Abstract
Native T1, extracellular volume fraction (ECV), and late gadolinium enhancement (LGE) characterize myocardial tissue and relate to patient prognosis in a variety of diseases, including pulmonary hypertension. The purpose of this study was to evaluate if left ventricle (LV) fibrosis measurements have prognostic value for cardiac outcomes in pulmonary hypertension subgroups. 54 patients with suspected pulmonary hypertension underwent right-heart catheterization and were classified into pulmonary hypertension subgroups: pre-capillary component (PreCompPH) and isolated post-capillary (IpcPH). Cardiac magnetic resonance imaging (MRI) scans were performed with the acquisition of balanced cine steady-state free precession, native T1, and LGE pulse sequences to measure cardiac volumes and myocardial fibrosis. Associations between cardiac events and cardiac MRI measurements were analyzed within PreCompPH and IpcPH patients. IpcPH: LV native T1 was higher in patients who experienced a cardiac event within two years vs. those who did not. In patients with LV native T1 > 1050 ms, the rate of cardiac events was higher. ECV and quantitative LGE did not differ between groups. PreCompPH: native T1, ECV, and quantitative/qualitative LGE did not differ between patients who experienced a cardiac event within two years vs. those who did not. LV native T1 may have potential value for forecasting cardiac events in IpcPH, but not in PreCompPH, patients.
1. Introduction
Pulmonary hypertension (PH) is a progressive, life-threatening, and multifactorial disease process that is characterized by an elevated resting mean pulmonary arterial pressure (mPAP) [1,2]. PH is broadly classified into pre-capillary PH (PrePH), isolated post-capillary PH (IpcPH), and combined pre- and post-capillary PH (CPH) based on clinical presentation and hemodynamic measurements [3,4]. Mechanistically, PrePH is defined as pulmonary vascular remodeling associated with an increase in pulmonary vascular resistance (PVR). IpcPH is defined as left-sided heart disease associated with increased pulmonary venous pressures (as measured by pulmonary capillary wedge pressure; PCWP). CPH represents a progression of IpcPH and is defined by concomitant increases in PVR and PCWP. While PrePH, IpcPH, and CPH all lead to right ventricular (RV) failure, elevated PVR heralds RV dysfunction in both PrePH and CPH (collectively termed: Pre-Capillary Component PH [5]; PreCompPH) while incipient left ventricular (LV) dysfunction characterizes the process in IpcPH. PH classification is used to guide optimal treatment and to predict prognosis [6].
Right heart catheterization (RHC) has been the gold-standard diagnostic and prognostic tool for PH. However, because of its invasive nature, there is increasing exploration of alternative prognostic markers in PH patients. In PH patients, cardiopulmonary exercise testing [7] and cardiac MRI measurements [8] have shown correlations with RHC measurements. Cardiac MRI, specifically, has been shown to provide sensitive biomarkers capable of distinguishing PH subgroups [9,10].
Ventricular fibrosis measurements have been shown to provide more sensitive prognoses in patients with LV diastolic dysfunction [11] and systemic sclerosis [12], compared to functional measurements alone. Cardiac MRI can be used to evaluate fibrosis through the detection of increased extracellular space (late gadolinium enhancement, LGE; extracellular volume fraction, ECV) through increased volume measures of extra- and intra-cellular space (native T1). These measures may have clinical value for outcome prediction in patients with cardiac disease [12,13,14,15]. Previous prognostic studies relate to RV functional measurements in PrePH [16,17,18].
Given the disparate pathophysiologic processes leading to an elevated pulmonary artery pressure, we hypothesized that LV fibrosis measurements would have prognostic significance in IpcPH, and not in PreCompPH.
2. Materials and Methods [10]
2.1. Subjects
Approval from the Institutional Review Board (IRB) was obtained, and all subjects provided written informed consent. The initial cohort was identical to that used in a previous study investigating 4D flow-derived velocities in PH patients [8]. Patients with suspected PH who had undergone standard-of-care RHC were identified. Patients with a mPAP ≥ 25 mmHg at rest or mPAP > 30 mmHg during exercise were recruited to undergo a cardiac MRI protocol with venous blood sampling for hematocrit within 28 days of cardiac catheterization. Patients were enrolled between August 2017 and March 2020. Exclusion criteria included: allergy to gadolinium-based contrast agents; severe kidney disease (estimated glomerular filtration rate < 30 mL/min/1.73 m2; acute kidney injury; kidney or liver transplant within 8 weeks; any contraindication to MRI (i.e., claustrophobia); pregnant or breastfeeding women; adults unable to provide consent; children; prisoners.
2.1.1. Right Heart Catheterization
Standard-of-care RHC was performed with a 7–9 French sheath via the internal jugular or femoral veins. A Swan-Ganz catheter, connected to an analog pressure recorder, was used to obtain mPAP, systolic PAP, diastolic PAP, pulmonary capillary wedge pressure (PCWP) and right ventricular cardiac output (QP). PVR (in Wood Units (WU)) was calculated using the formula: PVRRHC = ΔP/QP, where ΔP is the trans-pulmonary pressure gradient (ΔP = mPAP − PCWP) and QP is the flow in the pulmonary artery measured by the Fick principle using the pulmonary artery oxygen saturation.
2.1.2. Classification
PH patients were classified (MV: a cardiologist with 5 years of experience in cardiac imaging) based on a review of their clinical courses, therapeutic histories, and hemodynamic measurements. Patients’ clinical courses and therapeutic histories were considered in addition to hemodynamic measurements, during classification, because invasive parameters are known to vary with a patient’s fluid and metabolic status, and it has been suggested that hemodynamics should be interpreted in the context of the clinical picture [4]. Patients with pulmonary arterial hypertension, pulmonary hypertension due to chronic lung disease, and chronic thromboembolic PH) were considered pre-capillary PH [19]. Patients with pulmonary hypertension due to left heart disease were considered IpcPH or CPH, based on PVR measurements (PVR < 3 WU or PVR ≥ 3, respectively). CPH and PrePH patients were collectively designated as PreCompPH patients.
2.1.3. Clinical Data Collection
The electronic medical record (EMR) was reviewed to determine if a cardiac event occurred after patients’ cardiac MRI scans. Cardiac events were defined as any of the following: heart failure requiring intravenous diuretics; palpitations prompting inpatient observation; or coronary revascularization. If any of these events occurred, the elapsed time between the MRI scan and the event was recorded. Comorbidities (history of smoking, hypertension, hyperlipidemia, diabetes mellitus) and functional status within 4 months of MRI (New York Heart Association (NYHA) functional class, six-minute walk distance, brain natriuretic peptide (BNP), supplemental oxygen use, ascites, peripheral edema) were also retrieved from the EMR (CS: a medical student with 2 years of experience).
2.2. Cardiac MRI Data Acquisition [10]
The Cardiac MRI scans were performed by certified technicians using a 1.5 T MRI system (MAGNETOM Aera; Siemens Healthcare, Erlangen, Germany). A three-plane fast localization sequence was used to determine anatomic orientations for subsequent sequences, and four-chamber, two-chamber, and short-axis localizer views were obtained. The pre-contrast portion of the protocol consisted of multiplanar segmented balanced cine steady-state free precession (bSSFP) and native T1 mapping (Modified Look-Locker inversion recovery (MOLLI) technique). After contrast administration (Gadobutrol, 0.1 mL/kg), LGE and MOLLI T1 mapping sequences were performed (10 min and 15 min after contrast, respectively). The combined duration of the MRI sequences was 30 min.
The bSSFP cine acquisition was performed in the two-, three-, four-chamber, and short-axis orientations. Data were acquired during breath holds at end-expiration using retrospective electrocardiogram (ECG) gating. Cine sequence MRI parameters were as follows: field of view (FOV) read = 340 mm × 340 mm, spatial resolution = 1.8 mm × 1.8 mm × 6.0 mm, temporal resolution = 35.49 ms, flip angle = 80°, echo time/repetition time (TE/TR) = 1.16/35.49 ms.
The gradient recalled echo (GRE) bSSFP MOLLI acquisitions were collected pre- and post-contrast (delay = 10 min) with sequence parameters as follows: TE 1.33 ms, flip angle 35°, slice thickness 8.00 mm, pixel size = 1.0 × 1.0 mm2, Generalized Auto-calibrating Partial Parallel Acquisition (GRAPPA) with an acceleration factor, R = 2. Imaging reconstruction included the auto-calculation of parametric LV T1 maps.
The LGE pulse sequences were performed with either of two Phase-sensitive Inversion–Recovery (PSIR) pulse sequences: (1) a 2D inversion–recovery (IR) balanced steady-state free precession (bSSFP) pulse sequence (Echo spacing: 2.5 ms, TE 1.05 ms, flip angle 40°, slice thickness 6.0 mm, pixel size = 2.0 × 2.0 mm2, GRAPPA with an acceleration factor, R = 2) a segmented 2D IR gradient recalled echo (turboFLASH) pulse sequence (Echo spacing: 8.4 ms, TE 3.25 ms, flip angle 25°, slice thickness 6 mm, pixel size = 1.3 × 1.3 mm2, GRAPPA with an acceleration factor, R = 2).
2.3. Cardiac MRI Analysis [10]
2.3.1. Volumetric Measurements
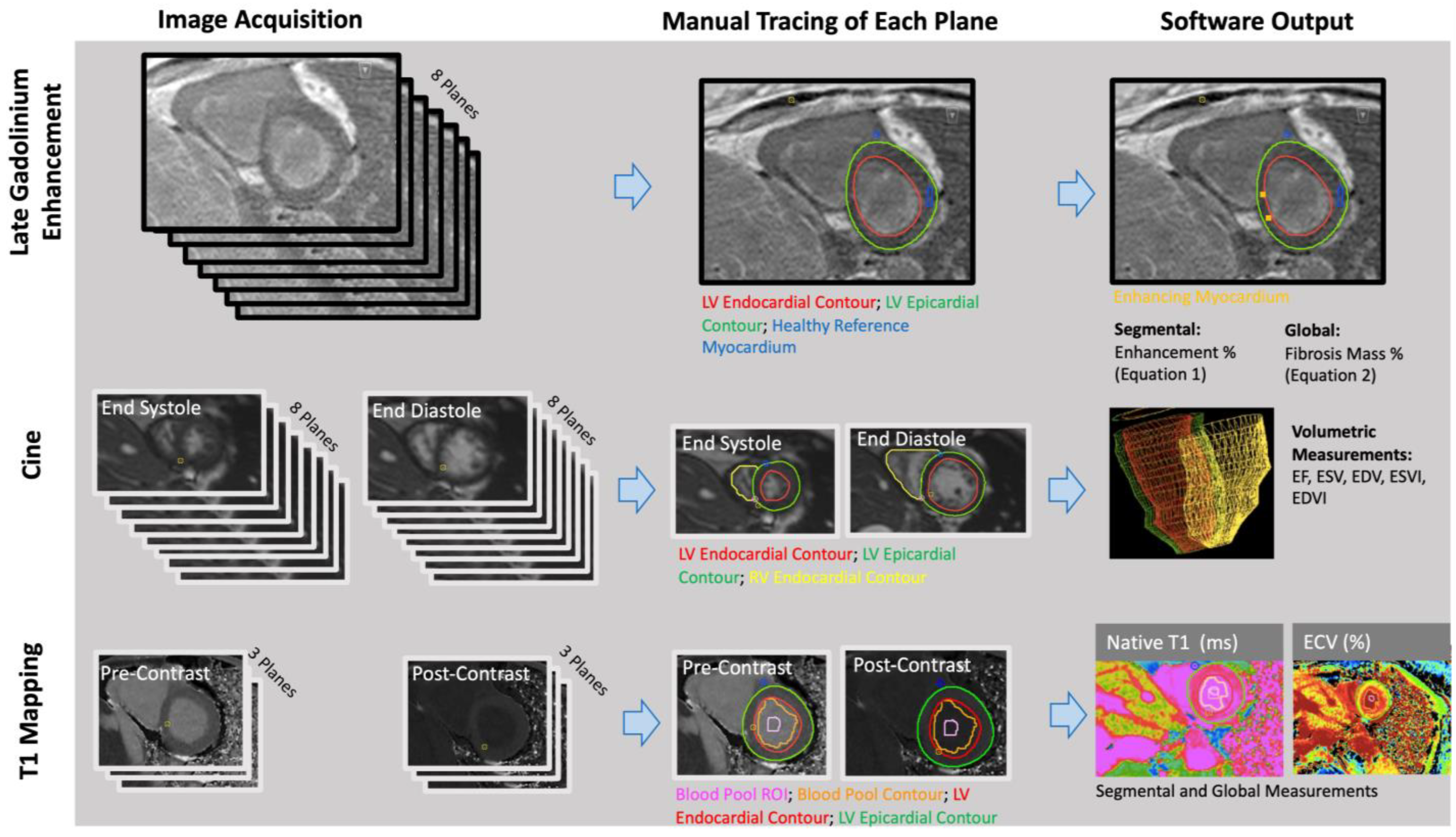
The RV and LV endocardial borders were manually contoured on short-axis bSSFP cine images at the peak end-diastolic and end-systolic time frames using CVI42 (Circle Cardiovascular Imaging, Calgary, AB, Canada) (Figure 1). CVI42 generated ejection fractions, end-diastolic volumes, and end-systolic volumes of the right and left ventricles (RVEF, RVEDV, RVESV; LVEF, LVEDV, LVESV). Each patient’s body surface area (BSA) was calculated using the Mosteller equation, and volumes were divided by BSA to get end-systolic and end-diastolic volume indices (RVEDVI, RVESVI, LVEDVI, LVESVI).
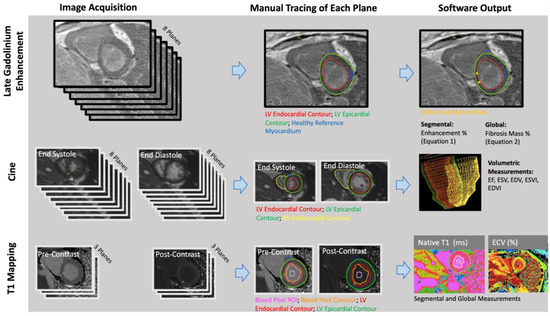
Figure 1.
Late gadolinium enhancement (LGE), volumetric, and parametric mapping measurements were obtained with similar workflows. The number of planes obtained and the traced regions differed for each analysis (Reprinted with permission from Ref. [10]. 2022, John W. Cerne).
2.3.2. Late Gadolinium Enhancement
Quantitative: A semi-automated technique was used to quantify myocardial fibrosis through manual thresholding of the short-axis LGE images on CVI42 (Figure 1). A research fellow (JWC: 1 year of experience in cardiothoracic imaging) performed manual tracings of the epicardial and endocardial LV borders. Regions of fibrosis were defined by reference to a manually delineated region of the normal myocardium. Voxels with intensities that were 4 standard deviations above the average intensity of normal myocardium were considered fibrosis. Images were anonymized and analyzed in a blinded fashion and in a random order.
For each scan, global LGE was calculated (Equation (1)) [20]
Qualitative: A radiologist (KS: 2 years of experience in cardiothoracic imaging) performed a qualitative analysis of LGE presence. Patients were determined to have: no LGE; or LGE at one insertional point; or LGE at both insertional points. To evaluate interobserver reliability, a second radiologist (BDA: 4 years of experience in cardiothoracic imaging) assessed the presence and location of LGE in a randomly selected subset of 10 subjects.
2.3.3. Native T1 Mapping
Manual segmentation of T1 maps was performed (AP: a research fellow with 3 years of experience in cardiothoracic imaging) on CVI42 (Figure 1). The LV epicardium/endocardium was manually contoured, and regions of interest within the blood pool cavity were demarcated on native t1 and postcontrast images (AP) (Figure 1). The basal, mid, and apical slices from pre- and post-contrast T1 maps were used with patients’ hematocrit values to obtain pixel-wise ECV values (Equation (2)).
Pixel-wise values were converted by the software into average values, on a segment-by-segment basis, for each of the American Heart Association (AHA)-defined myocardial segments. Segmental values were averaged to obtain global ECV and native T1 values for each scan [21].
2.4. Statistical Analysis
Descriptive statistics were recorded based on the nature of the compared variables: mean ± standard deviation (continuous variables, normal distribution); median (interquartile range) (continuous variable, non-normal distribution); frequency (percentage) (categorical variables). Shapiro–Wilk test was used to assess continuous variables for normality.
Continuous variables were compared between two groups with independent samples Student’s t-test preceded by Levene’s test (normally distributed) or Mann–Whitney U test (non-normally distributed). Chi-square statistics with Yates correction or Fisher’s exact test were used to compare categorical data, depending on the size of the groups being compared (>/=5 and <5, respectively). Kaplan–Meier curve analysis and log-rank test were used to assess cumulative cardiac events.
Statistical analyses were performed with SPSS (IBM corporation, Chicago, IL, USA). All tests were two-tailed with p < 0.05 considered statistically significant.
3. Results
3.1. Patient Characteristics
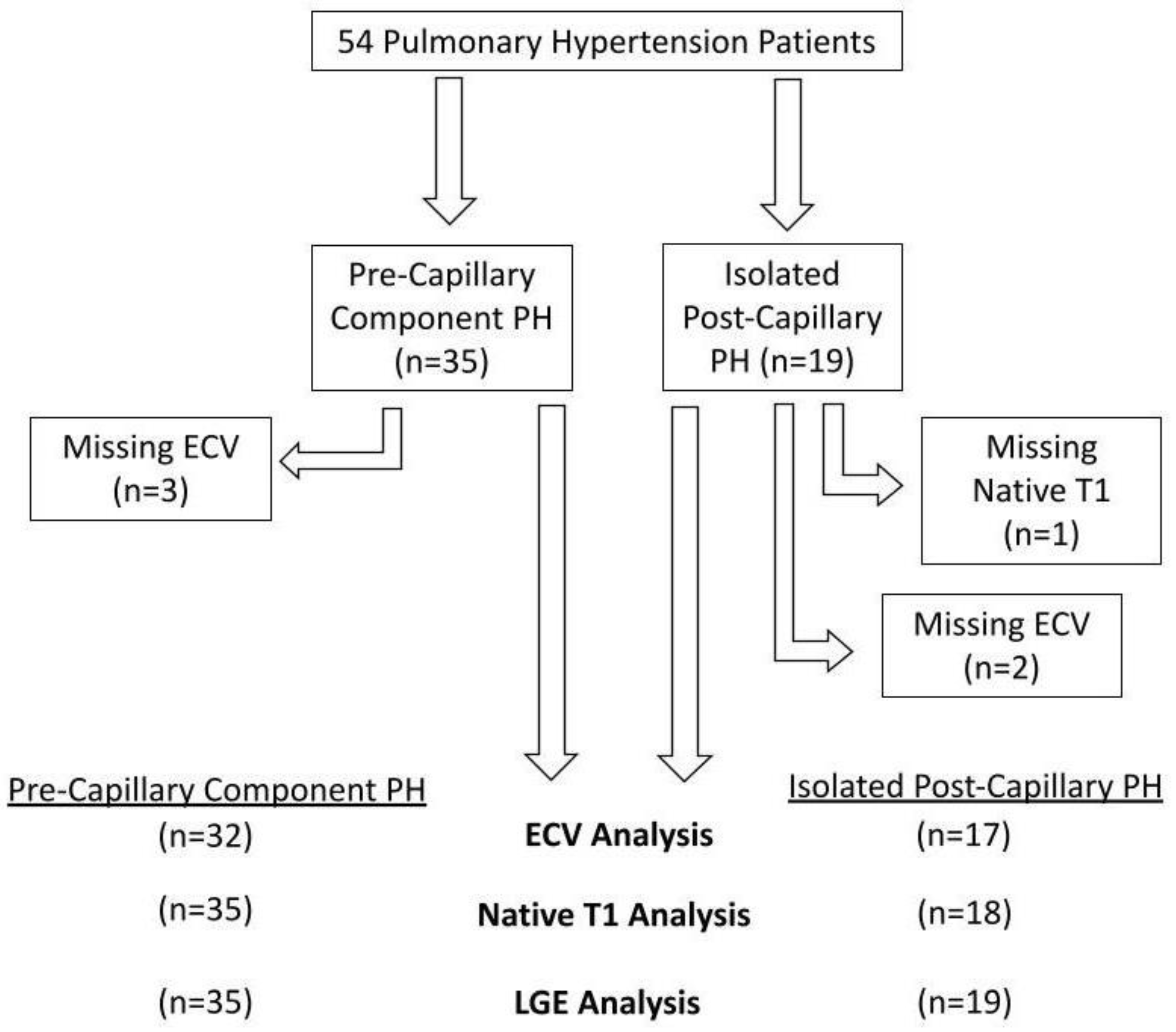
Fifty-four patients were involved in the assessment (35 PreCompPH and 19 IpcPH patients). Patients’ clinical information is depicted in Table 1. Functional status data within 4 months of the MRI scan was not found for all patients: 40/54 patients had a NYHA functional status designation; 12/54 patients had a six-minute walk distance; 30/54 patients had a BNP measurement. There was an inability to calculate ECV in five subjects due to the following reasons: missing post-contrast T1 MOLLI images (n = 2); severe zebra artifact (n = 1); misaligned slice positioning (n = 1); post-contrast dark blood misregistration (n = 1). Native T1 and volumetric indices could not be obtained due to zebra artifact in one subject (n = 1) (Figure 2). Receiver operating curve (ROC) analyses of mPAP and PVR within PreCompPH and within IpcPH patients showed no statistically significant area-under-curve (AUC) values for the occurrence of a cardiac event within 2 years of the cardiac MRI scan (Table 2 and Table 3).

Table 1.
Patient characteristics.
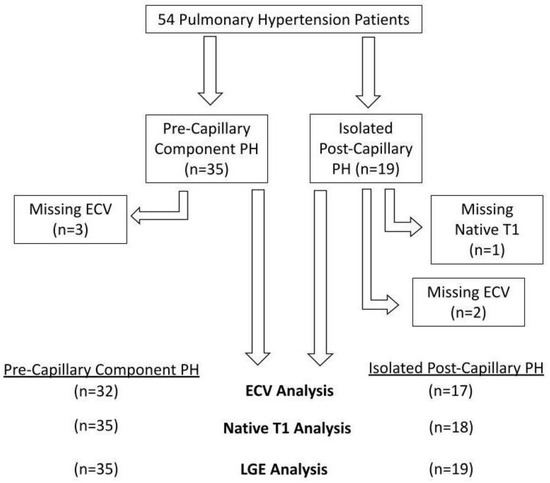
Figure 2.
Flow-chart detailing excluded data.

Table 2.
ROC analysis for the occurrence of a cardiac event within 2 years of cardiac MRI scan in pre-capillary component PH.

Table 3.
ROC analysis for the occurrence of a cardiac event within 2 years of cardiac MRI scan in isolated post-capillary PH.
3.2. Volumetric Measurements
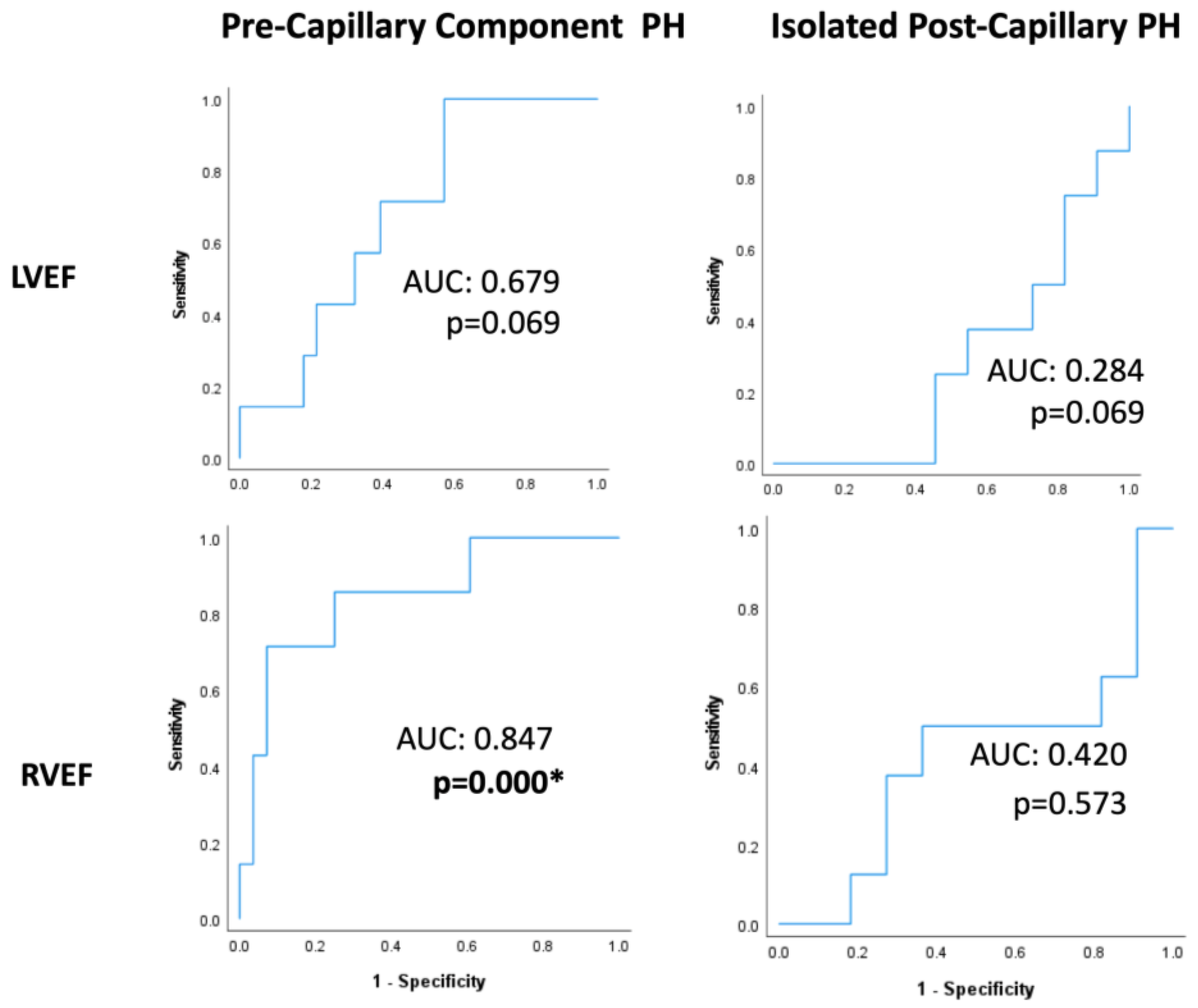
ROC analyses within PreCompPH patients showed no statistically significant AUC values for LVEDV (AUC = 0.419; p = 0.529), LVESV (AUC = 0.500; p = 1.000), LVEDVI (AUC = 0.324; p = 0.181), LVESVI (AUC = 0.500; p = 0.126), RVEDV (AUC = 0.571; p = 0.561), RVESV (AUC = 0.648; p = 0.236), or RVESVI (AUC = 0.632; p = 0.294) for the occurrence of a cardiac event within 2 years of the cardiac MRI scan. The AUC of LVEF approached significance (AUC = 0.679; p = 0.069), and the AUC of RVEF was statistically significant (AUC = 0.847; p = 0.000) (Table 2 and Figure 3).
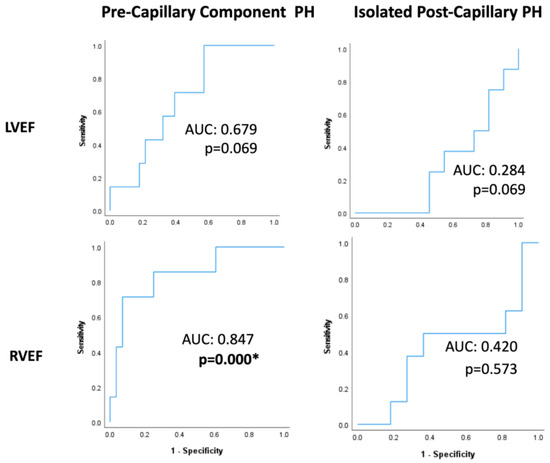
Figure 3.
Receiver-operating characteristic curves of left ventricle and right ventricle ejection fractions (LVEFs and RVEFs, respectively) within Pre-Capillary Component and Isolated Post-Capillary pulmonary hypertension (PH) patients. The binary classifier was the occurrence of a cardiac event within 2 years of cardiac MRI. RVEF showed a statistically significant classification ability within Pre-Capillary Component PH patients. * = p < 0.05.
ROC analyses within IpcPH patients showed no statistically significant AUC values for LVEDV (AUC = 0.386; p = 0.424), LVESV (AUC = 0.371; p = 0.360), LVEDVI (AUC = 0.443; p = 0.694), LVESVI (AUC = 0.429; p = 0.624), LVEF (AUC = 0.284; p = 0.069), RVEDV (AUC = 0.414; p = 0.552), RVESV (AUC = 0.371; p = 0.371), RVEDVI (AUC = 0.429; p = 0.650), RVESVI (AUC = 0.429; p = 0.650), or RVEF (AUC = 0.420; p = 0.573) (Table 3 and Figure 3).
3.3. Late Gadolinium Enhancement
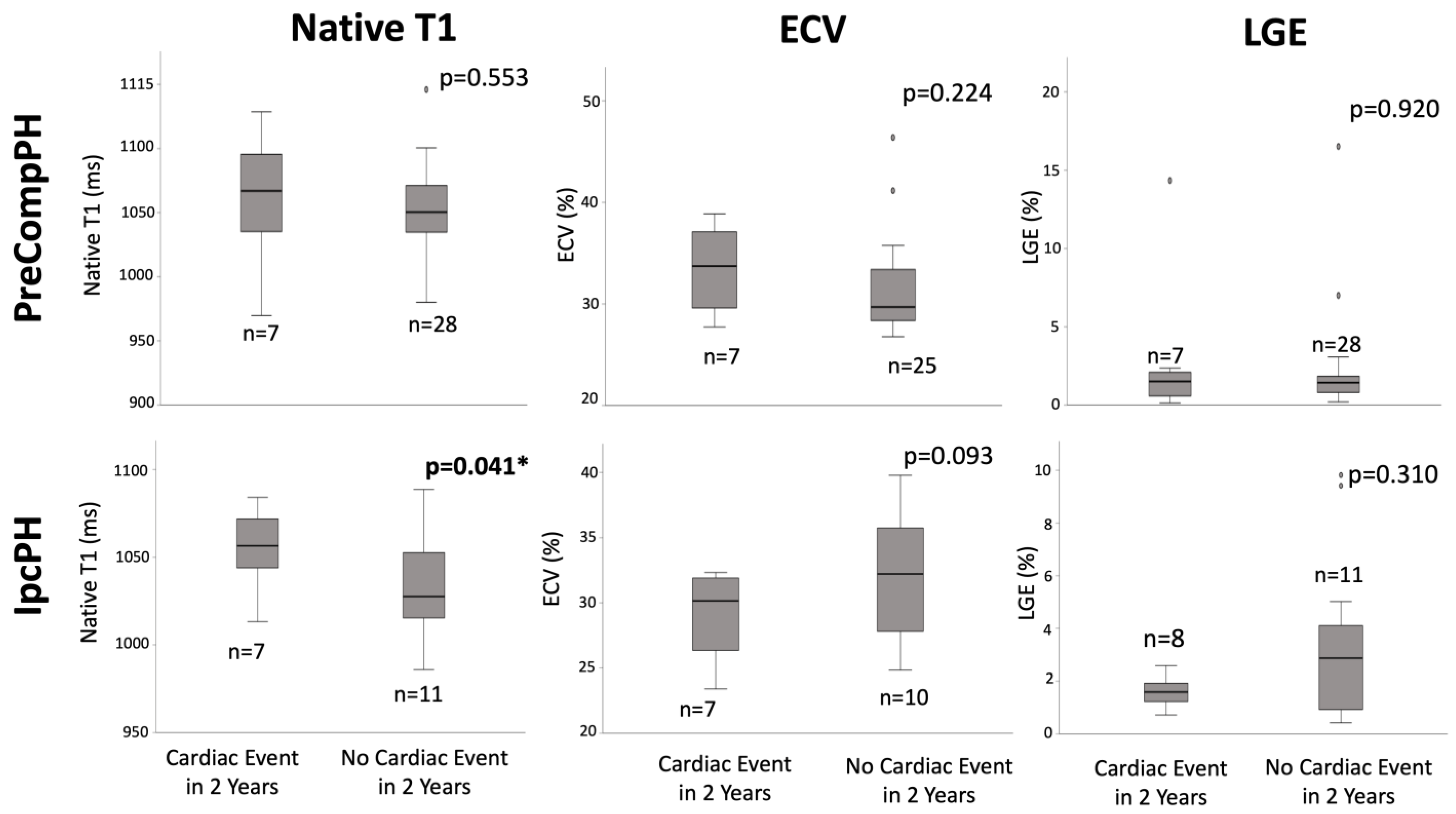
Quantitative: Within PreCompPH patients, there was no statistically significant difference in global fibrosis mass percent between the patients who experienced a cardiac event within two years (1.5 (0, 3.4)%) and those who did not (1.4 (0.2, 2.6)%) (p = 0.920). Similarly, within IpcPH patients, there was no statistically significant difference between the patients who experienced a cardiac event within 2 years (1.6 ± 0.6%) and those who did not (2.9 (0, 8.5)%) (Figure 4).
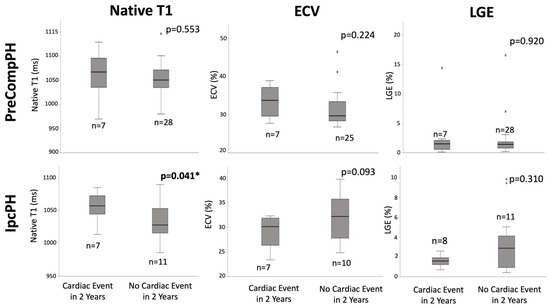
Figure 4.
Box-plot comparisons of native T1, extracellular volume fraction (ECV), and late gadolinium enhancement (LGE) between Pre-Capillary Component pulmonary hypertension (PreCompPH) and Isolated Post-Capillary pulmonary hypertension (IpcPH) patients. Mann-U Whitney Tests or Independent Samples t-Tests were used based on normality testing by Shapiro–Wilk Test (p < 0.05 or p > 0.05, respectively). * = p < 0.05.
Qualitative: Within PreCompPH patients, the distributions of the numbers of patients with no LGE at insertional points (IPs), LGE at one IP, and LGE at both IPs between patients who experienced a cardiac event within two years vs. those who did not were: 14% vs. 32% (p = 0.645); 86% vs. 61% (p = 0.380); and 0% vs. 7% (p = 1.000), respectively. Within IpcPH patients, the distributions of the numbers of patients with no LGE at IPs, LGE at one IP, and LGE at both IPs between patients who experienced a cardiac event within two years vs. those who did not were: 63% vs. 64% (p = 1.000); 13% vs. 36% (p = 0.338); 25% vs. 0% (p = 0.164) (Table 4).

Table 4.
Qualitative LGE analysis.
3.4. Native T1
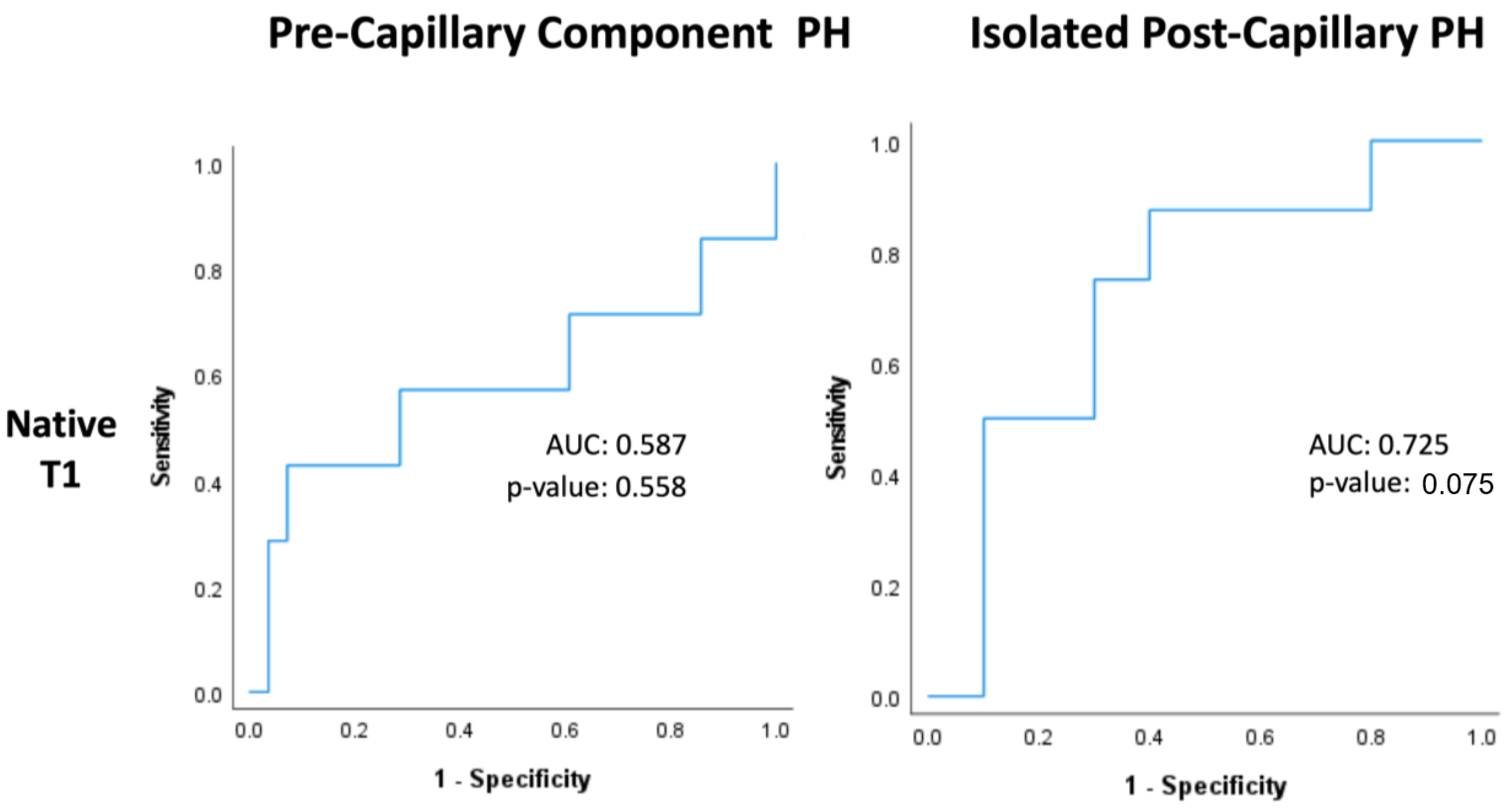
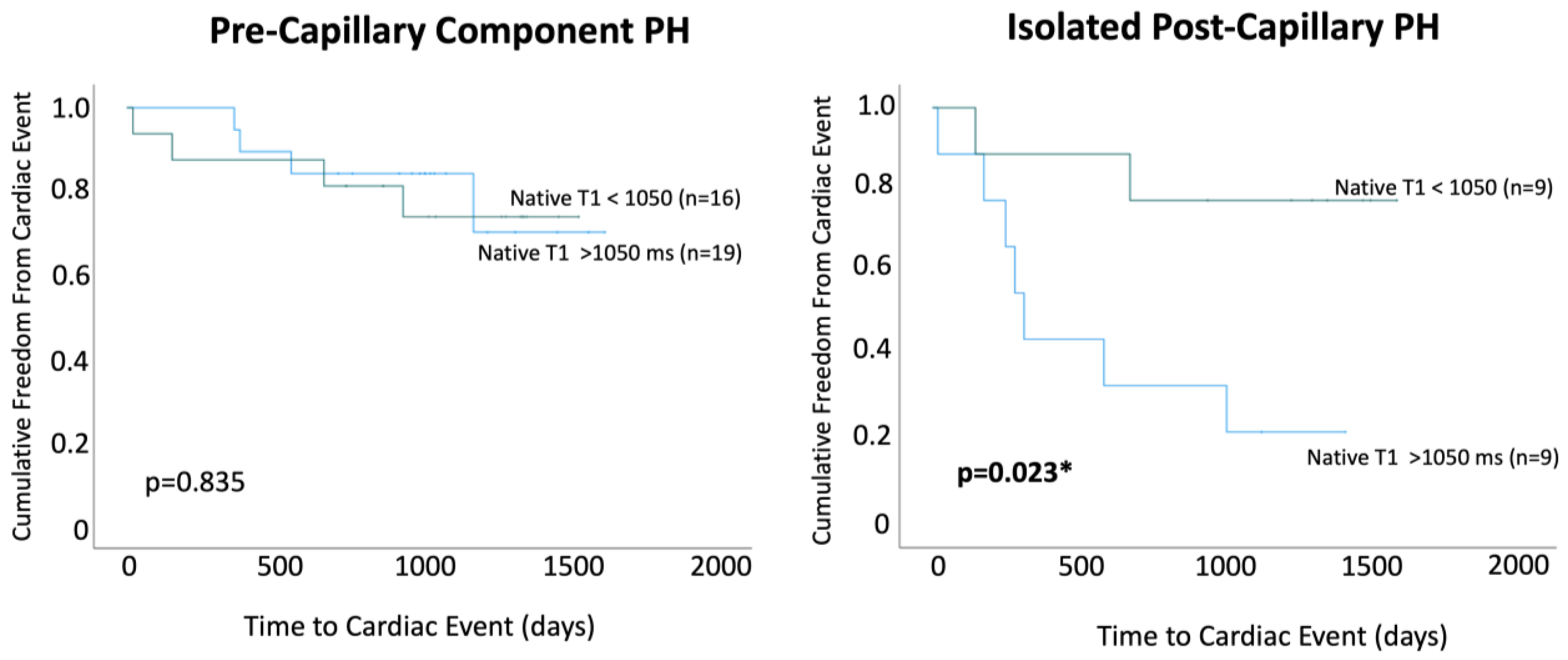
Within PreCompPH patients, the amount of LV native T1 in patients who experienced a cardiac event within two years was higher than the amount of native T1 in patients who did not experience a cardiac event within two years (1061.0 ± 53.4 ms vs. 1049.2 ± 33.9 ms), though without statistical significance (p = 0.553). ROC analysis showed an AUC of 0.587 for the association between LV native T1 and the occurrence of a cardiac event within two years of cardiac MRI (p = 0.558) (Figure 5). Kaplan–Meier survival analysis showed no statistically significant survival difference between patients with a LV native T1 <1050 ms (n = 16) and patients with a LV native T1 >1050 ms (n = 19) (p = 0.835) (Figure 6).
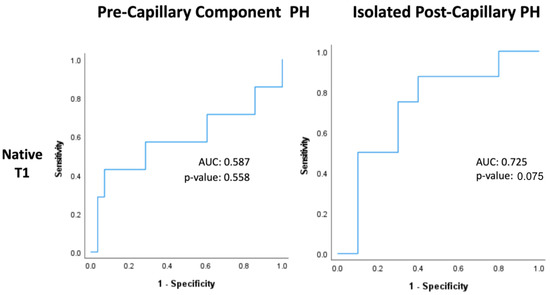
Figure 5.
Receiver-operating characteristic curves of native T1 within Pre-Capillary Component and Isolated Post-Capillary pulmonary hypertension (PH) patients. The binary classifier was the occurrence of a cardiac event within 2 years of cardiac MRI.
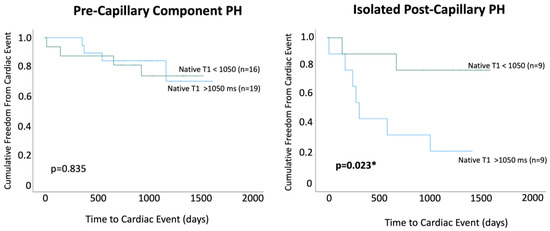
Figure 6.
Kaplan–Meier survival curves were used to compare patients with native T1 values < 1050 ms and >1050 ms within Pre-Capillary Component pulmonary hypertension (PH) and Isolated Post-Capillary pulmonary hypertension (PH) patient groups. * = p < 0.05.
Within IpcPH patients, there was a statistically significant higher amount of LV native T1 in patients who experienced a cardiac event within two years of cardiac MRI (1055.7 ± 24.2 ms vs. 1032.1 ± 28.9 ms) (p = 0.041) (Figure 4). ROC analysis showed an AUC of 0.725 for the association between LV native T1 and the occurrence of a cardiac event within two years of cardiac MRI (p = 0.075) (Figure 5). Kaplan–Meier survival analysis showed a statistically significant lower rate of survival in patients with LV native T1 >1050 ms (n = 9) compared to patients with LV Native T1 < 1050 ms (n = 9) (p = 0.023) (Figure 6).
3.5. Extracellular Volume Fraction
Within PreCompPH patients, there was no statistically significant difference in global LV ECV percent between the patients who experienced a cardiac event within two years of cardiac MRI (33.4 ± 4.4%) and those who did not (29.7 (24.4, 35.0)%) (p = 0.224) (Figure 4). ROC analysis showed an AUC of 0.632 for the association between LV ECV and the occurrence of a cardiac event within two years of cardiac MRI (p = 0.273).
Within IpcPH patients, there was no statistically significant difference in global LV ECV percent between the patients who experienced a cardiac event within two years of cardiac MRI (28.9 ± 3.5%) and those who did not (32.0 ± 5.1%) (p = 0.093). ROC analysis showed an AUC of 0.314 for the association between LV ECV and the occurrence of a cardiac event within two years of cardiac MRI (p = 0.168).
4. Discussion
The presented results show that fibrosis and volumetric assessments may be of value for the prediction of cardiac outcomes in PH patients, but this value may depend on whether a patient’s PH is marked by elevated PVR (and subsequent PreCompPH characterization). Volumetric and hemodynamic measurements appear less promising.
The results of this study show that LV native T1 measurements may be able to forecast cardiac events in IpcPH patients, but not in PreCompPH patients. No association was found between LGE or ECV and the occurrence of a cardiac event in either subset of PH patients. Though several fibrosis quantification techniques have been simultaneously studied for their potential to prognosticate muscular dystrophy patients [21], amyloidosis patients [22], and a heterogeneous group of patients referred for cardiac MRI [23], our study is the first study to simultaneously assess the relationship between prognosis and all three fibrosis measurement techniques in a PH cohort. In a retrospective cohort study of 223 patients with PAH, Saunders et al. [24] found that septal T1, RV insertion point T1, and LV free-wall T1 were not associated with mortality. In this study, 59 patients died during a follow-up period of 29 months which allowed for univariate Cox proportional hazards regression analysis. The present study differed as native T1 values were assessed by an LV global summary measurement; however, global native T1 of the LV has been previously shown to correlate with insertional point T1 (r = 0.75; p < 0.05) [25]. The results of our study are similar to the results of Saunders et al. as no outcome association was observed in PreCompPH (of which PAH is a subtype). Yet our results suggest that this may not be the case in IpcPH patients. Previous studies have reported a predictive relationship between native T1 measurements in outcomes. These works have studied all-comer patients [26,27], patients with diabetes [28], and non-ischemic dilated cardiomyopathy patients [29]. We believe these cohorts are more akin to IpcPH given the collective absence of elevated PVR (and likely absence of fulminant right heart failure) in these patient populations, in which right ventricular function has less impact on prognosis.
Our results showing a lack of statistically significant associations between LGE, ECV, and cardiac outcomes are consistent with the previous literature. In a study using native T1, ECV, and LGE measurement techniques in the non-infarcted myocardium of patients with coronary heart disease, native T1 and ECV were independent predictors of outcome [29]. Both tissue characterization techniques surpassed LGE assessment, and native T1 performed better than ECV. It was suggested that this may be due to effects other than fibrosis, such as inflammation, which affect native T1 values more than ECV. Our results suggest that this effect may not be limited to coronary artery disease patients, and that pathophysiological processes outside of fibrosis may be occurring in the LV of IpcPH patients. Furthermore, in a group of hypertrophic cardiomyopathy patients, 30% of LGE-negative segments showed an elevated native T1 time [30], suggesting that native T1 is a more sensitive biomarker than LGE. ECV, native T1, and LGE all purport to measure myocardial fibrosis, but they achieve this through surrogate fibrosis measurements. Native T1 appears most relatable to the outcome and may therefore be the best means of fibrosis approximation.
It has also become appreciated that RV functional measures assessed by cardiac MRI are associated with a functional state, exercise capacity, and survival in patients with PAH (a subset of PreCompPH) [31,32,33,34]. RVEF and RVESV have been shown capable of distinguishing PH severity grades [31], and the loss of RV function is associated with a poor outcome irrespective of any changes in PVR [34]. Whether RV functional measures continue to hold clinical value in IpcPH patients has been under-investigated. We indeed found that a lower RVEF was characteristic of PreCompPH patients who went on to experience a cardiac event in two years, but not of IpcPH experiencing a cardiac event in two years.
Interestingly, invasive measurements were not prognostic in IpcPH or PreCompPH patients. Elevated pulmonary artery pressures have previously been shown to be associated with outcomes in patients with left heart disease and PH [35]. PVR measurements greater than 2.2 Wood Units portend a worse prognosis in all subgroups of PH [36]. Previous works showing the relationship between hemodynamic measurements have focused on all-cause mortality [36,37,38] or heart-failure-specific hospitalizations [36], singularly. These assessments were not possible in our cohort, considering the small number of observed deaths and hospitalizations secondary to heart failure. The present study defined outcomes as cardiac events because previous associations between fibrosis measurements and cardiac outcomes have been observed in heart transplant patients [13]. In our small cohort, the inclusion of ‘cardiac event’ outcomes extending to palpitations and coronary revascularization was necessary to investigate the relationship between outcomes and several cardiac MRI parameters.
5. Limitations [8,10]
There are several limitations to our study. First, the small sample size of the present cohort, combined with a low death rate (6 out of 54 patients) during the follow-up period, precluded the use of regression and survival analyses in our study. Larger studies are needed to allow for robust statistical testing of our preliminary findings. Second, up to 28 days elapsed between cardiac MRI and RHC. Disease progression or remission may have occurred during this time. For this reason, patients were classified by a cardiology fellow with experience following PH patients. RHC values, volumetric measurements, and clinical factors were all considered during the classification process. Spruijt et al. [39] similarly classified idiopathic PAH, systemic scleroderma-related PH, and CTEPH all as PrePH-type, without reference to RHC measurements. It would have been ideal for RHC and cardiac MRI to have been collected close together, as has previously been done [40], but this was not possible given the recruitment protocol. This study is further limited by the recruitment protocol, as the mPAP threshold used to define PH has recently changed (in 2015, a resting mPAP >/= 25 mmHg defined PH [4]; in 2022, a resting mPAP of >20 mmHg defined PH [41]), with new guidelines established by the European Society of Cardiology and the European Respiratory Society. Our study used the older guidelines, as the recruitment protocol occurred during this time. It has been suggested that mPAP values used in isolation cannot characterize the PH clinical condition and do not define the pathological process per se [19]. Although our recruitment protocol may have been a more sensitive protocol for recruiting PH patients, the higher mPAP threshold precluded the inclusion of PH patients with milder diseases. Our conclusions may not hold relevance in these newly defined PH patients, with a resting mPAP between 20 and 25 mmHg.
6. Conclusions
This preliminary proof-of-concept study demonstrates that native T1 measurements of the LV may be able to forecast the occurrence of cardiac events in IpcPH patients, and that this ability does not persist in PreCompPH patients. RVEF does not appear to hold prognostic value in IpcPH patients but may be prognostic in PreCompPH patients.
Author Contributions
Conceptualization, J.W.C., J.C.C., A.R. and A.P.; formal analysis, J.W.C., A.P. and C.S.; classification of patients, M.V.; data curation, J.W.C., C.S., K.S., B.D.A. and A.P.; writing—original draft preparation, J.W.C.; writing—review and editing, J.W.C., C.S., J.C.C., A.R., A.P., B.D.A., R.J.A., M.V., K.S. and M.M.; funding acquisition, J.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Bayer HealthCare Pharmaceuticals Inc. (reference award number: Carr-IIR-US-2017-3822). All funding was directed towards the research protocol execution. The funding body played no role in the study design, data collection, data analysis, or manuscript writing.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Northwestern University (protocol code 00205106, approved 06/2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
JCC is the principal investigator for this Bayer funded study. JCC declares that he has previously participated in advisory boards for Bayer, Guerbet and Bracco. JCC participates in speaking roles sponsored by Bayer. JCC has received institutional research support sponsored by Bayer, Guerbet, and Siemens. MM has received research support from Siemens. MM has received research grants from Circle Cardiovascular imaging and Cryolife incorporated. BDA and RJA have performed consulting for Circle Cardiovascular Imaging. The other authors declare that they have no competing interests.
References
- Bogaard, H.J.; Abe, K.; Vonk Noordegraaf, A.; Voelkel, N.F. The right ventricle under pressure: Cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 2009, 135, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Setaro, J.F.; Cleman, M.W.; Remetz, M.S. The right ventricle in disorders causing pulmonary venous hypertension. Cardiol. Clin. 1992, 10, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.F.; Hoeper, M.M.; Gibbs, J.S.R.; Kaemmerer, H.; Pepke-Zaba, J.; Coghlan, J.G.; Scelsi, L.; D’Alto, M.; Olsson, K.M.; Ulrich, S.; et al. Pre-capillary, combined, and post-capillary pulmonary hypertension. J. Am. Coll. Cardiol. 2016, 68, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simmonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): Endorsed by: Association for European paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef]
- Guazzi, M.; Galiè, N. Pulmonary hypertension in left heart disease. Eur. Respir. Rev. 2012, 21, 338–346. [Google Scholar] [CrossRef]
- Assad, T.R.; Hemnes, A.R.; Larkin, E.K.; Glazer, A.M.; Xu, M.; Wells, Q.S.; Farber-Eger, E.H.; Sheng, Q.; Shyr, Y.; Harrel, F.E.; et al. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J. Am. Coll. Cardiol. 2016, 68, 2525–2536. [Google Scholar] [CrossRef] [PubMed]
- Habedank, D.; Obst, A.; Heine, A.; Stubbe, B.; Ewert, R. Correlation of hemodynamic and respiratory parameters in invasive cardiopulmonary Exercise Testing (iCPET). Life 2022, 12, 655. [Google Scholar] [CrossRef] [PubMed]
- Cerne, J.W.; Pathrose, A.; Gordon, D.Z.; Sarnari, R.; Veer, M.; Blaisdell, J.; Allen, B.D.; Avery, R.; Markl, M.; Ragin, A.; et al. Evaluation of pulmonary hypertension using 4D flow MRI. J. Magn. Reson. Imaging 2022, 56, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Salton, F.; Baratella, E.; Confalonieri, P.; Geri, P.; Pozzan, R.; Torregiani, C.; Bulla, R.; Confalonieri, M.; Matucci-Cerinic, M.; et al. An Overview of Different Techniques for Improving the Treatment of Pulmonary Hypertension Secondary in Systemic Sclerosis Patients. Diagnostics 2022, 12, 616. [Google Scholar] [CrossRef]
- Cerne, J.W.; Pathrose, A.; Sarnari, R.; Veer, M.; Chow, K.; Subedi, K.; Allen, B.D.; Avery, R.J.; Markl, M.; Carr, J.C. Left Ventricular Fibrosis Assessment by Native T1, ECV, and LGE in Pulmonary Hypertension Patients. Diagnostics 2023, 13, 71. [Google Scholar] [CrossRef]
- Wang, L.; Singh, H.; Mulyala, R.R.; Weber, J.; Barasch, E.; Cao, J.J. The association between left ventricular diastolic dysfunction and myocardial scar and their collective impact on all-cause mortality. J. Am. Soc. Echocardiogr. 2020, 33, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Mousseaux, E.; Agoston-Coldea, L.; Marjanovic, Z.; Stanciu, R.; Deligny, C.; Perdrix, L.; Boutouyrie, P.; Azarine, A.; Soulat, G.; Farge, D. Left ventricle replacement fibrosis detected by CMR associated with cardiovascular events in systemic sclerosis patients. J. Am. Coll. Cardiol. 2018, 71, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Chaikriangkrai, K.; Abbasi, M.A.; Sarnari, R.; Dolan, R.; Lee, D.; Anderson, A.S.; Ghafourian, K.; Khan, S.S.; Vorovich, E.E.; Rich, J.D.; et al. Prognostic value of myocardial extracellular volume fraction and T2-mapping in heart transplant patients. JACC Cardiovasc. Imaging 2020, 13, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Asano, R.; Ogo, T.; Morita, Y.; Kotoku, A.; Aoki, T.; Hirakawa, K.; Nakayama, S.; Ueda, J.; Tsuji, A.; Waddingham, M.T.; et al. Prognostic value of right ventricular native T1 mapping in pulmonary arterial hypertension. PLoS ONE 2021, 16, e0260456. [Google Scholar] [CrossRef]
- De Lazzari, M.; Cipriani, A.; Rizzo, S.; Famoso, G.; Giorgi, B.; Tarantini, G.; Thiene, G.; Tona, F.; Iliceto, S.; Basso, C.; et al. Right ventricular junctional late gadolinium enhancement correlates with outcomes in pulmonary hypertension. JACC Cardiovasc. Imaging 2019, 12, 936–938. [Google Scholar] [CrossRef]
- Sandoval, J.; Bauerle, O.; Palomar, A.; Gómez, A.; Martínez-Guerra, M.L.; Beltrán, M.; Guerrero, M.L. Survival in primary pulmonary hypertension. Validation of a prognostic equation. Circulation 1994, 89, 1733–1744. [Google Scholar] [CrossRef]
- D’Alonzo, G.E. Survival in patients with primary pulmonary hypertension: Results from a national prospective registry. Ann. Int. Med. 1991, 115, 343–349. [Google Scholar] [CrossRef]
- McLaughlin, V.V.; Shillington, A.; Rich, S. Survival in primary pulmonary hypertension: The impact of epoprostenol therapy. Circulation 2002, 106, 1477–1482. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Rahsepar, A.A.; Ghasemiesfe, A.; Suwa, K.; Dolan, R.S.; Shehata, M.L.; Korell, M.J.; Naresh, N.K.; Markl, M.; Collins, J.D.; Carr, J.C. Comprehensive evaluation of macroscopic and microscopic myocardial fibrosis by cardiac MR: Intra-individual comparison of gadobutrol versus gadoterate meglumine. Eur. Radiol. 2019, 29, 4357–4367. [Google Scholar] [CrossRef]
- Florian, A.; Ludwig, A.; Rösch, S.; Yildiz, H.; Sechtem, U.; Yilmaz, A. Myocardial fibrosis imaging based on T1-mapping and extracellular volume fraction (ECV) measurement in muscular dystrophy patients: Diagnostic value compared with conventional late gadolinium enhancement (LGE) imaging. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, X.; Feng, J.; Shen, K.-N.; Tian, Z.; Sun, J.; Yue-ying, M.; Cao, J.; Zheng-yu, J.; Li, J.; et al. The prognostic value of T1 mapping and late gadolinium enhancement cardiovascular magnetic resonance imaging in patients with light chain amyloidosis. J. Cardiovasc. Magn. Reson. 2018, 20, 2. [Google Scholar] [CrossRef]
- Yang, E.Y.; Khan, M.A.; Graviss, E.A.; Nguyen, D.T.; Bhimaraj, A.; Nambi, V.; Hoogeveen, R.C.; Ballantyne, C.M.; Zoghbi, W.A.; Shah, D.J. Relationship of extracellular volume assessed on cardiac magnetic resonance and serum cardiac troponins and natriuretic peptides with heart failure outcomes. Sci. Rep. 2019, 9, 20168. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.C.; Johns, C.S.; Stewart, N.J.; Oram, C.J.E.; Capener, D.A.; Puntmann, V.O.; Elliot, C.A.; Condliffe, R.C.; Kiely, D.G.; Graves, M.F.; et al. Diagnostic and prognostic significance of cardiovascular magnetic resonance native myocardial T1 mapping in patients with pulmonary hypertension. J. Cardiovasc. Magn. Reson. 2018, 20, 78. [Google Scholar] [CrossRef]
- Reiter, U.; Reiter, G.; Kovacs, G.; Adelsmayr, G.; Greiser, A.; Olschewski, H.; Fuchsjäger, M. Native myocardial T1 mapping in pulmonary hypertension: Correlations with cardiac function and hemodynamics. Eur. Radiol. 2017, 27, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.C.; Piehler, K.; Meier, C.G.; Testa, S.M.; Klock, A.M.; Aneizi, A.A.; Shakesprere, J.; Kellman, P.; Shroff, S.G.; Schwartzman, D.S.; et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation 2012, 126, 1206–1216. [Google Scholar] [CrossRef]
- Kammerlander, A.A.; Marzluf, B.A.; Zotter-Tufaro, C.; Aschauer, S.; Duca, F.; Bachmann, A.; Knechtelsdorfer, K.; Wiesinger, M.; Pfaffenberger, S.; Greiser, A.; et al. T1 mapping by CMR imaging: From histological validation to clinical implication. JACC Cardiovasc. Imaging 2016, 9, 14–23. [Google Scholar] [CrossRef]
- Wong, T.C.; Piehler, K.M.; Kang, I.A.; Kadakkal, A.; Kellman, P.; Schwartzman, D.S.; Mulukutla, S.R.; Simon, M.A.; Shroff, S.G.; Kuller, L.H.; et al. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur. Heart J. 2014, 35, 657–664. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carr-White, G.; Jabbour, A.; Yu, C.-Y.; Gebker, R.; Kelle, S.; Rolf, A.; Zitzmann, S.; Peker, E.; D’Angelo, T.; et al. Native T1 and ECV of noninfarcted myocardium and outcome in patients with coronary artery disease. J. Am. Coll. Cardiol. 2018, 71, 766–778. [Google Scholar] [CrossRef]
- Kato, S.; Nakamori, S.; Bellm, S.; Jang, J.; Basha, T.; Maron, M.; Manning, W.J.; Nezafat, R. Myocardial Native T1 Time in Patients With Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2016, 118, 1057–1062. [Google Scholar] [CrossRef]
- Alunni, J.-P.; Degano, B.; Arnaud, C.; Tétu, L.; Blot-Soulétie, N.; Didier, A.; Otal, P.; Rousseau, H.; Chabbert, V. Cardiac MRI in pulmonary artery hypertension: Correlations between morphological and functional parameters and invasive measurements. Eur. Radiol. 2010, 20, 1149–1159. [Google Scholar] [CrossRef]
- Trip, P.; Rain, S.; Handoko, M.L.; van der Bruggen, C.; Bogaard, H.J.; Marcus, J.T.; Boonstra, A.; Westerhof, N.; Vonk-Noordegraaf, A.; de Man, F.S. Clinical relevance of right ventricular diastolic stiffness in pulmonary hypertension. Eur. Respir. J. 2015, 45, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- van Wolferen, S.A.; Marcus, J.T.; Boonstra, A.; Marques, K.M.J.; Bronzwaer, J.G.F.; Spreeuwenberg, M.D.; Postmus, P.E.; Vonk-Noordegraaf, A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur. Heart J. 2007, 28, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, M.C.; Kind, T.; Marcus, J.T.; Mauritz, G.-J.; Heymans, M.W.; Bogaard, H.-J.; Boonstra, A.; Marques, K.M.K.; Westerhof, N.; Vonk-Noordegraaf, A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J. Am. Coll. Cardiol. 2011, 58, 2511–2519. [Google Scholar] [CrossRef]
- Strange, G.; Playford, D.; Stewart, S.; Deague, J.A.; Nelson, H.; Kent, A.; Gabbay, E. Pulmonary hypertension: Prevalence and mortality in the Armadale echocardiography cohort. Heart 2012, 8, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Brittain, E.L.; Hess, E.; Waldo, S.W.; Barón, A.E.; Huang, S.; Goldstein, R.H.; Assad, T.; Wertheim, B.W.; Alba, G.A.; et al. Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 873–884. [Google Scholar] [CrossRef]
- Corte, T.J.; Wort, S.J.; Gatzoulis, M.A.; Macdonald, P.; Hansell, D.M.; Wells, A.U. Pulmonary vascular resistance predicts early mortality in patients with diffuse fibrotic lung disease and suspected pulmonary hypertension. Thorax 2009, 64, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Benza, R.L.; Miller, D.P.; Gomberg-Maitland, M.; Frantz, R.P.; Foreman, A.J.; Coffey, C.S.; Frost, A.; Barst, R.J.; Badesch, D.B.; Elliot, C.G.; et al. Predicting survival in pulmonary arterial hypertension: Insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010, 122, 164–172. [Google Scholar] [CrossRef]
- Spruijt, O.A.; Vissers, L.; Bogaard, H.-J.; Hofman, M.B.M.; Vonk-Noordegraaf, A.; Marcus, J.T. Increased native T1-values at the interventricular insertion regions in precapillary pulmonary hypertension. Int. J. Cardiovasc. Imaging 2016, 32, 451–459. [Google Scholar] [CrossRef]
- Vanderpool, R.R.; Pinsky, M.R.; Naeije, R.; Deible, C.; Kosaraju, V.; Bunner, C.; Mathier, M.A.; Lacomis, J.; Champion, H.C.; Simon, M.A. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart 2015, 101, 37–43. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).