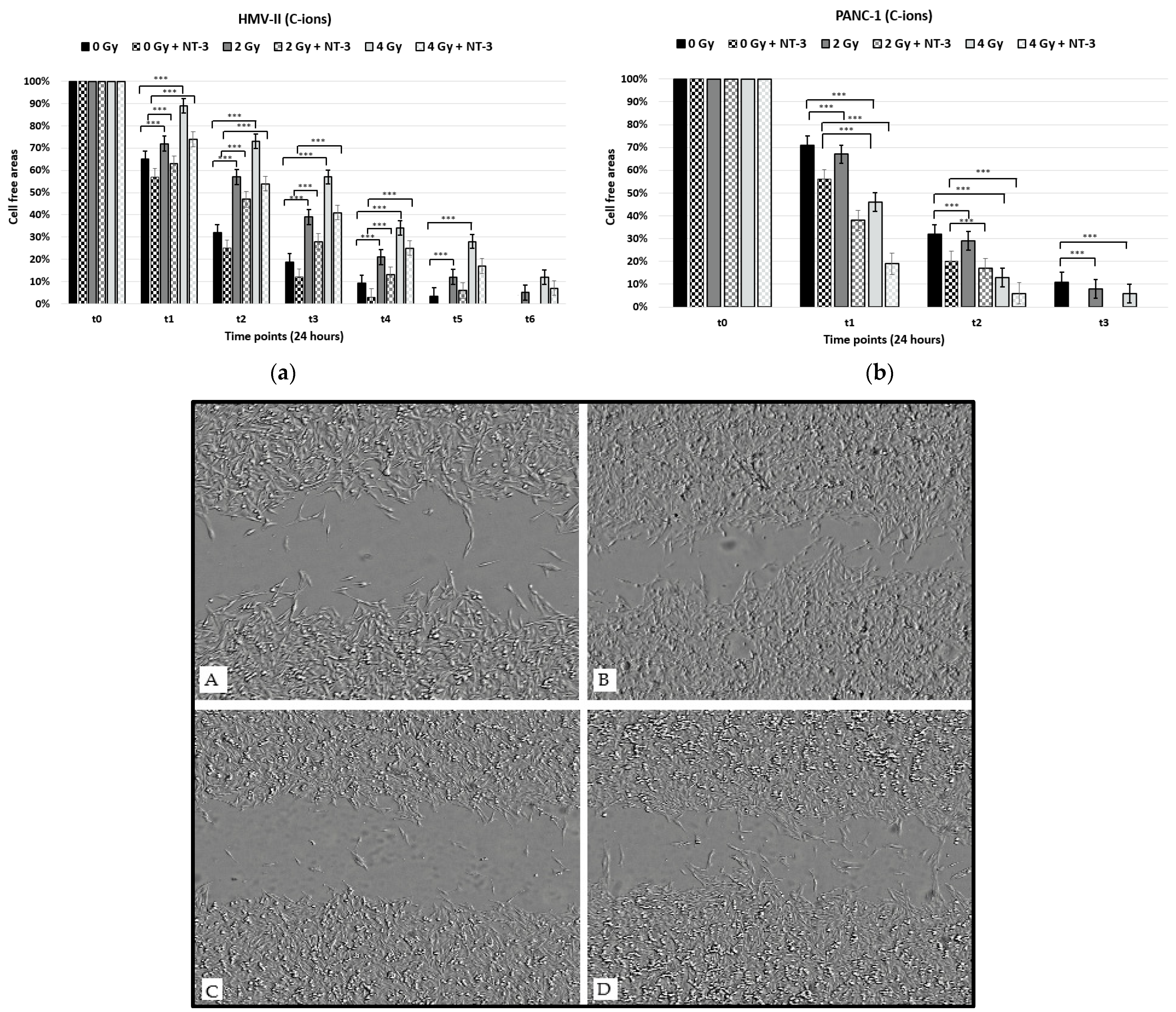
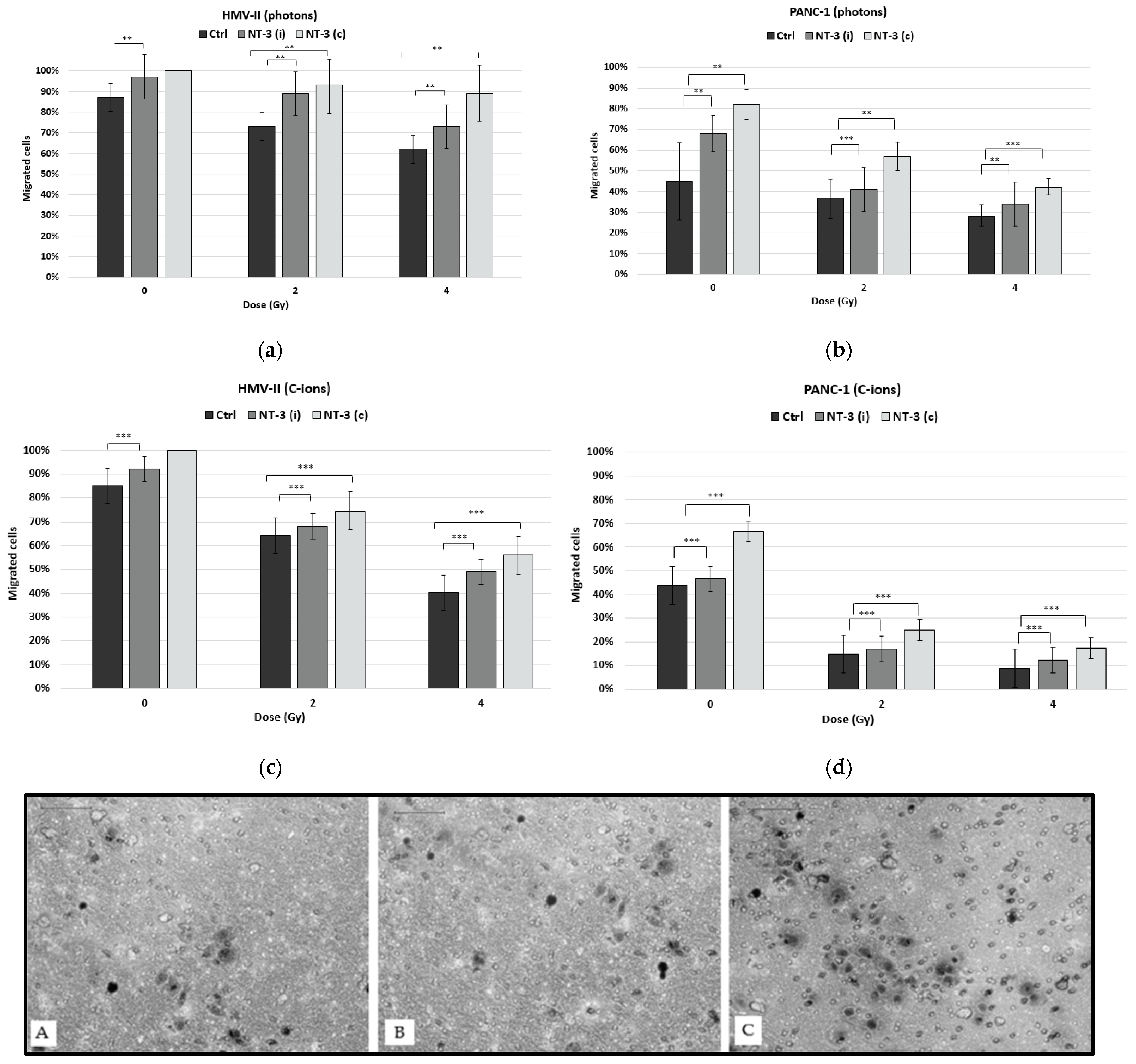
3. Results
3.1. Cell Viability and Proliferation
3.2. Cell Migration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, F.; Saidou, J.; Watabe, K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front. Biosci. Landmark Ed. 2010, 15, 166–179. [Google Scholar] [CrossRef]
- Demir, I.E.; Friess, H.; Ceyhan, G.O. Nerve-cancer interactions in the stromal biology of pancreatic cancer. Front. Physiol. 2012, 3, 97. [Google Scholar] [CrossRef]
- Taș, F.; Erturk, K. Neurotropism as a prognostic factor in cutaneous melanoma patients. Neoplasma 2018, 65, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Benda, J.A.; Platz, C.E.; Anderson, B. Malignant Melanoma of the Vulva: A Clinical-Pathologic Review of 16 Cases. Int. J. Gynecol. Pathol. 1986, 5, 202–216. [Google Scholar] [CrossRef]
- Prasad, M.L.; Patel, S.G.; Busam, K.J. Primary mucosal desmoplastic melanoma of the head and neck. Am. J. Clin. Pathol. 2002, 118, 648. [Google Scholar] [CrossRef] [PubMed]
- Massi, G.; Leboit, P.E. Neurotropic Melanoma. In Histological Diagnosis of Nevi and Melanoma; Steinkopff: Heidelberg, Germany, 2004. [Google Scholar] [CrossRef]
- Tan, X.; Sivakumar, S.; Bednarsch, J.; Wiltberger, G.; Kather, J.N.; Niehues, J.; de Vos-Geelen, J.; Iersel, L.V.-V.; Kintsler, S.; Roeth, A.; et al. Nerve fibers in the tumor microenvironment in neurotropic cancer—Pancreatic cancer and cholangiocarcinoma. Oncogene 2021, 40, 899–908. [Google Scholar] [CrossRef]
- Chatzistefanou, I.; Lubek, J.; Markou, K.; Ord, R.A. The role of perineural invasion in treatment decisions for oral cancer patients: A review of the literature. J. Cranio-Maxillofac. Surg. 2017, 45, 821–825. [Google Scholar] [CrossRef]
- O′Regan, K.; Breen, M.; Ramaiya, N.; Jagannathan, J.; DiPiro, P.J.; Hodi, F.S.; Abbeele, A.D.V.D. Metastatic mucosal melanoma: Imaging patterns of metastasis and recurrence. Cancer Imaging 2013, 13, 626–632. [Google Scholar] [CrossRef]
- Brown, I.S. Pathology of Perineural Spread. J. Neurol. Surg. B Skull Base 2016, 77, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Narayan, P.; Flynn, J.; Zhang, Z.; Gillespie, E.F.; Mueller, B.; Xu, A.J.; Cuaron, J.; McCormick, B.; Khan, A.J.; Cahlon, O.; et al. Perineural invasion as a risk factor for locoregional recurrence of invasive breast cancer. Sci. Rep. 2021, 11, 12781. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, Z.L.; Gao, X.L.; Wu, J.S.; Liang, X.H.; Tang, Y.L. Functions of chemokines in the perineural invasion of tumors (Review). Int. J. Oncol. 2018, 52, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzi, E.; Angioni, R.; Molon, B.; Calì, B. Chemokines and Chemokine Receptors: Orchestrating Tumor Metastasization. Int. J. Mol. Sci. 2019, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Raman, D.; Baugher, P.J.; Thu, Y.M.; Richmond, A. Role of chemokines in tumor growth. Cancer Lett. 2007, 256, 137–165. [Google Scholar] [CrossRef]
- Marchesi, F.; Piemonti, L.; Mantovani, A.; Allavena, P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010, 21, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Winkler, F. Insights and opportunities at the crossroads of cancer and neuroscience. Nature 2022, 24, 1454–1460. [Google Scholar] [CrossRef]
- Ohta, T.; Numata, M.; Tsukioka, Y.; Futagami, F.; Kayahara, M.; Kitagawa, H.; Nakamura, Y. Neurotrophin-3 expression in human pancreatic cancers. J. Pathol. 1997, 181, 405–412. [Google Scholar] [CrossRef]
- Weeraratna, A.T.; Arnold, J.T.; George, D.J.; DeMarzo, A.; Isaacs, J.T. Rational basis for Trk inhibition therapy for prostate cancer. Prostate 2000, 45, 140–148. [Google Scholar] [CrossRef]
- Yamauchi, J.; Chan, J.R.; Shooter, E.M. Neurotrophin 3 activation of TrkC induces Schwann cell migration through the c-Jun N-terminal kinase pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 14421–14426. [Google Scholar] [CrossRef]
- Kawashiro, S.; Yamada, S.; Okamoto, M.; Ohno, T.; Nakano, T.; Shinoto, M.; Shioyama, Y.; Nemoto, K.; Isozaki, Y.; Tsuji, H.; et al. Multi-institutional Study of Carbon-ion Radiotherapy for Locally Advanced Pancreatic Cancer: Japan Carbon-ion Radiation Oncology Study Group (J-CROS) Study 1403 Pancreas. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1212–1221. [Google Scholar] [CrossRef]
- Liermann, J.; Naumann, P.; Hommertgen, A.; Pohl, M.; Kieser, M.; Debus, J.; Herfarth, K. Carbon ion radiotherapy as definitive treatment in non-metastasized pancreatic cancer: Study protocol of the prospective phase II PACK-study. BMC Cancer 2020, 20, 947. [Google Scholar] [CrossRef]
- Broerse, J.; Barendsen, G.W.; Van Kersen, G.R. Survival of Cultured Human Cells after Irradiation with Fast Neutrons of Different Energies in Hypoxic and Oxygenated Conditions. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1968, 13, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, H.; Xing, M.; Qin, J.; Zhang, H.; Liu, Y.; Zhang, L.; Zhang, C.; Tian, Z.; Gao, X.; et al. Carbon Ion Induces Cell Death and G2/M Arrest Through pRb/E2F1Chk2/Cdc2 Signaling Pathway in X-ray Resistant B16F10 Melanoma Cells. Dose-Response 2022, 20, 15593258221092364. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Okonogi, N.; Wakatsuki, M.; Kato, S.; Kiyohara, H.; Karasawa, K.; Ohno, T.; Nakano, T.; Kamada, T.; Shozu, M.; et al. Long-Term Outcomes of Carbon-Ion Radiotherapy for Malignant Gynecological Melanoma. Cancers 2019, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Liermann, J.; Shinoto, M.; Syed, M.; Debus, J.; Herfarth, K.; Naumann, P. Carbon ion radiotherapy in pancreatic cancer: A review of clinical data. Radiother. Oncol. 2020, 147, 145–150. [Google Scholar] [CrossRef]
- Moncharmont, C.; Levy, A.; Guy, J.B.; Falk, A.T.; Guilbert, M.; Trone, J.C. Radiation-enhanced cell migration/invasion process: A review. Crit. Rev. Oncol. Hematol. 2014, 92, 133–142. [Google Scholar] [CrossRef]
- Fujita, M.; Otsuka, Y.; Yamada, S.; Iwakawa, M.; Imai, T. X-ray irradiation and Rho-kinase inhibitor additively induce invasiveness of the cells of the pancreatic cancer line MIAPaCa-2, which exhibits mesenchymal andamoeboid motility. Cancer Sci. 2011, 102, 792–798. [Google Scholar] [CrossRef]
- Fujita, M.; Otsuka, Y.; Imadome, K.; Endo, S.; Yamada, S.; Imai, T. Carbon-ion radiation enhances migration ability and invasiveness of the pancreatic cancer cell PANC-1, in vitro. Cancer Sci. 2012, 103, 677–683. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Wang, W.; Wang, J.; Zhang, J.; Liu, J.; Yang, T.; Yang, Y.; Wei, J.; Lei, D.; et al. NT-3/TrkC Axis Contributes to the Perineural Invasion and the Poor Prognosis in Human Salivary Adenoid Cystic Carcinoma. J. Cancer 2019, 10, 6065–6073. [Google Scholar] [CrossRef]
- Malo, M.E.; Bryan, R.A.; Shuryak, I.; Dadachova, E. Morphological changes in melanized and non-melanized Cryptococcus neoformans cells post exposure to sparsely and densely ionizing radiation demonstrate protective effect of melanin. Fungal Biol. 2018, 122, 449–456. [Google Scholar] [CrossRef]
- Garbe, C.; Eigentler, T.K.; Keilholz, U.; Hauschild, A.; Kirkwood, J.M. Systematic Review of Medical Treatment in Melanoma: Current Status and Future Prospects. Oncol. 2011, 16, 5–24. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Zhang, Y.; Ye, Y.; Qi, Y.; Hu, T.; Pan, X. Carbon ion radiotherapy with complete tumor regression for primary malignant melanoma of female urethra orifice: A case report. J. Int. Med. Res. 2022, 50, 03000605211072795. [Google Scholar] [CrossRef] [PubMed]
- Barcellini, A.; Vitolo, V.; Facoetti, A.; Fossati, P.; Preda, L.; Fiore, M.R.; Vischioni, B.; Iannalfi, A.; Bonora, M.; Ronchi, S.; et al. Feasibility of Carbon Ion Radiotherapy in the Treatment of Gynecological Melanoma. In Vivo 2019, 33, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, S.; Ronchi, S.; Barcellini, A.; Bonora, M.; Vischioni, B.; Vitolo, V.; Villa, R.; Del Vecchio, M.; Licitra, L.; Orlandi, E. Toxicity of carbon ion radiotherapy and immune checkpoint inhibitors in advanced melanoma. Radiother. Oncol. 2021, 164, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef]
- National Cancer Institute. Surveillance Epidemiology and End Results Cancer Statistics Review 1975–2006; National Cancer Institute: Rockville, MD, USA, 2011. [Google Scholar]
- Rau, B.M.; Moritz, K.; Schuschan, S.; Alsfasser, G.; Prall, F.; Klar, E. R1 resection in pancreatic cancer has significant impact on long-term outcome in standardized pathology modified for routine use. Surgery 2012, 152, S103–S111. [Google Scholar] [CrossRef]
- Barugola, G.; Falconi, M.; Bettini, R.; Boninsegna, L.; Casarotto, A.; Salvia, R.; Bassi, C.; Pederzoli, P. The determinant factors of recurrence following resection for ductal pancreatic cancer. JOP 2007, 8, 132–140. [Google Scholar]
- Seshacharyulu, P.; Baine, M.J.; Souchek, J.; Menning, M.; Kaur, S.; Yan, Y.; Ouellette, M.M.; Jain, M.; Lin, C.; Batra, S.K. Biological determinants of radioresistance and their remediation in pancreatic cancer. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2017, 1868, 69–92. [Google Scholar] [CrossRef]
- Trappetti, V.; Fazzari, J.; Fernandez-Palomo, C.; Scheidegger, M.; Volarevic, V.; Martin, O.; Djonov, V. Microbeam Radiotherapy—A Novel Therapeutic Approach to Overcome Radioresistance and Enhance Anti-Tumour Response in Melanoma. Int. J. Mol. Sci. 2021, 22, 7755. [Google Scholar] [CrossRef]
- Espenel, S.; Vallard, A.; Rancoule, C.; Garcia, M.-A.; Guy, J.-B.; Chargari, C.; Deutsch, E.; Magné, N. Melanoma: Last call for radiotherapy. Crit. Rev. Oncol. 2016, 110, 13–19. [Google Scholar] [CrossRef]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- Deborde, S.; Wong, R.J. How Schwann cells facilitate cancer progression in nerves. Cell. Mol. Life Sci. 2017, 74, 4405–4420. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Zhang, B.-Y.; Zhou, B.; Zhu, C.-Z.; Sun, L.-Q.; Feng, Y.-J. Perineural invasion of cancer: A complex crosstalk between cells and molecules in the perineural niche. Am. J. Cancer Res. 2019, 9, 1–21. [Google Scholar]
- Chan, J.R.; Cosgaya, J.M.; Wu, Y.J.; Shooter, E.M. Glucocorticoids and progestins signal the initiation and enhance the rate of myelin formation. Proc. Natl. Acad. Sci. USA 2001, 95, 10459–10464. [Google Scholar] [CrossRef]
- Cosgaya, J.M.; Chan, J.R.; Shooter, E.M. The neurotrophin receptor p75NTR as a positive modulator of myelination. Science 2002, 298, 1245–1248. [Google Scholar] [CrossRef]
- Elsayed, M.; Abdelrahim, M. The Latest Advancement in Pancreatic Ductal Adenocarcinoma Therapy: A Review Article for the Latest Guidelines and Novel Therapies. Biomedicines 2021, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Jethwa, K.R.; Neibart, S.S.; Truty, M.J.; Jabbour, S.K.; Hallemeier, C.L. Patterns of Recurrence After Primary Local Therapy for Pancreatic Ductal Adenocarcinoma—A Critical Review of Rationale and Target Delineation for (Neo)Adjuvant Radiation Therapy. Pract. Radiat. Oncol. 2022, 12, e463–e473. [Google Scholar] [CrossRef]
- Bakst, R.L.; Lee, N.; He, S.; Chernichenko, N.; Chen, C.-H.; Linkov, G.; Le, H.C.; Koutcher, J.; Vakiani, E.; Wong, R.J. Radiation Impairs Perineural Invasion by Modulating the Nerve Microenvironment. PLoS ONE 2012, 7, e39925. [Google Scholar] [CrossRef] [PubMed]
- Frydenlund, N.; Mahalingam, M. Desmoplastic Melanoma, Neurotropism, and Neurotrophin Receptors—What We Know and What We Do Not. Adv. Anat. Pathol. 2015, 22, 227–241. [Google Scholar] [CrossRef]
- Zhu, S.; Mendenhall, W.M. Radiotherapy for Melanoma with Perineural Invasion: University of Florida Experience. Cancer Investig. 2018, 36, 389–394. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charalampopoulou, A.; Barcellini, A.; Frittitta, G.E.; Fulgini, G.; Ivaldi, G.B.; Magro, G.; Liotta, M.; Orlandi, E.; Pullia, M.G.; Tabarelli de Fatis, P.; et al. In Vitro Effects of Photon Beam and Carbon Ion Radiotherapy on the Perineural Invasion of Two Cell Lines of Neurotropic Tumours. Life 2023, 13, 794. https://doi.org/10.3390/life13030794
Charalampopoulou A, Barcellini A, Frittitta GE, Fulgini G, Ivaldi GB, Magro G, Liotta M, Orlandi E, Pullia MG, Tabarelli de Fatis P, et al. In Vitro Effects of Photon Beam and Carbon Ion Radiotherapy on the Perineural Invasion of Two Cell Lines of Neurotropic Tumours. Life. 2023; 13(3):794. https://doi.org/10.3390/life13030794
Chicago/Turabian StyleCharalampopoulou, Alexandra, Amelia Barcellini, Giuseppe Emanuele Frittitta, Giorgia Fulgini, Giovanni Battista Ivaldi, Giuseppe Magro, Marco Liotta, Ester Orlandi, Marco Giuseppe Pullia, Paola Tabarelli de Fatis, and et al. 2023. "In Vitro Effects of Photon Beam and Carbon Ion Radiotherapy on the Perineural Invasion of Two Cell Lines of Neurotropic Tumours" Life 13, no. 3: 794. https://doi.org/10.3390/life13030794
APA StyleCharalampopoulou, A., Barcellini, A., Frittitta, G. E., Fulgini, G., Ivaldi, G. B., Magro, G., Liotta, M., Orlandi, E., Pullia, M. G., Tabarelli de Fatis, P., & Facoetti, A. (2023). In Vitro Effects of Photon Beam and Carbon Ion Radiotherapy on the Perineural Invasion of Two Cell Lines of Neurotropic Tumours. Life, 13(3), 794. https://doi.org/10.3390/life13030794








