Abstract
The literature search was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) protocol in the PubMed, Cochrane Library, LILACS, EBSCO, Scielo, between 2012 and 2022. The methodological quality was assessed by using the Newcastle–Ottawa Study Quality Assessment Scale. Mean differences and 95% confidence intervals were calculated and combined in meta-analyses. A total of 1202 participants were included in this systematic review (690 with TMD; 512 without TMD), with 22 articles being included in the qualitative analysis. Only three studies enabled the comparative analysis of the results. Ten articles showed a high methodological quality and a low risk of bias, and twelve had a low methodological quality and an increased risk of bias. The meta-analysis showed that the differences between the intervention and control groups were not statistically significant for the percentage overlapping coefficient of the anterior temporal muscle, for the masseter, and for the torque coefficient. The parameters analyzed with the compound technique for chewing showed altered mandibular functions in individuals with TMD. With the EMG method, it was possible to suggest that TMD in adult individuals causes compensatory muscle behaviors, and several changes in the masticatory function were found.
1. Introduction
Temporomandibular disorders (TMDs) involve the pathologies of the orofacial region with neoplastic, traumatic, and/or musculoskeletal disorders [1]. TMD involves signs and symptoms such as joint and/or muscle pain, limited mandibular movement, altered masticatory muscle function, and joint noises [2]. The manifestation of one of these factors, or the combination of several, may adversely influence the performance of stomatognathic functions, namely chewing and swallowing [3]. Epidemiological studies indicate a high prevalence of TMD of approximately 31% in adults and the elderly population, with the most prevalent temporomandibular joint disorders (TMJs) being the disc displacement with reduction (DDwR) [4]. Some studies have shown that TMD affects more female individuals, i.e., in a ratio of 3 to 1 [5,6,7,8,9].
Pain is one of the most common symptoms described by TMD subjects [10]. The literature describes that these individuals suffer from masticatory function limitations [11] because their mandibular movements adapt in a conscious or unconscious attempt to avoid painful stimuli. Difficulty swallowing hard foods and tiredness after chewing have been reported in TMD patients [4,12]. Changes in the masticatory muscles’ recruitment and an increased asymmetry between the right and left sides were also reported when comparing individuals suffering from TMD to asymptomatic ones [3]. As chewing is one of the essential functions of the stomatognathic system [13], it is critical to understand the functional and clinical changes related to TMD, as well as their consequences. Some TMJ can influence the normal functioning of mastication, altering its type and pattern [14,15,16]. These alterations can cause numerous problems in need of treatment from several clinical specialists, such as speech therapists, physiotherapists, and dentists, among others [17,18,19,20]. Often, eating limitations match the self-reports of jaw pain, fatigue, or jaw noises during biting [21,22,23] and some physical examination findings, such as decreased activation, strength, or endurance of the masticatory muscles and/or diminished force production [23]. A recent study revealed that unilateral TMD involves an alteration of the preferred chewing side, being also accompanied by TMJ remodeling [24]. Furthermore, the correlation between TMD and mandibular kinematics range of movements parameters, such as maximum mouth opening, lateralization, and maximum protrusion/retrusion, has been described, and it was found that values tend to be increased or decreased depending on the type of TMD [19].
Surface electromyography (sEMG) is a technique that contributes to a better knowledge of muscle physiology and assists in the differential diagnosis and monitoring of TMD [25]. This diagnostic tool can assess the behavior of muscles intervening in the TMJ at rest and during human jaw motion [26]. Surface electromyography (sEMG) is a reliable and valid tool to evaluate muscle activity and, therefore, may be useful in the evaluation of TMD patients. sEMG detects electrical potentials and, on this account, may conceivably be employed in TMD diagnosis [27]. A chewing compound is considered one of the most valuable test materials to evaluate the ability to chew and assess the parameters of masticatory efficiency. It has stability in quality and uniformity as a manufactured product and can be produced on a large scale [28]. Masticatory efficiency (ME) can be defined as the ability to fragment food within a given time interval and can be measured by an individual’s ability to fractionate natural or artificial foods [29].
It is important for professionals to know the clinical manifestations of TMD and to understand the influence of TMD on the individual’s habitual chewing. Surface electromyography (sEMG) is a diagnostic tool that ensures reliable and valid evaluation of muscle activity. It detects electrical potentials and, on this account, may conceivably be employed in TMD diagnosis [30]. Collecting accurate data on the temporomandibular complex is important to create and adapt the treatment to each case, evaluating the previously mentioned variables. This study aimed to summarize the scientific evidence regarding the assessment of masticatory function in adult individuals with TMD, using two different techniques, namely chewing material and electromyography, as well as evaluate the methodological quality of the included studies and to perform a meta-analysis.
2. Materials and Methods
The literature search was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) protocol. The research question was defined as follows: “Do individuals with TMD present changes in masticatory function, evaluated through a chewing compound or an electromyographic technique when compared with individuals without TMD?” The scientific question was structured according to the acronym PECO (Population, Exposure, Comparison, and Outcome; see Table 1), establishing the primary inclusion criteria for the studies that were selected a priori. No language limitation was set.

Table 1.
PECO Acronym.
The exclusion criteria were set as follows: (1) the presence of with systemic diseases, degenerative diseases or neuromuscular disorders; (2) unavailable full text; (3) TMD diagnostic tools other than RDC-TMD or DC-TMD; (4) participants with malocclusions; and (5) participants wearing dental prostheses.
2.1. Information Sources and Search Strategies
The search was conducted in different databases (Cochrane Library, PubMed, LILACS, EBSC, and SciELO) to include all relevant literature on this topic. The search strategy was established before starting the database query. A register of unpublished or in-progress studies, called “grey literature” (ClinicalTrials.gov), assessed on the 16 June 2022, was also consulted to minimize publication bias. The search strategy was based on the combination of medical terms (Mesh) and keywords relating to the following concepts: “Temporomandibular disorders, electromyography, mastication, masticatory efficiency, chewing, bite force.” The complete search strategy is available in Appendix A, Appendix B, Appendix C, Appendix D, Appendix E, Appendix F and Appendix G.
2.2. Study Selection
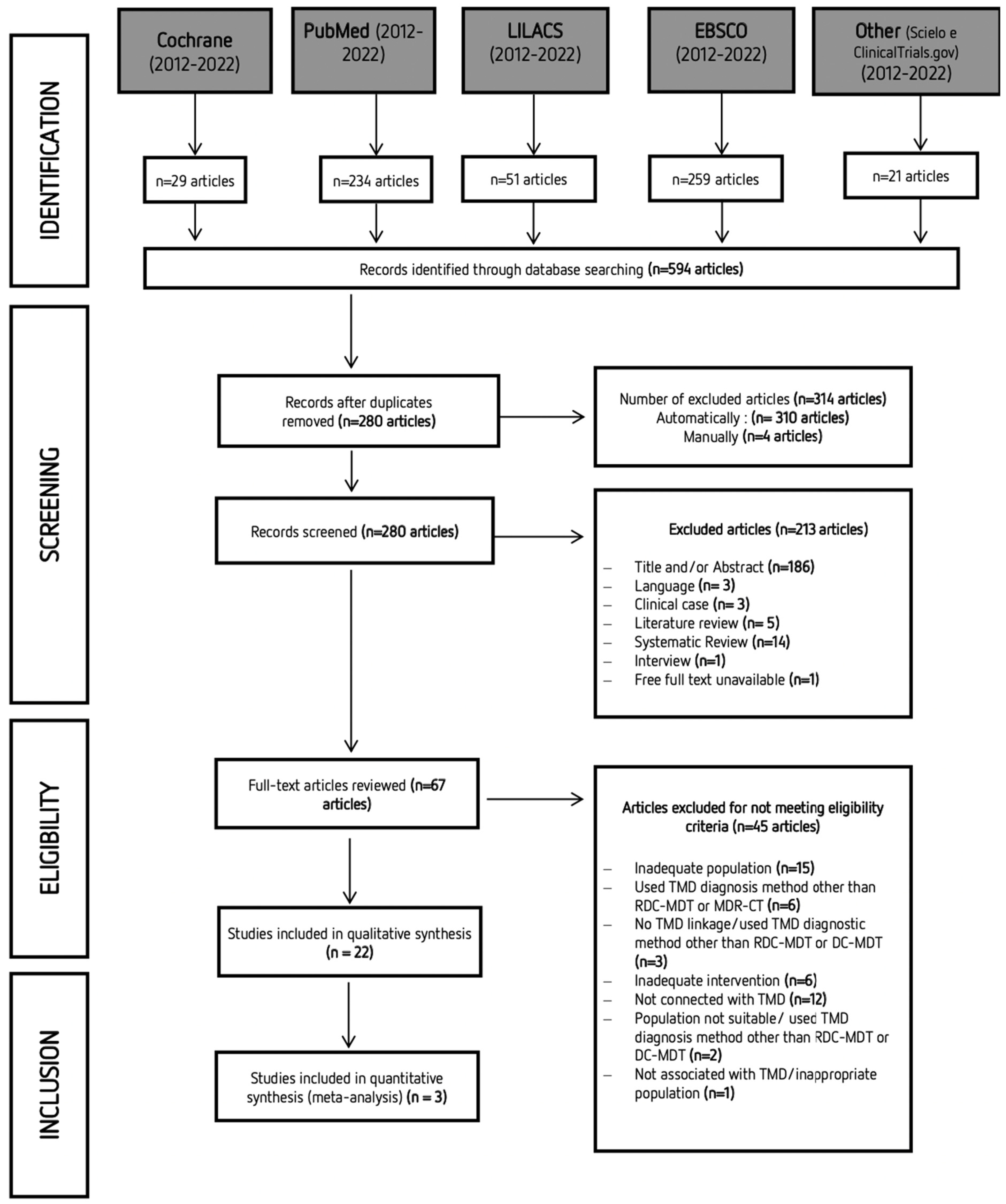
A systematic search was performed with articles published between 2012 and 2022. The last online search was performed on the 16 June 2022. However, because different databases were explored, it is frequent to find duplicate articles. Study selection was initially carried out independently by two researchers (S and VM) via title and abstract reading. Studies that did not complete the eligibility criteria were discarded. In the second phase of this selection, the same investigators independently applied the same eligibility criteria to the full texts, compared decisions, and resolved differences by discussion and consultation with experienced investigators (TP and MP) whenever consensus could not be reached. The process of identifying, screening, and excluding studies followed the strategy shown in Figure 1. Most of the studies that Mendeley did not identify as duplicates had minor changes in the title or the original language.
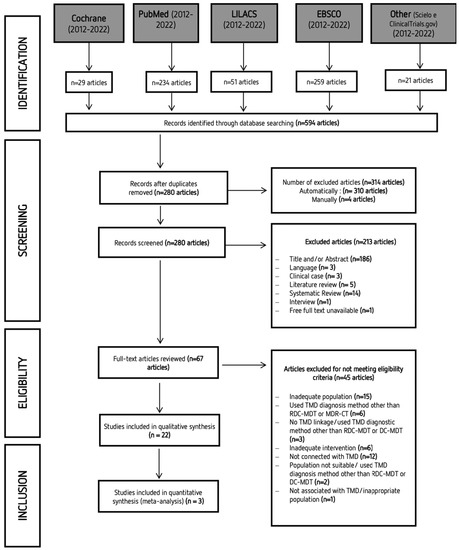
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of the search strategy and obtained results.
2.3. Data Extraction
Data extraction is displayed in Table 2, where information such as study design, sample, age, diagnostic method, chewing evaluation method, variables analyzed, and results is shown. Data extraction was carried out independently by two researchers (S and VM); any disagreement was solved by discussion, and when necessary, a third author (TP) was consulted.

Table 2.
Descriptive characteristics of the studies included.
2.4. Data Analysis
The meta-analysis was performed by using the Review Manager software, version 5.4. Forest plots were created to present the combined estimates for which two or more studies had similar EMG signal collection and processing methods. Statistical heterogeneity among studies was assessed by using the I2 test. Forest plots were created to present the combined estimates for which two or more studies had similar EMG signal collection and processing methods. Statistical heterogeneity among studies was assessed by using the I2 test. A value of I2 > 50% is considered to indicate large heterogeneity. In the presence of large heterogeneity, a random-effects model was used; otherwise, a fixed-effects model was used. The result was considered statistically significant when the p-value was <0.05, or if the 95% CI (confidence interval) about the mean differences did not cross 0 (zero).
2.5. Risk of Bias in the Studies
This systematic review used the Newcastle–Ottawa scale (NOS) to assess the methodological quality of the included studies. NOS presents 3 parameters: selection, comparability, and outcomes. A study can be given a maximum of one star for each numbered item in the selection and outcomes. A maximum of two stars can be given for comparability, with a maximum score of 9, indicating the highest quality studies [53]. Two researchers (S and VM) did this assessment independently and in duplicate. Again, any disagreements were solved through discussion with experienced researchers (TP and MP).
3. Results
3.1. Studies Selection
After completing the first stage of search in databases, a total of 596 studies were obtained. After eliminating duplicate articles, the remaining 280 articles were assessed via title and abstract reading. Only 67 articles were retained for full-text reading. Twenty-two articles were included for the qualitative analysis. The characteristics of the selected studies are summarized in Table 3. A total of 1202 participants were included in the studies that were analyzed in this systematic review (690 with TMD; 512 without TMD).

Table 3.
Summary of the result of the methodological quality assessment of the included studies.
3.2. Study Characteristics
Fifteen of the selected articles used the RDC/TMD protocol to diagnose TMD [31,34,37,38,39,40,41,42,43,44,45,50,54,55,56], and the seven others used DC/TMD [32,33,35,36,48,51,52]. Mapelli et al. (2016) [38] was the only study that presented, within the group defined as “with TMD,” a subdivision according to the pathology’s severity: moderate and severe. The publication date criterium revealed six articles > 6 years [31,42,43,50,54,55], eight articles between 3 and 6 years [34,38,39,40,41,44,45,48], and eight from the last 3 years [32,33,35,36,37,51,52,56]. Studies using electromyography to assess chewing function are more recent [32,35,37,51] than those using chewing gums [31,32,54,55]. All articles are available in English, and the authors’ country of origin and the sample are usually the same. We found a significant predominance of studies from Brazil (n = 16) [3,31,33,34,35,36,38,39,40,41,42,43,44,50,52,56]. Our list of countries includes Italy [32,51], China [45], Poland [48] and Turkey [37,54]. Regarding the protocol used to assess masticatory function, all studies performed static and/or dynamic tests in MVC (Maximum Voluntary Contraction), and six also evaluated the stomatognathic system during mandibular rest [34,36,41,48,50,52]. Concerning gender, thirteen studies only included female subjects [31,32,33,34,39,40,41,42,43,48,51,52,55]. Regarding the sample size of the studied populations, of the twenty-two selected articles, two studies [40,43] presented small samples (n = 22), contrasting with the two articles [32,39], which presented a higher total sample (n = 104). The other studies have intermediate sample sizes, ranging between 25 [37] and 100 [48] individuals.
3.3. Risk of Bias in the Studies
The risk of bias in the included studies and the description of the aspects contained in the NOS scale are summarized in Table 4. When analyzing the risk of bias, it was shown that ten studies presented high methodological quality and low risk of bias [32,33,34,35,41,50,51,52,55,56], and twelve were classified as being of low quality [31,36,37,38,39,40,42,43,44,45,48,54].

Table 4.
Summaries of studies included in present review.
3.4. Masticatory Function
The masticatory function was evaluated by different aspects/instruments in the included studies. Some studies evaluated the muscular activity through the electrical intensity of masseter and temporal muscles [31,33,34,35,36,37,38,39,41,43,44,45,48,50,51,52,55], the symmetry [31,32,33,38,42,51,52,55], and the synergy [32,38,51,52], and others evaluated the masticatory efficiency [44,54]. Besides the EMG and masticatory compound, the included studies described the masticatory function with a computerized mandibular scanner in two studies [36,54], a digital dynamometer in two studies [40,55]; in one, the authors used a pressure transducer [50]; in one, a bite force transducer [46]; in another one, an ultrasonography [50]; in one study, they used a sonography [36]; in one, a vibraphone [37]; in one, a computerized digital occlusal with T-Scan III [37]; and at last, in one study, a mandible kinesiograph [54].
3.4.1. Muscle Activity in MVC
Twelve studies [31,34,35,36,37,38,39,41,45,48,51,52] compared the muscular intensity between TMD and control subjects. An increase in electrical intensity was found in two of them [45,51]. In both, the TMD groups presented greater values of muscular activation than the control group [45]. In addition, in one of those [51], higher activation of masseter muscle was only observed in TMD patients in comparison with the control group. However, seven studies found that TMD leads to reduced values of muscular activation for both muscles [35,36,38,39,41,48,52], happening more frequently in the temporal [48] or more in the masseter [39]. In the other three studies [31,34,37], no relevant differences were found between the studied groups. Five articles [31,32,38,51,52] also evaluated muscle force symmetry/coordination, where three of them showed statistical differences between both groups [31,38,52], revealing that subjects with TMD presented greater asymmetry of both muscles [31] or specifically in one of them (temporalis [38] or masseter [52]). They also presented significantly larger unbalanced contractile activities of the contralateral masseter and temporal muscles. Four studies [32,38,51,52] assessed muscle synergy, and all of them showed that TMD leads to greater muscle activity asynergy between the pairs of muscles (masseter or temporal muscles). Four studies [39,40,41,45] evaluated the median frequency during MVC, and three [39,40,45] of those studies did not find any statistical difference between TMD and control groups; however, the fourth study [41] found a reduced frequency. Only one study [48] evaluated the frequency index with the aid of EMG during MVC and found a diminution of these parameters in the sample with TMD in comparison with the healthy individuals.
3.4.2. Muscle Activity at Rest
Four studies [36,48,50,52] assessed the electrical intensity at rest, but only one [48] showed increased muscular activity in both muscles, more in the masseter than in the temporalis, compared to the control group. One study also evaluated the muscle force symmetry/coordination [52] and showed a decrease in the symmetry index only for the temporal muscle.
3.4.3. Muscle Activity in Dynamic
Six studies [33,37,38,43,44,55] compared the electrical intensity between TMD and control groups. A statistical difference was found in two of them [33,44], where the TMD group presented greater electrical intensity values than the control group, specifically during the agonist phase in non-habitual chewing [33]. However, during the agonist phase in habitual chewing, TMD leads to decreased muscular activation values for the masseter muscle [33]. The other studies [37,38,43,55] did not find any relevant differences between the studied groups. Of the two studies that evaluated the “global activity” and “activity/cycle of chewing” [38,55], only one found significantly higher values of these parameters in the TMD group [55]. Five studies [32,33,38,42,55] also evaluated muscle symmetry/coordination, and all showed that TMD leads to a more asymmetrical activity; one study specified that it happened only in the temporal muscle [42], and another one only during the habitual chewing [33]. Three studies [33,35,42] assessed muscle synergy, but only two observed a decrease in the synergy between the masseter and temporalis muscles of both sides in the TMD group. Moreover, the TMD group showed a greater relative energy than the control group [33,35]. One article [38] measured the Functional Index (FI) during chewing and found that the global functioning condition of the masticatory system decreased to form the control group to the TMD group. In one study, chewing was examined by using the Functional Index (FI), and it was found that the TMD group’s overall masticatory system function was lower than the one of the control group. The TMD group showed a longer chewing stroke duration than the control group [35].
3.5. Results by Chewing Analysis
The chewing process was analyzed in six studies [31,35,44,54,55,56]. From these, one tested chewing through capsule with fuscin [44], and five used cookies [31,35,55,56] or gelatin cubes [54].
Chewing analysis using chewing compound:
OMES-Score: Four studies [31,35,55,56] agreed with the fact that subjects with TMD have greater chewing difficulty than the control group, presenting a decreased OMES-score.
Chewing stroke duration and the number of stokes: These parameters were increased in the TMD group in comparison to the control group [44].
Frequency index: Two studies [38,51] evaluated the frequency of chewing and did not suggest any statistical difference between TMD and control groups.
Masticatory efficiency: The two studies [44,54] that studied “masticatory efficiency” found contradictory data; one [44] concluded that TMD leads to an increase in masticatory efficiency, and the other [54] presented a decreased for the same parameter.
Meta-Analysis
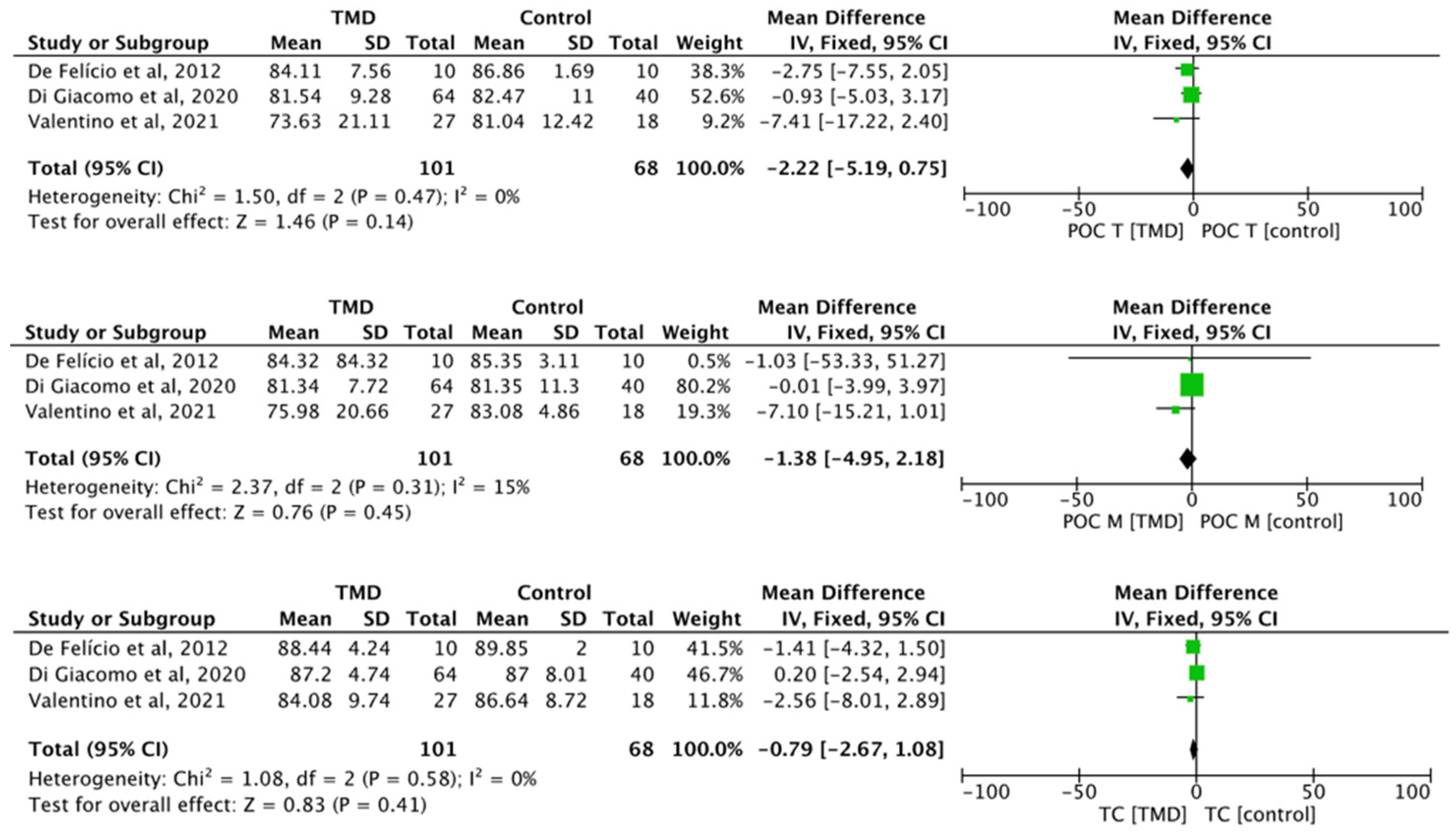
The differences between TMD and control for the parameters’ percentage overlapping coefficient of the anterior temporal muscle (POC T) (MD −2.22, 95% CI −5.19 to 0.75; I2 = 0%; p = 0.47; n = 3), percentage overlapping coefficient of the masseter muscle (POC M) (MD −1.38, 95% CI −4.95 to 2.18; I2 = 0%; p = 0.31; n = 3), and torque coefficient (TOC) (MD −0.79, 95% CI −2.67 to 1.08; I2 = 0%; p = 0.58; n = 3) did not present any statistical significance (Figure 2).
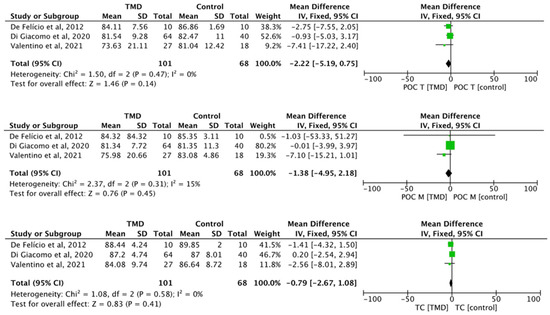
Figure 2.
Forest plot of POC T, POC M, and TC of electromyographic signal with TMD compared to healthy controls. Values represent effect sizes (weighted mean differences) and 95% confidence intervals (CI). SD, standard deviation; I2, I-squared. Black diamond represents the overall effect estimate of the meta-analysis. Green squares represent each studies individual standardized mean difference (SMD) and the extending lines the confidence intervals [31,32,51].
4. Discussion
To the best of the authors’ knowledge, this is the first systematic review that compares two assessment methods to analyze masticatory function in adult individuals with and without TMD. Many studies used sEMG to assess the functional status of the masticatory muscles in individuals with TMD [17,18,20,47,49,57,58,59,60,61,62,63]. However, fewer used chewing compounds for this analysis [31,35,44,54,55,56]. From the 22 included studies, 19 presented different degrees of association between TMD and chewing function [31,32,33,35,36,37,39,40,41,42,44,48,50,51,52,54,55,56]. When using the chewing compound method, one study found that TMD leads to an increase in the masticatory efficiency with the fuchsin capsules [44], whereas another one found the opposite result with a decreased masticatory efficiency with gelatin cubes [54]. Peroz et al. (2002) [64] reported that pain promotes a tendency to chew food more cautiously, thus obtaining smaller pieces, leading to an increased chewing time and, consequently, greater chewing efficiency. For this reason, we are unable to reach a conclusion regarding the direction of the impact on the masticatory efficiency, only that it seems to be influenced by the presence of TMD. Nonetheless, it can be suggested that, clinically, it may be useful to use the masticatory compound to highlight any chewing-pattern changes. For future studies, we may recommend a more careful description of the sample depending on the subgroups, allowing a better comparison. Homogenization of the type of masticatory compound is necessary, too, since it is known that masticatory behavior can be altered depending on the foods’ texture [65]. The sequence of a mastication cycle is constituted by a set of movements that occur from food ingestion all the way to swallowing it. Although the number of cycles required to chew the same type of food is relatively constant for the same individual, it has sizeable inter-individual variations [66]. With the chewing-gum method, we found that individuals with TMD exhibited an increase in the number of chewing cycles and the time required to perform a cycle compared to healthy individuals [44]. This does not mean that TMD individuals have better chewing function, but it is possible to suggest that the patient developed this adaptation to prevent pain exacerbation [67]. Nonetheless, when the frequency index was used, no alteration was found [38,51]. Authors should preferably use the masticatory frequency index because it expresses the normalization of the number of masticatory cycles in relation to the execution time, since this discrepancy could be avoided by using the same parameter [51].
Regarding the analysis through sEMG, six studies [33,37,38,43,44,55] evaluated the electrical intensity of muscles during chewing and showed discrepancies in the results. Four of them found no differences between groups, and the other two showed that the electrical intensity of mastication muscles in individuals with TMD is greater than the intensity found in the healthy control group [33,44]. This increased intensity may not be associated with greater muscle strength but rather with the recruitment of new motor units to compensate for any asymmetry [38] and thus, improving neuromuscular coordination that is needed for the masticatory movements [68]. These variations may be the result of the use of a different sEMG pattern or chewing evaluation technique. In fact, mastication patterns differ during masticatory activity [33], and the differences in muscular intensity between TMD subjects and healthy individuals are more evident when collected under guided conditions (unusual and unilateral chewing) [40]. All the studies showed that individuals with TMD entail impaired orofacial motor functions, which may be related to the asymmetry of muscle activity, inducing a change in the mandibular movement itself [69]. All the articles that studied the symmetry during dynamic setup showed that patients with TMD disorders presented an altered muscular contraction [32,33,38,42,55], being more asymmetric during masticatory activity, specifically in the temporal muscle [42] or only during the habitual chewing [33]. Two studies [55,56] found that TMD patients showed impairment of orofacial motor functions, with alterations in the recruitment of masseter and temporal muscles during chewing. Two studies [55,70] reported the importance of this topic, considering that the general population which presents any sign or symptom of TMD may have the chewing process affected [71]. The analysis of the muscular electrical activity during MVC did not show any differences between the control and the TMD groups in terms of activation of one or both muscles. It permitted researchers to identify if one muscle is more activated than the other. An increase in electrical intensity was found in two studies [45,51] where the TMD group presented greater values of muscular activation than the control group [45]. In addition, in the second article [51], only a higher activation of masseter muscle was observed in TMD patients in comparison with the control group. Some of the studies included in this systematic review and previous studies [35,36,38,39,41,48,52] found that TMD subjects have lower activities during MVC than normal subjects associated with a reduction of the number of masticatory cycles [48]. Those findings may be due to the lower efficiency of masticatory muscles [32] and the easy muscle fatigue [36,39,40,41,45]. The other studies [31,34,37] found no relevant differences between the studied groups. In future studies, it would be pertinent to select the sample through the TMD type presented by each individual in order to reduce discrepancies. In the analysis of symmetry, five studies have also evaluated muscle symmetry/coordination [31,32,38,51]; three of them showed larger unbalanced contractile activities of the contralateral masseter and temporal muscles between both groups [31,38,52], while the other two did not [32,51]. These symmetry changes may serve as an incentive for future research since these effects may suggest that individuals with TMD tend to present a functional alteration reflected in masticatory muscles coordination. However, a marked asynergia was noted in TMD subjects since a preponderance of activity was found in the masseter muscle [51] or the temporalis muscle [32,38]. Except for one article [48], all studies that evaluated the parameters in the mandibular resting position concluded that individuals with TMD showed no differences in muscular intensity compared with individuals without TMD [33,36,41,50]. Thus, using sEMG in the mandibular resting position does not prove to be the ideal method for TMD diagnosis. However, it is widely found in the literature in the stomatognathic system evaluation.
The different methods of sEMG signal capture, processing, and analysis constituted an important limiting factor for the comparative analysis of the results described in the articles selected in this systematic review. All articles described different sEMG capture and processing protocols. Such variations in methodological procedures hinder data analysis and indicate the need for a standardized protocol regarding the sEMG signal capture, processing, and analysis for the temporalis and masseter muscles. When analyzing the methodological quality, we noted that thirteen of the included studies had low methodological quality, with a high risk of bias. To reduce such a risk of bias, we propose that, in the future, we suggest including the imperative description of the control-group selection method. Moreover, several studies only defined individuals as “with or without TMD.” They did not subdivide individuals according to the classification of the different types of TMD (although a diagnosis was made by widely used validated questionnaires used in research and clinical context). Each kind of TMD may interfere with different parameter changes used to determine masticatory function [55]. On the other hand, we can mention the scarce number of articles available in the literature that used chewing compounds compared with those concerning electromyography. This entails more limited comparability of data and a need for further studies to analyze the chewing function of TMD individuals by chewing compounds. Hence, we can say that, besides the fact that there is a great variety of chewing materials that can be used, few studies fulfilled the inclusion criteria to integrate this present review. The main reasons for these exclusions were an inadequate sample age and a different methodology for TMD diagnosis other than RDC/DC-TMD. This shows the need for future studies to agree on comparable research findings. The chewing-gum methodology is considered to be a suitable method for evaluating chewing patterns, mainly because of the processing easiness and the standardized tests in contrast to natural foods [72]. In the experimental procedure itself, except for one article that assessed laterotrusive movements [37], the dynamic tests were all recorded during habitual chewing or non-habitual chewing (either following a metronome or forced unilateral type). When performed with unilateral or non-habitual mastication, this allows for avoiding possible compensatory adjustments that may arise during contraction of the masticatory muscles, thus obtaining a more stable pattern in muscle recruitment [33]. It also permitted researchers to avoid that the individuals choose their preferred chewing pattern, attributing greater comfort, as happens in habitual chewing [73]. Another limitation verified throughout this systematic review is related to the sample size and diversity of the individuals included in the selected studies. Some of the selected studies had a minimal number of subjects [40,43]. All the studies that evaluated mastication by using the electromyography method used different electromyographs, as well as different frequency domains. The normalization of these parameters would allow for homogenization of the results obtained in those studies.
As for the procedure, we may suggest for future studies a standardization of the methodologies used, either through the electromyography method or with the chewing gum, in order to obtain standardized and comparable results in all the studies carried out.
5. Conclusions
Through this review of the literature, we found that the parameters analyzed with the compound technique for chewing showed altered mandibular functions in individuals with TMD. With the EMG method, it was possible to suggest that TMD in adult individuals causes compensatory muscle behaviors. Multiple modifications of the masticatory function were reported, including an asymmetrical and lesser synergy pattern of muscle contractions compared to individuals without TMD. However, it is important to note that a clear association between TMD and chewing disorders could not be determined categorically. Several factors, including sample selection, subjects’ clinical conditions, and research techniques, are particularly important in explaining it.
Author Contributions
V.M., conception and design of the work, acquisition, analysis and interpretation of the data, drafted the work, and was the main author of the present manuscript; S.D.R., conception and design of the work, acquisition, analysis and interpretation of the data, and substantively revised it; M.P., conception and design of the work, revision of the work; S.M. revision of the work.; A.S.G., revision of the work M.G., performed statistical analysis and interpretation of the data and substantively revised it; T.P., conception and design of the work, analysis and interpretation of the data and revision of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This work received funding from CESPU—Cooperativa de Ensino Superior Politécnico e Universitário under the project “AlignAgen-GI2-CESPU-2022”.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Cochrane Library

Table A1.
The search strategy used in the Cochrane Library.
Table A1.
The search strategy used in the Cochrane Library.
| Date of Last Survey: 16 June 2022 06:54:34 | ||
|---|---|---|
| #1 | Mesh descriptor: [temporomandibular] explode all trees | 1874 |
| #2 | «Disorder» (word variations were searched) | 90,869 |
| #3 | #1 and #2 | 609 |
| #4 | Electromyography | 6447 |
| #5 | «Mastication» (word variations were searched) | 911 |
| #6 | (chewing) | 2682 |
| #7 | #5 OR #6 | 3101 |
| #8 | #3 AND #4 AND #7 | 11 |
| #9 | bite force | 312 |
| #10 | masticatory efficiency | 106 |
| #11 | mastication cycle | 32 |
| #12 | #9 OR #10 OR #11 | 436 |
| #13 | #3 AND #12 | 20 |
| #14 | #8 OR #13 | 29 |
Appendix B. PubMed

Table A2.
The search strategy used on PubMed.
Table A2.
The search strategy used on PubMed.
| Last Research Date: 16 June 2022 | |||
| 1 | temporomandibular | “temporomandibular” [All Fields] | 30,251 |
| 2 | disorder | “disease” [MeSH Terms] OR “disease” [All Fields] OR “disorder” [All Fields] OR “disorders” [All Fields] OR “disorder s” [All Fields] OR “disordes” [All Fields] | 64,562 |
| 3 | (#1) AND (#2) | “temporomandibular” [All Fields] AND “disorder” [All Fields] | 18,224 |
| 4 | electromyography | “electromyography” [MeSH Terms] OR “electromyography” [All Fields] OR “electromyographies” [All Fields] | 90,672 |
| 5 | (mastication) OR (chewing) | “masticated” [All Fields] OR “masticates” [All Fields] OR “masticating” [All Fields] OR “mastication” [MeSH Terms] OR “mastication” [All Fields] OR “masticate” [All Fields] OR “mastications” [All Fields] OR “masticator” [All Fields] OR “chewings” [All Fields] OR “chews” [All Fields] OR “mastication” [MeSH Terms] OR “mastication” [All Fields] OR “chewed” [All Fields] OR “chewing” [All Fields] | 25,572 |
| 6 | ((#3) AND (#4)) AND (#5) | “temporomandibular” [All Fields] AND “disorder” [All Fields] AND (“electromyography” [MeSH Terms] OR “mastication” [MeSH Terms] OR “chewing” [All Fields])) | 137 |
| 7 | bite force | “bite force” [MeSH Terms] OR (“bite” [All Fields] AND “force” [All Fields]) OR “bite force” [All Fields] | 5028 |
| 8 | masticatory efficiency | “masticatory” [All Fields] AND (“efficiences” [All Fields] OR “efficiency” [MeSH Terms] OR “efficiency” [All Fields] OR “efficiencies” [All Fields] OR “efficient” [All Fields] OR “efficiently” [All Fields] OR “efficients” [All Fields]) | 711 |
| 9 | mastication cycle | (“masticated” [All Fields] OR “masticates” [All Fields] OR “masticating” [All Fields] OR “mastication” [MeSH Terms] OR “mastication” [All Fields] OR “masticate” [All Fields] OR “mastications” [All Fields] OR “masticator” [All Fields]) AND (“cycle” [All Fields] OR “cycle s” [All Fields] OR “cycled” [All Fields] OR “cycles” [All Fields] OR “cycling” [All Fields] OR “cyclings” [All Fields]) | 1090 |
| 10 | ((#7) OR (#9)) OR (#10) | “bite force” [MeSH Terms] OR (“masticatory” [All Fields] AND (“efficiences” [All Fields] OR “efficiency” [MeSH Terms] “cycles” [All Fields] | 6450 |
| 11 | (#3) AND (#10) | “temporomandibular” [All Fields] AND “disorder” [All Fields] AND “bite force” [MeSH Terms] OR (“masticatory” [All Fields] AND (“efficiences” [All Fields] OR “efficiency” [MeSH Terms] “cycles” [All Fields] | 407 |
| 12 | (#6) OR (#11) | “temporomandibular” [All Fields] AND “disorder” [All Fields] AND (“electromyography” [MeSH Terms] OR “mastication” [MeSH Terms] OR “chewing” [All Fields])) OR “temporomandibular” [All Fields] AND “disorder” [All Fields] AND “bite force” [MeSH Terms] OR (“masticatory” [All Fields] AND (“efficiences” [All Fields] OR “efficiency” [MeSH Terms] “cycles” [All Fields] | 508 |
| 13 | (#6) OR (#11) | “temporomandibular” [All Fields] AND “disorder” [All Fields] AND (“electromyography” [MeSH Terms] OR “mastication” [MeSH Terms] OR “chewing” [All Fields])) OR “temporomandibular” [All Fields] AND “disorder” [All Fields] AND “bite force” [MeSH Terms] OR (“masticatory” [All Fields] AND (“efficiences” [All Fields] OR “efficiency” [MeSH Terms] “cycles” [All Fields] | 234 |
Appendix C. EBSCO

Table A3.
The search strategy used on EBSCO.
Table A3.
The search strategy used on EBSCO.
| # | Consulta | Limitadores/Expansores | última Execução Por | Resultados |
|---|---|---|---|---|
| S9 | S1 AND S8 | Limitadores—data de Publicação: 20110101-20211231 Expansores—Aplicar assuntos equivalentes Modos de pesquisa—Booleana/Frase | Interface—EBSCOhost research Databases Ecrã e Pesquisa—Pesquisa Avançada Base de dados—CINAHL with Full Text; Dentistry & Oral Sciences Sources; MEDLINE Complete | 259 |
| S8 | S1 AND S7 | Limitadores—aplicar assuntos equivalentes Modos de pesquisa—Booleana/Frase | Interface—EBSCOhost research Databases Ecrã e Pesquisa—Pesquisa Avançada Base de dados—CINAHL with Full Text; Dentistry & Oral Sciences Sources; MEDLINE Complete | 511 |
| S7 | Mastication cycle OR masticatory efficiency OR bite force | Limitadores—aplicar assuntos equivalentes Modos de pesquisa—Booleana/Frase | Interface—EBSCOhost research Databases Ecrã e Pesquisa—Pesquisa Avançada Base de dados—CINAHL with Full Text; Dentistry & Oral Sciences Sources; MEDLINE Complete | 7073 |
| S6 | S4 AND S5 | Limitadores—aplicar assuntos equivalentes Modos de pesquisa—Booleana/Frase | Interface—EBSCOhost research Databases Ecrã e Pesquisa—Pesquisa Avançada Base de dados—CINAHL with Full Text; Dentistry & Oral Sciences Sources; MEDLINE Complete | 280 |
| S5 | S1 AND S2 | Limitadores—aplicar assuntos equivalentes Modos de pesquisa—Booleana/Frase | Interface—EBSCOhost research Databases Ecrã e Pesquisa—Pesquisa Avançada Base de dados—CINAHL with Full Text; Dentistry & Oral Sciences Sources; MEDLINE Complete | 1032 |
| S4 | Mastication OR chewing | Limitadores—aplicar assuntos equivalentes Modos de pesquisa—Booleana/Frase | Interface—EBSCOhost research Databases Ecrã e Pesquisa—Pesquisa Avançada Base de dados—CINAHL with Full Text; Dentistry & Oral Sciences Sources; MEDLINE Complete | 37,514 |
| S3 | Temporomandibular AND disorder OR electromyography | Limitadores—aplicar assuntos equivalentes Modos de pesquisa—Booleana/Frase | Interface—EBSCOhost research Databases Ecrã e Pesquisa—Pesquisa Avançada Base de dados—CINAHL with Full Text; Dentistry & Oral Sciences Sources; MEDLINE Complete | 138,322 |
| S2 | electromyography | Limitadores—aplicar assuntos equivalentes Modos de pesquisa—Booleana/Frase | Interface—EBSCOhost research Databases Ecrã e Pesquisa—Pesquisa Avançada Base de dados—CINAHL with Full Text; Dentistry & Oral Sciences Sources; MEDLINE Complete | 112,218 |
| S1 | Temporomandibular AND disorder | Limitadores—aplicar assuntos equivalentes Modos de pesquisa—Booleana/Frase | Interface—EBSCOhost research Databases Ecrã e Pesquisa—Pesquisa Avançada Base de dados—CINAHL with Full Text; Dentistry & Oral Sciences Sources; MEDLINE Complete | 27,136 |
Appendix D. Scielo

Table A4.
The search strategy used on Scielo.
Table A4.
The search strategy used on Scielo.
| Id. | Busca | Resultados |
|---|---|---|
| #11 | Expressão: (#6) OR (#8) Filtros aplicados: (Ano de publicação: 2018) (Ano de publicação: 2019) (Ano de publicação: 2017) (Ano de publicação: 2016) (Ano de publicação: 2020 (Ano de publicação: 2015) (Ano de publicação: 2014) (Ano de publicação: 2011) | 20 |
| #10 | Expressão:(((((temporomandibular) AND (disorder) AND network:org AND network:rve) OR (electromyography AND network:org AND network:rve) AND network:org AND network:rve) AND ((mastication) OR (chewing) AND network:org AND network:rve) AND network:org AND network:rve) OR (((temporomandibular) AND (disorder) AND network:org AND network:rve) AND (bite force) OR (masticatory efficiency) OR (mastication cycle) NAD network:org AND network:rve) AND network:org AND network:rve) AND network:org AND network:rve | 1 |
| #9 | Expressão: (#6) OR (#8) Filtros aplicados: | 39 |
| #8 | Expressão: (#1) OR (#7) Filtros aplicados: | 1 |
| #7 | Expressão: (BITE FORCE) OR (masticatory efficiency) OR (mastication cycle) Filtros aplicados: | 109 |
| #6 | Expressão: (#4) OR (#5) Filtros aplicados: | 38 |
| #5 | Expressão: (MASTICATION) OR (CHEWING) Filtros aplicados: | 780 |
| #4 | Expressão: (#1) OR (#2) Filtros aplicados: | 997 |
| #3 | Expressão: ( Filtros aplicados: | 0 |
| #2 | Expressão: ELECTROMYOGRAPHY Filtros aplicados: | 759 |
| #1 | Expressão:(TEMPOROMANDIBULAR) OR (DISORDER) Filtros aplicados: | 259 |
Appendix E. ClinicalTrials.gov

Table A5.
The search strategy used on ClinicalTrials.gov.
Table A5.
The search strategy used on ClinicalTrials.gov.
| Terms Synonyms | Search Results |
|---|---|
| Electromyography Electromyogram | 5 studies -- |
| Masticatory efficiency | 2 studies |
| efficiency | 4 studies |
| masticatory | 6 studies |
| Temporomandibular Disorder TEMPOROMANDIBULAR JOINT DISORDER Temporomandibular joint dysfunction Temporomandibular dysfunction Temporomandibular Joint Disease Costen Syndrome Dysfunction tmj Mandibular dysfunction Pain-dysfunction syndrome Temporomandibular Joint Syndrome TMJ Disease TMJ Disorder Tmj dysfunction | 8 studies 7 studies 7 studies 1 studies 1 studies -- -- -- -- -- -- -- -- |
| Disorder Diseases condition | 8 studies 8 studies -- |
| Temporomandibular | 8 studies |
Appendix F. Excluded Articles

Table A6.
Articles excluded through after applying selection and eligibility.
Table A6.
Articles excluded through after applying selection and eligibility.
| Articles Excluded-Selection (n = 213) | |
|---|---|
| n = 186 | Title and/abstract |
| n = 3 | Language (Russian) |
| n = 3 | Case report |
| n = 5 | Literature review |
| n = 14 | Systematic review |
| n = 1 | Interview |
| n = 1 | Free complete text unavailable |
| Articles Excluded—Eligibility (n = 45) | |
| n = 15 | Inappropriate sample |
| n = 6 | Used other TMD diagnostic method than RDC-TMD and DC-TMD |
| n = 3 | Without link with TDM/Used other TMD diagnostic method than RDC-TMD and DC-TMD |
| n = 6 | Inappropriate intervention |
| n = 12 | Without link with TDM |
| n = 2 | Used other TMD diagnostic method than RDC-TMD and DC-TMD Inappropriate sample |
| n = 1 | Without link with TDM Inappropriate sample |
Appendix G. Description of the Aspects Contained in the Newcastle–Ottawa Quality Assess
Ment Scale

| Autor/Year | |
|---|---|
| Selection 1—Representativeness of the sample |
|
| 2—Sample size |
|
| 3—Non-respondents |
|
| 4—Ascertainment of the exposure (risk factor) |
|
| Comparability: The subjects in different outcome groups are comparable based on the study design or analysis. Confounding factors are controlled. |
|
| Outcomes 1—Assessment of the outcome |
|
| 2—Statistical test |
|
| TOTAL |
According to the Newcastle-Ottawa Scale (NOS) criteria. Quality score: Overall scores were given (good, fair, and poor). Good quality: 3 or 4 stars (*) in the selection domain AND 1 or 2 stars in the comparability domain and 2 or 3 stars in the outcome domain; Fair quality: 2 stars in the selection domain and 1 or 2 stars in the comparability domain and 2 or 3 stars in the outcome/exposure domain; poor quality: 0 or 1 star in the selection domain OR 0 stars in the comparability domain OR 0 or 1 stars in the outcome/exposure domain.
References
- Cascone, P.; Gennaro, P.; Gabriele, G.; Chisci, G.; Mitro, V.; De Caris, F.; Iannetti, G. Temporomandibular synovial chondromatosis with numerous nodules. J. Craniofacial Surg. 2014, 25, 1114–1115. [Google Scholar] [CrossRef]
- Kapos, F.P.; Exposto, F.G.; Oyarzo, J.F.; Durham, J. Temporomandibular disorders: A review of current concepts in aetiology, diagnosis and management. Oral Surg. 2020, 13, 321–334. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Porto de Toledo, I.; Dutra, K.L.; Stefani, F.M.; Porporatti, A.L.; Flores-Mir, C.; De Luca Canto, G. Association between chewing dysfunctions and temporomandibular disorders: A systematic review. J. Oral Rehabil. 2018, 45, 819–835. [Google Scholar] [CrossRef]
- De Figueiredo, V.M.G.; Cavalcanti, A.L.; De Farias, A.B.L.; Do Nascimento, S.R. Prevalence of signs, symptoms and associated factors in patients with temporomandibular dysfunction. Acta Sci.-Health Sci. 2009, 31, 159–163. [Google Scholar] [CrossRef]
- Halpern, L.R.; Levine, M.; Dodson, T.B. Sexual Dimorphism and Temporomandibular Disorders (TMD). Oral Maxillofac. Surg. Clin. North Am. 2007, 19, 267–277. [Google Scholar] [CrossRef]
- Zanin, M.C.; Garcia, D.M.; Rocha, E.M.; de Felício, C.M. Orofacial Motor Functions and Temporomandibular Disorders in Patients With Sjögren’s Syndrome. Arthritis Care Res. 2020, 72, 1057–1065. [Google Scholar] [CrossRef]
- Østensjø, V.; Moen, K.; Storesund, T.; Rosén, A. Prevalence of Painful Temporomandibular Disorders and Correlation to Lifestyle Factors among Adolescents in Norway. Pain Res. Manag. 2017, 2017, 2164825. [Google Scholar] [CrossRef]
- Greenbaum, T.; Dvir, Z.; Emodi-Perlman, A.; Reiter, S.; Rubin, P.; Winocur, E. The association between specific temporomandibular disorders and cervicogenic headache. Musculoskelet. Sci. Pract. 2021, 52, 102321. [Google Scholar] [CrossRef]
- Yap, A.U.J.; Dworkin, S.F.; Chua, E.K.; List, T.; Tan, K.B.C.; Tan, H.H. Prevalence of temporomandibular disorder subtypes, psychologic distress, and psychosocial dysfunction in Asian patients. J. Orofac. Pain 2003, 17, 21–28. [Google Scholar] [CrossRef]
- Dubner, R.; Ohrbach, R.; Dworkin, S.F. The Evolution of TMD Diagnosis: Past, Present, Future. J. Dent. Res. 2016, 95, 1093–1101. [Google Scholar] [CrossRef]
- de Felício, C.M.; de Oliveira Melchior, M.; da Silva, M.A.M.R.; dos Santos Celeghini, R.M. Masticatory performance in adults related to temporomandibular disorder and dental occlusion. Pro-Fono 2007, 19, 151–158. [Google Scholar] [CrossRef]
- Douglas, C.R.; Avoglio, J.L.V.; de Oliveira, H. Stomatognathic adaptive motor syndrome is the correct diagnosis for temporomandibular disorders. Med. Hypotheses 2010, 74, 710–718. [Google Scholar] [CrossRef]
- de Andrade, R.A.; da Cunha, M.D.; Reis, A.M.; da Costa dos Santos Reis, A.M. Morphofunctional analysis of the stomatognathic system in conventional complete dentures users from the Integrated Health Center. Rev. CEFAC 2017, 19, 712–725. [Google Scholar] [CrossRef]
- Sodhi, A.; Naik, S.; Pai, A.; Anuradha, A. Rheumatoid arthritis affecting temporomandibular joint. Contemp. Clin. Dent. 2015, 6, 124–127. [Google Scholar] [CrossRef]
- Guarda-Nardini, L.; Olivo, M.; Ferronato, G.; Salmaso, L.; Bonnini, S.; Manfredini, D. Treatment effectiveness of arthrocentesis plus hyaluronic acid injections in different age groups of patients with temporomandibular joint osteoarthritis. J. Oral Maxillofac. Surg. 2012, 70, 2048–2056. [Google Scholar] [CrossRef]
- Nosouhian, S.; Haghighat, A.; Mohammadi, I.; Shadmehr, E.; Davoudi, A.; Badrian, H. Temporomandibular Joint Hypermobility Manifestation Based on Clinical Observations. J. Int. Oral Health JIOH 2015, 7, 1–4. [Google Scholar]
- Ardizone, I.; Celemin, A.; Aneiros, F.; Del Rio, J.; Sanchez, T.; Moreno, I. Electromyographic study of activity of the masseter and anterior temporalis muscles in patients with temporomandibular joint (TMJ) dysfuction: Comparison with the clinical dysfunction index. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e14–e19. [Google Scholar] [CrossRef][Green Version]
- Fuentes, A.D.; Sforza, C.; Miralles, R.; Ferreira, C.L.; Mapelli, A.; Lodetti, G.; Martin, C. Assessment of electromyographic activity in patients with temporomandibular disorders and natural mediotrusive occlusal contact during chewing and tooth grinding. CRANIO J. Craniomandib. Sleep Pract. 2017, 35, 152–161. [Google Scholar] [CrossRef]
- Kordass, B.; Hugger, A.; Bernhardt, O. Correlation between computer-assisted measurements of mandibular opening and closing movements and clinical symptoms of temporomandibular dysfunction. Int. J. Comput. Dent. 2012, 15, 93–107. [Google Scholar]
- De Felício, C.M.; Mapelli, A.; Sidequersky, F.V.; Tartaglia, G.M.; Sforza, C. Mandibular kinematics and masticatory muscles EMG in patients with short lasting TMD of mild-moderate severity. J. Electromyogr. Kinesiol. 2013, 23, 627–633. [Google Scholar] [CrossRef]
- Bakke, M. Bite Force and Occlusion. Semin. Orthod. 2006, 12, 120–126. [Google Scholar] [CrossRef]
- Dinsdale, A.; Forbes, R.; Thomas, L.; Treleaven, J. “What if it doesn’t unlock?”: A qualitative study into the lived experiences of adults with persistent intra-articular temporomandibular disorders. Musculoskelet. Sci. Pract. 2021, 54, 102401. [Google Scholar] [CrossRef]
- van der Bilt, A. Assessment of mastication with implications for oral rehabilitation: A review. J. Oral Rehabil. 2011, 38, 754–780. [Google Scholar] [CrossRef]
- Roberts, W.E.; Stocum, D.L. Part II: Temporomandibular Joint (TMJ)—Regeneration, Degeneration, and Adaptation. Curr. Osteoporos. Rep. 2018, 16, 369–379. [Google Scholar] [CrossRef]
- Szyszka-Sommerfeld, L.; Lipski, M.; Woźniak, K. Surface Electromyography as a Method for Diagnosing Muscle Function in Patients with Congenital Maxillofacial Abnormalities. J. Healthc. Eng. 2020, 2020, 8846920. [Google Scholar] [CrossRef]
- Shousha, T.; Alayat, M.; Moustafa, I. Effects of low-level laser therapy versus soft occlusive splints on mouth opening and surface electromyography in females with temporomandibular dysfunction: A randomized-controlled study. PLoS ONE 2021, 16, e0258063. [Google Scholar] [CrossRef]
- Szyszka-Sommerfeld, L.; Machoy, M.; Lipski, M.; Woźniak, K. The Diagnostic Value of Electromyography in Identifying Patients With Pain-Related Temporomandibular Disorders. Front. Neurol. 2019, 10, 108. [Google Scholar] [CrossRef]
- Shiga, H.; Kobayashi, Y.; Arakawa, I.; Shonai, Y. Selection of food and chewing side for evaluating masticatory path stability. Odontology 2003, 91, 26–30. [Google Scholar] [CrossRef]
- Ishii, T.; Narita, N.; Endo, H. Evaluation of jaw and neck muscle activities while chewing using EMG-EMG transfer function and EMG-EMG coherence function analyses in healthy subjects. Physiol. Behav. 2016, 160, 35–42. [Google Scholar] [CrossRef]
- Troka, M.; Wojnicz, W.; Szepietowska, K.; Podlasiński, M.; Walerzak, S.; Walerzak, K.; Lubowiecka, I. Towards classification of patients based on surface EMG data of temporomandibular joint muscles using self-organising maps. Biomed. Signal Process. Control 2022, 72, 103322. [Google Scholar] [CrossRef]
- De Felício, C.M.; Ferreira, C.L.P.; Medeiros, A.P.M.; Rodrigues Da Silva, M.A.M.; Tartaglia, G.M.; Sforza, C. Electromyographic indices, orofacial myofunctional status and temporomandibular disorders severity: A correlation study. J. Electromyogr. Kinesiol. 2012, 22, 266–272. [Google Scholar] [CrossRef]
- Di Giacomo, P.; Ferrato, G.; Serritella, E.; Polimeni, A.; Di Paolo, C. Muscular pattern in patients with temporomandibular joint disc displacement with reduction: An electromyographical assessment. Clin. Ther. 2020, 171, e414–e420. [Google Scholar] [CrossRef]
- Fassicollo, C.E.; Graciosa, M.D.; de Medeiros, D.L.; Soares, L.P.; Mochizuki, L.; Ries, L.G.K. Standardized mastication increases the coordination in masticatory activity in women with chronic temporomandibular joint disorders: A case control study. Manual Ther. Posturol. Rehabil. J. 2020, 1–7. [Google Scholar] [CrossRef]
- Fassicollo, C.E.; Graciosa, M.D.; Graefling, B.F.; Ries, L.G.K. Temporomandibular dysfunction, myofascial, craniomandibular and cervical pain: Effect on masticatory activity during rest and mandibular isometry. Rev. Dor 2017, 18, 250–254. [Google Scholar] [CrossRef]
- Fassicollo, C.E.; Garcia, D.M.; Machado, B.C.Z.; de Felício, C.M. Changes in jaw and neck muscle coactivation and coordination in patients with chronic painful TMD disk displacement with reduction during chewing. Physiol. Behav. 2021, 230, 113267. [Google Scholar] [CrossRef]
- Helena, A.; Santos, D.L. Influence of Temporomandibular Disorder in the Stomatognatic System: Electromyography, Mandibular. J. Dent. Oral Biol. 2021, 5, 1173. [Google Scholar]
- Karakis, D.; Bagkur, M.; Toksoy, B. Comparison of Simultaneously Recorded Computerized Occlusal Analysis and Surface Electromyographic Activity of Masticatory Muscles Between Patients with Unilateral TMD. Int. J. Prosthodont. 2021, 34, 554–559. [Google Scholar] [CrossRef]
- Mapelli, A.; Zanandréa Machado, B.C.; Giglio, L.D.; Sforza, C.; De Felício, C.M. Reorganization of muscle activity in patients with chronic temporomandibular disorders. Arch. Oral Biol. 2016, 72, 164–171. [Google Scholar] [CrossRef]
- Pires, P.F.; Rodrigues-Bigaton, D. Evaluation of integral electromyographic values and median power frequency values in women with myogenous temporomandibular disorder and asymptomatic controls. J. Bodyw. Mov. Ther. 2018, 22, 720–726. [Google Scholar] [CrossRef]
- Pitta, N.C.; Nitsch, G.S.; Machado, M.B.; de Oliveira, A.S. Activation time analysis and electromyographic fatigue in patients with temporomandibular disorders during clenching. J. Electromyogr. Kinesiol. 2015, 25, 653–657. [Google Scholar] [CrossRef]
- Ries, L.G.K.; Graciosa, M.D.; Soares, L.P.; Sperandio, F.F.; Santos, G.M.; Degan, V.V.; Gadotti, I.C. Effect of time of contraction and rest on the masseter and anterior temporal muscles activity in subjects with temporomandibular disorder. CoDAS 2016, 28, 155–162. [Google Scholar] [CrossRef][Green Version]
- Ries, L.G.K.; Graciosa, M.D.; De Medeiros, D.L.; Pacheco, S.C.D.S.; Fassicolo, C.E.; Graefling, B.C.F.; Degan, V.V. Influence of craniomandibular and cervical pain on the activity of masticatory muscles in individuals with Temporomandibular Disorder. CoDAS 2014, 26, 389–394. [Google Scholar] [CrossRef][Green Version]
- Machado, M.B.; Nitsch, G.S.; Pitta, N.C.; de Oliveira, A.S. Tempo de ativação muscular em portadoras de disfunção temporomandibular durante a mastigação. Audiol.-Commun. Res. 2014, 19, 202–207. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, C.A.; Melchior, M.; de Oliveira Melchior, M.; Magri, L.V.; Mestriner, W., Jr.; Mazzetto, M.O. Is the masticatory function changed in patients with temporomandibular disorder? Braz. Dent. J. 2015, 26, 181–185. [Google Scholar] [CrossRef]
- Xu, L.; Fan, S.; Cai, B.; Fang, Z.; Jiang, X. Influence of sustained submaximal clenching fatigue test on electromyographic activity and maximum voluntary bite forces in healthy subjects and patients with temporomandibular disorders. J. Oral Rehabil. 2017, 44, 340–346. [Google Scholar] [CrossRef]
- Yin, Y.; Yin, Y.; He, S.; Xu, J.; You, W.; You, W.; Li, Q.; Li, Q.; Long, J.; Long, J.; et al. The neuro-pathophysiology of temporomandibular disorders-related pain: A systematic review of structural and functional MRI studies. J. Headache Pain 2020, 21, 78. [Google Scholar] [CrossRef]
- Schindler, H.J.; Kordaß, B.; Hugger, A.; Hugger, S.; Schindler, H.J.; Kordaß, B.; Hugger, A. Surface EMG of the Masticatory Muscles (Part 2): Fatigue Testing, Mastication Analysis and Influence of Different Factors Oberflächen-EMG der Kaumuskulatur (Teil 2): Klinische Relevanz im Fatigue-Test, bei der Kauanalyse und bei unterschiedlichen Einfluss. Int. J. Comput. Dent. 2013, 16, 37–58. [Google Scholar]
- Sójka, A.; Huber, J.; Hędzelek, W.; Wiertel-Krawczuk, A.; Szymankiewicz-Szukała, A.; Seraszek-Jaros, A.; Kulczyk, A.; Wincek, A.; Sobieska, M. Relations between the results of complex clinical and neurophysiological examinations in patients with temporomandibular disorders symptoms. CRANIO J. Craniomandib. Sleep Pract. 2018, 36, 44–52. [Google Scholar] [CrossRef]
- Ferrario, V.F.; Sforza, C.; Miani, A., Jr.; D’Addona, A.; Barbini, E. Electromyographic activity of human masticatory muscles in normal young people. Statistical evaluation of reference values for clinical applications. J. Oral Rehabil. 1993, 20, 271–280. [Google Scholar] [CrossRef]
- Silva Andresen Strini, P.J.; Silva Andresen Strini, P.J.; De Souza Barbosa, T.; Duarte Gavião, M.B. Assessment of thickness and function of masticatory and cervical muscles in adults with and without temporomandibular disorders. Arch. Oral Biol. 2013, 58, 1100–1108. [Google Scholar] [CrossRef]
- Valentino, R.; Cioffi, I.; Vollaro, S.; Cimino, R.; Baiano, R.; Michelotti, A. Jaw muscle activity patterns in women with chronic TMD myalgia during standardized clenching and chewing tasks. Cranio 2021, 39, 157–163. [Google Scholar] [CrossRef]
- Fassicollo, C.E.; Graefling, B.C.F.; Ries, L.G.K. Correlations between masticatory muscle activity, quality of life, and dysfunction severity in women with chronic temporomandibular disorder. Braz. J. Pain 2019, 2, 225–231. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa Quality Assessment Scale; Ottawa Hospital Research Institute: Ottawa, ON, USA, 2014; pp. 2–4. [Google Scholar]
- Kümbüloğlu, O.; Saracoglu, A.; Bingöl, P.; Hatipoğlu, A.; Ozcan, M. Clinical study on the comparison of masticatory efficiency and jaw movement before and after temporomandibular disorder treatment. Cranio 2013, 31, 190–201. [Google Scholar] [CrossRef]
- Ferreira, C.L.P.; Machado, B.C.Z.; Borges, C.G.P.; Rodrigues Da Silva, M.A.M.; Sforza, C.; De Felício, C.M. Impaired orofacial motor functions on chronic temporomandibular disorders. J. Electromyogr. Kinesiol. 2014, 24, 565–571. [Google Scholar] [CrossRef]
- Marim, G.C.; Machado, B.C.Z.; Trawitzki, L.V.V.; de Felício, C.M. Tongue strength, masticatory and swallowing dysfunction in patients with chronic temporomandibular disorder. Physiol. Behav. 2019, 210, 112616. [Google Scholar] [CrossRef]
- Manfredini, D.; Cocilovo, F.; Stellini, E.; Favero, L.; Guarda-Nardini, L. Surface electromyography findings in unilateral myofascial pain patients: Comparison of painful vs non painful sides. Pain Med. 2013, 14, 1848–1853. [Google Scholar] [CrossRef]
- Lauriti, L.; Motta, L.J.; de Godoy, C.H.L.; Biasotto-Gonzalez, D.A.; Politti, F.; Mesquita-Ferrari, R.A.; Fernandes, K.P.S.; Bussadori, S.K. Influence of temporomandibular disorder on temporal and masseter muscles and occlusal contacts in adolescents: An electromyographic study. BMC Musculoskelet. Disord. 2014, 15, 123. [Google Scholar] [CrossRef]
- Caria, P.H.F.; Bigaton, D.R.; de Oliveira, A.S.; Bérzin, F. Fatigue analysis in the masseters and temporalis muscles in patients with temporomandibular disorder during short period of mastication. Acta Odontológica Latinoam. 2009, 22, 87–91. [Google Scholar]
- Herpich, C.M.; Leal-Junior, E.C.P.; Amaral, A.P.; de Paiva Tosato, J.; dos Santos Glória, I.P.; Garcia, M.B.S.; Barbosa, B.R.B.; El Hage, Y.; Arruda, É.E.C.; de Paula Gomes, C.Á.F.; et al. Effects of phototherapy on muscle activity and pain in individuals with temporomandibular disorder: A study protocol for a randomized controlled trial. Trials 2014, 15, 491. [Google Scholar] [CrossRef]
- Tosato, J.D.P.; Caria, P.H.F. Electromyographic activity assessment of individuals with and without temporomandibular disorder symptoms. J. Appl. Oral Sci. 2007, 15, 152–155. [Google Scholar] [CrossRef]
- Nickel, J.C.; Gonzalez, Y.M.; McCall, W.D.; Ohrbach, R.; Marx, D.B.; Liu, H.; Iwasaki, L.R. Muscle organization in individuals with and without pain and joint dysfunction. J. Dent. Res. 2012, 91, 568–573. [Google Scholar] [CrossRef]
- Angeles-Medina, F.; Nuño-Licona, A.; Alfaro-Moctezuma, P.; Osorno-Escareño, C. Development and application of reflexodent in the quantitative functional evaluation of chewing control in patients with temporomandibular joint dysfunction and a control group. Arch. Med. Res. 2000, 31, 197–201. [Google Scholar] [CrossRef]
- Peroz, I.; Tai, S. Masticatory performance in patients with anterior disk displacement without reduction in comparison with symptom-free volunteers. Eur. J. Oral Sci. 2002, 110, 341–344. [Google Scholar] [CrossRef]
- Shiozawa, M.; Taniguchi, H.; Hayashi, H.; Hori, K.; Tsujimura, T.; Nakamura, Y.; Ito, K.; Inoue, M. Differences in Chewing Behavior during Mastication of Foods with Different Textures. J. Texture Stud. 2013, 44, 45–55. [Google Scholar] [CrossRef]
- van der Bilt, A.; Engelen, L.; Pereira, L.J.; van der Glas, H.W.; Abbink, J.H. Oral physiology and mastication. Physiol. Behav. 2006, 89, 22–27. [Google Scholar] [CrossRef]
- Bakke, M.; Hansdottir, R. Mandibular function in patients with temporomandibular joint pain: A 3-year follow-up. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2008, 106, 227–234. [Google Scholar] [CrossRef]
- Tarata, M.T. Mechanomyography versus electromyography, in monitoring the muscular fatigue. BioMedical Eng. OnLine 2003, 2, 3. [Google Scholar] [CrossRef]
- Wang, C.; Yin, X. Occlusal risk factors associated with temporomandibular disorders in young adults with normal occlusions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 419–423. [Google Scholar] [CrossRef]
- dos Santos Berni, K.C.; Dibai-Filho, A.V.; Pires, P.F.; Rodrigues-Bigaton, D. Accuracy of the surface electromyography RMS processing for the diagnosis of myogenous temporomandibular disorder. J. Electromyogr. Kinesiol. 2015, 25, 596–602. [Google Scholar] [CrossRef]
- Santana-Mora, U.; Cudeiro, J.; Mora-Bermúdez, M.J.; Rilo-Pousa, B.; Ferreira-Pinho, J.C.; Otero-Cepeda, J.L.; Santana-Penín, U. Changes in EMG activity during clenching in chronic pain patients with unilateral temporomandibular disorders. J. Electromyogr. Kinesiol. 2009, 19, e543–e549. [Google Scholar] [CrossRef]
- Slagter, A.P.; Olthoff, L.W.; Steen, W.H.; Bosman, F. Comminution of food by complete-denture wearers. J. Dent. Res. 1992, 71, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Pasinato, F.; de Oliveira, A.G.; Santos-Couto-Paz, C.C.; Zeredo, J.L.L.; de Paula Bolzan, G.; Macedo, S.B.; Corrêa, E.C.R. Study of the kinematic variables of unilateral and habitual mastication of healthy individuals. Codas 2017, 29, e20160074. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).